Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


618 Topics in atmospheric thermodynamics
tail represents molecules with enough velocity to exceed the escape velocity. The giant
planets can therefore be expected to be rich in the lightest volatiles, hydrogen and helium,
which is indeed the case. By comparison, Titan has a much higher ^ value, so that even
though its temperature, given by its solar distance, is similar to that of Saturn, its atmosphere
is dominated by the much heavier species N
2
. The value of the parameter for the Galilean
satellites of Jupiter is higher still, consistent with the fact that not even N
2
molecules have
remained gravitationally bound.
Among the three large terrestrial planets Earth has a significantly lower ^ value than
Venus and Mars. This is part of the explanation for why Earth is so much richer in water
than the other two planets. However, the fact that Europa, Ganymede, Callisto and even the
small icy moons of Saturn are richer in water than Earth suggests that the temperature at
which a volatile species condenses and/or freezes is also an important factor in determining
whether H
2
O can remain gravitationally bound to the planet.
The apparently simple physical picture suggested by Fig. 13.1 becomes complicated
when we consider it in detail. For instance, the characteristic temperature that determines
whether molecules have enough kinetic energy to escape is not the temperature at the
planet’s surface (which I used in the figure), but rather the temperature at an elevation at
which atmospheric density is low enough that molecular collisions are unlikely, so that a
molecule that is moving faster than the escape velocity will indeed escape before it has a
chance of colliding with another molecule. The elevation where this becomes true, and the
temperature at that elevation, are not as simply defined as the surface temperature (or the
equilibrium temperature, Worked Example 13.1). Also, the escape mechanism on which
equation (13.1) is based, which is known as thermal escape, is not the only way in which a
planet can lose its atmosphere, and perhaps not even the dominant way. Further discussion of
these topics is beyond the scope of this book but clear mathematical descriptions, including
rigorous treatments of thermal and other escape mechanisms, can be found, for example,
in Hunten (1973), Chamberlain and Hunten (1987), Zahnle and Kasting (1986), Zahnle et
al. (1990) and Bohren and Albrecht (1998).
Should we expect the mass of a planet’s atmosphere to be related in some simple fashion
to the parameter ^? Atmospheric pressure at a planet’s surface is given by the weight of the
gas column. Calling the atmospheric mass density per unit of area m
a
, the mean atmospheric
pressure at the planet’s surface, P
0
, is given by:
P
0
=m
a
g, (13.2)
where g is the gravitational acceleration at the planet’s surface, assumed to remain constant
throughout the atmospheric thickness. Substituting numerical values of P
0
and g for the
five solid planetary bodies with substantial atmospheres we find that atmospheric mass
densities vary by six orders of magnitude, as follows:
Venus ∼1.1 ×10
6
kg m
−2
Titan ∼1.1 ×10
5
kg m
−2
Earth ∼1.0 ×10
4
kg m
−2
Mars ∼1.7 ×10
2
kg m
−2
Triton ∼2.1 ×10
0
kg m
−2

619 13.1 Gravitational binding of planetary atmospheres
Comparing these values with Fig. 13.1 reveals no correlation between atmospheric mass
and ^. What determines atmospheric mass, then? We begin by considering planetary accre-
tion, the bulk of which must have taken place while the growing solid bodies were immersed
in the solar nebula. Rocky planets are likely to have had primordial atmospheres of solar
composition, i.e. dominated by hydrogen and helium. These primordial atmospheres persist
in the giant planets (which have a rocky core – the image that comes to mind is that of a
dandelion), but they have been lost from the solid planets (think of dandelions after you
blow on them). Loss of primordial atmospheres probably took place by a combination of
gradual processes, such as thermal and non-thermal escape (see Hunten, 1973; Chamber-
lain & Hunten, 1987), and catastrophic processes such as atmospheric blowoff caused by
large impacts (Pepin, 1997). This physical picture is supported by the observed abundances
and isotopic compositions of noble gases in planetary atmospheres (Pepin, 2006). It is
unlikely that whatever atmosphere the proto-Earth may have had would have been capable
of surviving the Moon-forming event.
