Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


588 Non-equilibrium thermodynamics
where D
i
is the interdiffusion coefficient of species 1 and 2 in a binary mixture.
Note that identity (12.38) does not mean that the interdiffusion coefficient is constant:
equations (12.37) show that it is a function of the concentration product c
1
c
2
. Because the
phenomenological coefficient L is a constant, however, it is in principle possible to calcu-
late the interdiffusion coefficient for any arbitrary composition from measurements of tracer
diffusion coefficients of one of the two species. From this we can infer that interdiffusion
coefficients are at most of the same order of magnitude as tracer diffusion coefficients. For
example, if we take species 1 as the trace component we have (equation (12.25)):
D
1,tr
=
LR
c
1,tr
, (12.39)
where c
1,tr
is a trace concentration of 1, and D
1,tr
is the corresponding tracer diffusion
coefficient. Using 12.37 we calculate the interdiffusion coefficient for c
1
!c
1,tr
:
D
i
=D
1,tr
c
1,tr
Vc
1
c
2
, (12.40)
which is at most of the same order as D
1,tr
, and generally smaller. It follows that the
conclusions that we reached in Worked Example 12.1 are valid for chemical diffusion in
general, not just tracer diffusion.
Consider now a slightly different case, in which rather than balancing the number of
particles, as in equation (12.31), the opposing flows of matter keep the volume of the
system constant. Equation (12.31) is then modified as follows:
v
1
J
1
+v
2
J
2
=0, (12.41)
where v
1
, v
2
are the partial molar volumes of the two species. In this case we find that the
diffusion coefficients are given by:
D
1
=
LR
X
2
v
1
c
1
D
2
=
LR
X
1
v
2
c
2
(12.42)
(note that the dimension of L is not the same as in equations (12.37), which of course follows
from the fact that we are now considering volume fluxes rather than particle fluxes). The
interdiffusion coefficient is now given by:
D
i
=v
1
D
1
=v
2
D
2
, (12.43)
which, if the two species have the same partial molar volume, as is the case for ideal gases,
collapses to (12.38).
The fact that binary diffusion can be fully described with a single phenomenological
coefficient (equations (12.37)or(12.42)) means that there are no “cross-flow” terms, or,
in other words, that the Onsager reciprocal relationship does not apply to binary diffusion.
This is so because the chemical potential gradient of each component is unequivocally
determined by the concentration gradient of the other. Reciprocal matter flow terms appear
in systems of three or more components (Exercise 12.1), in which there is at least one
concentration that can vary independently.