Present day atmospheres are therefore “secondary”, in the sense that they were acquired
after, and perhaps long after, planetary accretion was substantially completed, and the pri-
mordial atmospheres had been lost. The present day atmospheric masses and compositions
are therefore determined by the relative rates of addition, removal and modification of
individual volatile species since loss of the nebular atmosphere. Buildup of the secondary
atmospheres must have occurred via a combination of late accretion of volatile-rich mate-
rials (e.g. comets) and volcanic outgassing. The rates of these two processes during the
formative stages of the present-day atmospheres, almost certainly more than 4 billion years
ago, are very difficult to pin down with any degree of certainty. Yet those rates are largely
responsible for determining the initial masses and chemical compositions of the secondary
atmospheres. Crucially, the compositions of volcanic gases and cometary volatiles are likely
to have been different, and are poorly constrained.
Removal of volatile species is perhaps easier to constrain. It takes place by three distinct
pathways: escape to space, condensation, and chemical reaction with the planet’s surface
materials. As we saw above, the effectiveness of the first of these pathways depends at least
in part on the planet’s mass (i.e. its gravitational attraction) and distance from the Sun (i.e.
temperature), and may selectively remove light volatile species. For instance, we can expect
that molecular and atomic hydrogen escape planetary atmospheres much more effectively
than, say, molecular nitrogen or carbon dioxide, and that hydrogen loss will be more severe
from Venus and Mars than from Earth (Fig. 13.1). Hydrogen-bearing molecules such as
H
2
O and CH
4
are photodissociated in the upper atmosphere (Section 12.4.2). Escape of the
resulting hydrogen atoms is equivalent to an irreversible loss of water or methane, and an
increase in the oxidation state of the planet’s surface. Replenishment of these species in the
atmosphere by evaporation of liquid or solid reservoirs provides a continuous pathway for
planetary desiccation and oxidation (Chapter 14), which may have gone to near completion
in Venus and perhaps somewhat less in Mars.
Condensation of volatile species removes them from the atmosphere and tends to protect
them from escape. The process is particularly efficient if volatile species freeze, as in
Europa, Ganymede, Callisto and Triton. The surfaces of these bodies can be thought of
as collapsed atmospheres, that have been protected from escape by virtue of the very low
vapor pressures in equilibrium with solid phases. Since the rate of photodissociation is
proportional to concentration (e.g. equation (12.83)), the low vapor pressure of H
2
Oin
equilibrium with a planetary surface composed of ice hinders hydrogen loss.

620 Topics in atmospheric thermodynamics
Volatile species can also be removed from the atmosphere by reaction with surface mate-
rials. One example is precipitation of carbonates by reaction of atmospheric CO
2
with
aqueous cations leached from silicate minerals. This process is thought to have scavenged
10–60 bars’ worth of CO
2
from the terrestrial atmosphere (see for example, Walker, 1985;
Kasting & Ackerman, 1986; Kasting, 1987; Tajika & Matsui, 1993), which would other-
wise have present-day composition, mass, surface pressure and temperature not all that
different from those of Venus (and Gustav Mahler would not have existed, which would
have made the universe a much poorer place). Another example is the effect of Fe
2+
as a
sink for atmospheric O
2
. Oxidation of Fe
2+
has sequestered oxygen liberated by photodis-
sociation of H
2
O in the Martian atmosphere over billions of years, generating the strongly
oxidized Martian surface. At least in the case of Earth, present day atmospheric composi-
tion is also the result of modification by biological activity, including photosynthesis and
also unnecessary and irresponsible use of pick-up trucks, minivans, jet-skis, snowmobiles,
street lighting, insanely cold air conditioning, suffocating heating, and other sources of
anthropogenic greenhouse gases.
13.2 Equilibrium thermodynamics in a gravitational field
The equations of equilibrium thermodynamics that we have discussed to this point ignore
the existence of a gravitational field. This is generally acceptable locally, for example,
in a laboratory setting or at the scale of heterogeneous phase equilibrium in a planetary
body. However, if we are interested in the equilibrium distribution of species in a planetary
atmosphere, then the effect of the gravitational force must be taken into account. Each of
the terms in the fundamental equation for Gibbs free energy:
dG =−SdT +V dP +
i
µ
i
dn
i
(13.3)
is the product of a pair of conjugate variables, one intensive and the other extensive. In
every case the intensive variable can be thought of as a field, such that gradients in the
field drive displacement of the corresponding extensive quantity. Thus, a temperature gra-
dient generates heat flow, or, equivalently, entropy flow; a pressure gradient causes volume
change, and a gradient in chemical potential drives mass transfer. Another way of stating
these conditions is that dT = 0 implies thermal equilibrium, dP = 0 implies mechanical
equilibrium relative to expansion work, and dµ = 0 implies chemical equilibrium.
If the system is immersed in a non-uniform gravitational field then there is an additional
contribution to its energy, which arises from the work associated with displacement of matter
in the gravitational field. This is mechanical work, but it is distinct from the expansion work
that is encapsulated in the V dP term in equation (13.3). If Φ is the gravitational potential (=
gravitational potential energy per unit mass, equation (1.8)) at a point, and m
i
the molecular
weight of component i, then the product m
i
Φdn
i
is the work associated with an infinitesimal
change in the amount of component i at the point. This contribution must be included in
the Gibbs free energy of a system embedded in a gravitational field, which now becomes:
dG =−SdT +V dP +
i
µ
i
+m
i
Φ
dn
i
. (13.4)

621 13.2 Equilibrium thermodynamics in a gravitational field
By analogy with equation (5.24), a system in a gravitational field is in equilibrium relative
to transfer of component i if:
d
µ
i
+m
i
Φ
=0 (13.5)
or, as molecular weight is a constant:
dµ
i
+m
i
dΦ =0. (13.6)
In the absence of a gravitational field, or if the field can be considered to be uniform,
dΦ = 0 and we recover (5.24). However, if dΦ = 0, which is the general case, and is
in particular true in the neighborhood of planetary bodies, then equilibrium with respect
to mass transfer requires that dµ = 0. Thus, equilibrium distribution of matter in a non-
vanishing gravitational field implies a gradient in chemical potentials. Moreover, because
chemical potential is in general a function of temperature, pressure and composition, it
follows that gradients in at least some of these variables must exist in matter at equilibrium
in a gravitational field.
We can write the total change in chemical potential of component i as follows:
dµ
i
=
∂µ
i
∂T
P ,X
i
dT +
∂µ
i
∂P
T ,X
i
dP +
j=i
∂µ
i
∂X
j
P ,T
dX
j
(13.7)
or, by using (5.37) and (5.38), in terms of partial molar entropy and partial molar volume:
dµ
i
=−s
i
dT +v
i
dP +
j=i
∂µ
i
∂X
j
P ,T
dX
j
. (13.8)
Substituting in (13.6):
−s
i
dT +v
i
dP +
j=i
∂µ
i
∂X
j
P ,T
dX
j
+m
i
dΦ =0, (13.9)
which is the equilibrium condition for each component i of a multicomponent phase
immersed in a gravitational field. Summing over all components we get the equilibrium
condition for the phase:
−SdT +VdP +
i
X
i
j=i
∂µ
i
∂X
j
P ,T
dX
j
+MdΦ = 0, (13.10)
where S and V are the molar entropy and volume of the phase, and M is the molecular weight
of the phase (=weighted average of the molecular weights of the phase components). From
the Gibbs–Duhem equation (6.7) we have:
i
X
i
dµ
i
P ,T
=
i
X
i
j=i
∂µ
i
∂X
j
P ,T
dX
j
=0. (13.11)
So (13.10) simplifies to:
−SdT +VdP +MdΦ = 0. (13.12)

622 Topics in atmospheric thermodynamics
These differential equations are general, but they are not necessarily simple to solve. In
particular, the entropy term always introduces significant conceptual and computational
difficulties. Here we restrict ourselves to isothermal processes. At constant temperature,
and noting that density, ρ = M/V, we re-write (13.12) as follows:
dP
dΦ
=−ρ, (13.13)
which is the condition of hydrostatic equilibrium. In order to see this, use equation (1.8)to
calculate dΦ/dr, apply the chain rule to calculate dP/dr, and compare to equation (2.34).
13.2.1 Pressure in a one-component isothermal atmosphere.
Atmospheric scale height
Let us assume that a planetary atmosphere is composed of a single ideal gas species, so
that M is constant with elevation, and that the atmosphere is isothermal (see also Exercise
13.1). From the ideal gas EOS we have:
ρ =
PM
RT
. (13.14)
Substituting in (13.13):
dP
P
=−
M
RT
dΦ (13.15)
and integrating:
P
P
0
=exp
−
M
RT
(
Φ −Φ
0
)
, (13.16)
where P
0
, Φ
0
could be the pressure and gravitational potential at the planet’s surface, for
instance. With this convention, as we move up in the planet’s atmosphere it is Φ>Φ
0
(recall equation (1.8)), so that P < P
0
, as expected. Using the approximate expression for
gravitational potential energy that we discussed in Worked Example 1.1, and using h > 0
for elevation above the planet’s surface we get:
P = P
0
exp
−
Mgh
RT
. (13.17)
Provided that atmospheric temperature does not vary greatly, so that it makes sense to
talk of a “characteristic” temperature for the atmosphere, this equation provides a first
approximation to the variation in atmospheric pressure with elevation. In particular, it leads
to the definition of the scale height of the atmosphere, H :
H ≡
RT
Mg
(13.18)
so that:
P = P
0
exp
−
h
H
(13.19)

623 13.2 Equilibrium thermodynamics in a gravitational field
Thus, when elevation changes by H atmospheric pressure varies by a factor e. This gives
an indication of how quickly an atmosphere “fades” with elevation: the greater the scale
height, the more the atmosphere extends into space. Substituting appropriate values for the
five planetary bodies with substantial atmospheres in equation (13.18) we calculate scale
heights of: 7.9 km for Earth, 10.7 km for Mars, 13.3 km for Triton, 14.9 km for Venus and
19.8 km for Titan. Of all the bodies with air in the Solar System the Earth is the one that
“holds” its atmosphere closer to the solid surface. At the other end are Venus and Titan,
that have the most “stretched out” atmospheres, although for different reasons (see equation
(13.18)): Venus because of its high temperature, and Titan because of its low gravitational
acceleration. In the case of Triton the low gravitational acceleration is offset by the very
low temperature. Note that the definition of scale height, equation (13.18), is similar to
that of the parameter ^, equation (13.1), except that H is a dimensional quantity, and the
composition of the atmosphere is accounted for by means of the mean molecular weight,
M . The scale height is, however, undefined for an airless body, but ^ is independent of the
existence of an atmosphere.
13.2.2 Compositional stratification in a fluid immersed in a gravitational field
Consider a fluid composed of an arbitrary number of chemical species. The chemical
potential of species i is given by:
µ
i
=µ
0,i
+RT ln f
i
(13.20)
from which, at constant temperature:
dµ
i
=RT d ln f
i
. (13.21)
Substituting in (13.6):
RT d ln f
i
+m
i
dF =0 (13.22)
and integrating:
f
i
=f
i,0
exp
−
m
i
RT
(
Φ −Φ
0
)
(13.23)
where f
i,0
is the fugacity at the reference level, not the standard state fugacity. This rela-
tionship is general, and must be satisfied by every species in a multicomponent gas phase
immersed in a gravitational field. Suppose that the atmosphere behaves as an ideal gas. We
can then substitute partial pressure for fugacity, which simplifies things considerably. By
using, as in the previous example, h for elevation relative to the planet’s surface we find:
p
i
=p
i,0
exp
−
m
i
gh
RT
, (13.24)
where p
i
is the partial pressure of species i.
If we now consider two species in the gas phase, call them 1 and 2, with differentmolecular
weights we see that the ratio between the partial pressures of the two species varies as a
function of elevation as:
p
1
p
2
=
p
1,0
p
2,0
exp
(
m
2
−m
1
)
gh
RT
, (13.25)
624 Topics in atmospheric thermodynamics
0 0.02 0.04 0.06 0.08 0.1 0.1
2
0
10
20
30
40
X (CH
4
)
Elevation over Titan’s surface (km)
0 0.5 1 1.5
Atmospheric pressure (bar)
X(CH
4
)
P
H
Fig. 13.2
Variation with height of atmospheric pressure and methane mol fraction in Titan’s atmosphere, assuming surface
pressure =1.5 bar and constant temperature =90 K. H is the atmospheric scale height (equation (13.18)).
which shows that the composition of the gas phase will be stratified according to the
molecular weights (or densities) of the component species. For instance, if m
2
> m
1
, then
the ratio p
1
/p
2
increases with elevation. The gas becomes enriched in the lighter component
towards the top – which of course we already knew, but we can now quantify the effect.
Equation (13.25) shows that the extent to which a fluid phase fractionates in a gravitational
field varies directly with the difference in molecular weights of the component species and
with the gravitational acceleration, and inversely with temperature.
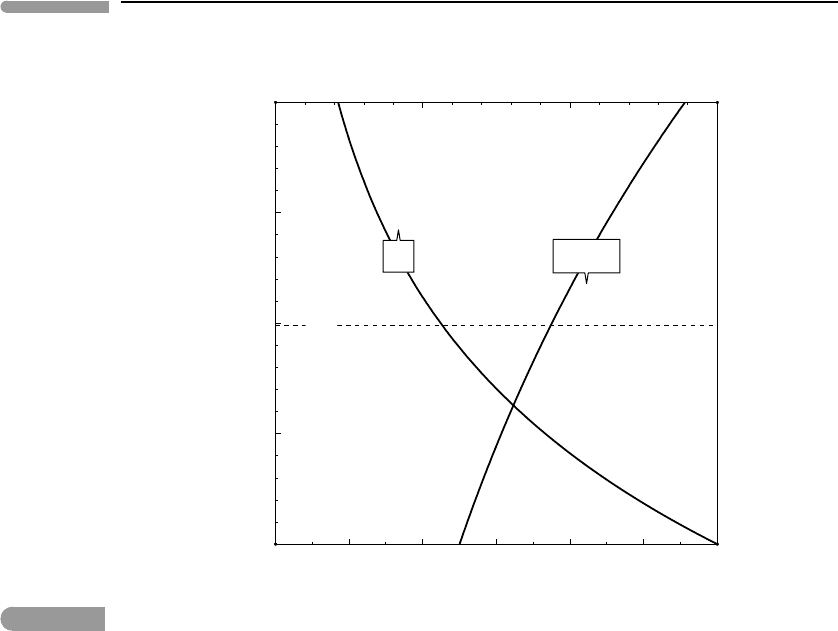
Figure 13.2 shows an application to Titan’s atmosphere. We assume that the atmosphere
consists of a binary mixture of CH
4
and N
2
, with X(CH
4
) at the surface equal to 0.05,
a surface pressure of 1.5 bar and a characteristic atmospheric temperature of 90 K. The
partial pressure of each gas as a function of elevation is calculated with (13.24), the
mol fraction of methane is X(CH
4
) = p(CH
4
)/(p(CH
4
) + p(N
2
)), and the total atmo-
spheric pressure is p(CH
4
) + p(N
2
). The latter value is virtually indistinguishable from
the value calculated with (13.19) because, even if the methane/nitrogen ratio changes sub-
stantially with elevation, nitrogen is always the dominant species. The mol fraction of
methane more than doubles between the surface and the upper atmosphere, which is impor-
tant given that methane is destroyed by photochemical reactions in the upper atmosphere
(Section 12.4.2), leading to irreversible hydrogen loss. Since the photochemical reaction
rate is proportional to methane concentration, atmospheric stratification enhances the rate of
hydrogen loss.
625 13.3 Radiative energy transfer
13.3 Radiative energy transfer
Although advection is an important heat transfer mechanism in some atmospheric layers,
solar heating of atmospheres and planetary surfaces, and heat loss of planets to space, take
place by radiation. We therefore devote this section to a discussion of radiative heat transfer,
and apply it to construct a simple quantitative model of greenhouse warming.
13.3.1 Fundamental concepts and equations of thermal radiation
All materials, at any temperature, emit and absorb electromagnetic radiation. The rates
at which a body emits and absorbs electromagnetic energy are not necessarily the same,
however. If they are then there is no net conversion between internal and electromagnetic
energies, but if, say, the rate of emission is higher than the rate of absorption then internal
energy is converted to electromagnetic energy, and conversely if absorption outpaces emis-
sion. At the microscopic level, conversion between internal energy and electromagnetic
radiation corresponds to exchanges between the vibrational, rotational and/or translational
kinetic energy modes of atoms and molecules on one side and energy of photons on the
other.
Here we will assume that all radiant surfaces behave as diffuse emitters, which means
that they emit radiation with the same intensity in all directions. This is not necessarily
true in nature, but this simplification makes it possible to avoid a significant amount of
terminology, algebra and solid geometry, and concentrate on the fundamental physics of
radiative heat transfer. We define the irradiance, F, as the total flux (energy per unit area
per unit time) of electromagnetic radiation over the entire spectrum and traveling in all
directions. The monochromatic irradiance, F
λ
, is the energy flux for radiation of a single
wavelength λ. The two variables are obviously related by:
F =
∞
0
F
λ
dλ. (13.26)
It is often necessary to distinguish between emitted and incident energy flux, which we will
do explicitly (special terms exist for these different quantities, but we will not introduce
them here).
Electromagnetic radiation travels unimpeded in vacuum, but it interacts with matter.
Interactions between radiant energy and matter can be described in terms of the absorptivity,
A
λ
, the transmissivity, Θ
λ
, and the reflectivity, R
λ
, of the medium, all of which are functions
of wavelength. These macroscopic parameters arise from microscopic interactions between
photons and particles of matter (molecules for the range of wavelengths of interest in
planetary processes, see Section 13.3.3). Each of these parameters varies between 0 and 1
and represents the fraction of the irradiance of wavelength λ that is absorbed, transmitted
and reflected, respectively. Their sum is always equal to 1, i.e.:
A
λ
+Θ
λ
+R
λ
=1 (13.27)
and also:
A +Θ +R =1, (13.28)

626 Topics in atmospheric thermodynamics
where A, Θ and R are the total absorptivity, transmissivity and reflectivity, integrated over
all wavelengths. These equations state energy conservation: the total incident flux of radiant
energy must be accounted for in terms of a fraction that is reflected, a fraction that is absorbed
and a fraction that is transmitted.
The spectrum and intensity of the electromagnetic radiation emitted by a body depends
on its temperature and on another macroscopic parameter called the emissivity, / (/ ≤ 1),
which is a function of temperature and of wavelength. Ablack body is defined as a substance
that emits and absorbs the maximum possible intensity of radiation at all wavelengths and
in all directions. Thus, for a black body A
λ
= /
λ
=1 and Θ
λ
= R
λ
= 0, for all wavelengths
and at all temperatures. The black body monochromatic emission flux, F
∗
λ
, also called the
spectral emissive power, is given by Planck’s radiation law:
F
∗
λ
=
2πhc
2
λ
5
e
hc
λk
B
T
−1
, (13.29)
where h is Planck’s constant, k
B
is Boltzmann’s constant and c is the speed of light. This
equation was first proposed semi-empirically by Planck in 1901 and became one of the
foundational pillars of quantum mechanics. Its derivation is beyond the scope of this book
but can be found, for example, in Incropera and DeWitt (1996) or Jones (2000). Equation
(13.29) is a function of two variables, temperature and wavelength. It yields the spectrum
of the electromagnetic radiation emitted by a black body at a constant temperature T, i.e.
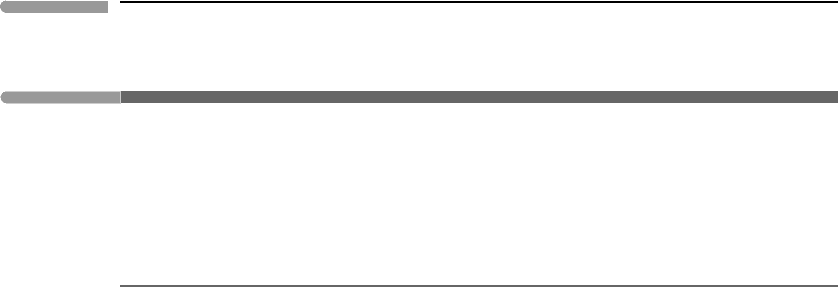
the distribution of emitted energy flux as a function of wavelength (Fig. 13.3). The curve is
a “skewed bell”. From the peak, radiation flux falls off steeply towards shorter wavelengths
and more gently towards longer wavelengths. The wavelength at which the maximum flux
occurs can be found by taking the derivative dF
λ
∗
/dλ and equating it to zero (see Exercise
13.3). The result, known as Wien’s displacement law, is:
λ
peak
≈
2898
T
(13.30)
with T in Kelvin and λ in µm. The peak shifts towards shorter wavelengths with increasing
temperature.
Figure 13.3 shows the regions into which the electromagnetic spectrum is conventionally
subdivided within the interval 10
−2
–10
4
µm. The Sun radiates (approximately) as a black
body at a temperature of ∼6000 K. Because radiation emitted at this temperature peaks
at ∼0.5 µm it was evolutionarily advantageous for life on Earth to develop acute sensory
organs that respond to wavelengths in this region. For this reason we call the 0.4–07 µm
range visible radiation. It also happens that animal perception senses radiation extending
from the near ultraviolet to the near infrared (roughly, 0.1–10 µm) as heat. Conventionally,
we extend this range to the far infrared (∼100 µm) and call the range 0.1–100 µm thermal
radiation. However, any electromagnetic radiation that has a spectrum given by Planck’s
equation (13.29) is strictly speaking thermal radiation.
The integral of F
λ
∗
over all wavelengths (i.e. the area under the curves in Fig. 13.3)
yields the total black body emission flux, F
∗
, at temperature T and across the full spectrum.
Substituting (13.29)in(13.26) and solving the integral (see Exercise 13.4) we get:
F
∗
=
∞
0
F
∗
λ
dλ =σT
4
. (13.31)
627 13.3 Radiative energy transfer
0.01 0.1 1 10 100 1000 1000
0
10
–17
10
–12
10
–7
F*
λ
(MW m
–2
µm
–1
)
10
–2
10
3
Wavelength (µm)
ultraviolet
infrared microwavev.
thermal radiation
6000 K
278 K
35 K
2.7 K
Fig. 13.3
Blackbody emission power spectrum calculated with Planck’s radiation law (equation (13.29)) for the temperature of
the solar photosphere (∼6000 K), the terrestrial equilibrium temperature (278 K), the temperature at which the
Earth’s internal energy flux would radiate to space if the Sun disappeared (35 K) and the cosmic microwave
background radiation (2.7 K). The narrow wavelength interval labeled “v.” is what we call visible radiation, because
we evolved around a star whose spectrum peaks in this region, making sensory organs that respond to these
wavelengths evolutionarily advantageous.
This is Stefan–Boltzmann’s law (equation (2.1), with / = 1). From the integral it also
follows that the Stefan–Boltzmann constant, σ , is given by (Exercise 13.4):
σ =
2
15
π
5
h
−3
c
−2
k
4
B
. (13.32)
13.3.2 Radiant energy exchange
The geometry sketched in Fig. 13.4 yields some results that are useful in solving problems
of thermal radiation that arise in planetary sciences. We consider a spherical body of radius
r
b
and surface area a
b
, at a uniform temperature T
b
, concentric with a spherical cavity of
radius r
c
!r
b
and surface area a
c
, at temperature T
c
. We assume that the intervening space
has transmissivity Θ =1 and we will initially also assume that both the sphere and the cavity
are black bodies, i.e. A
λ
= /
λ
= 1 for all λ. From equation (13.31) and the surface areas
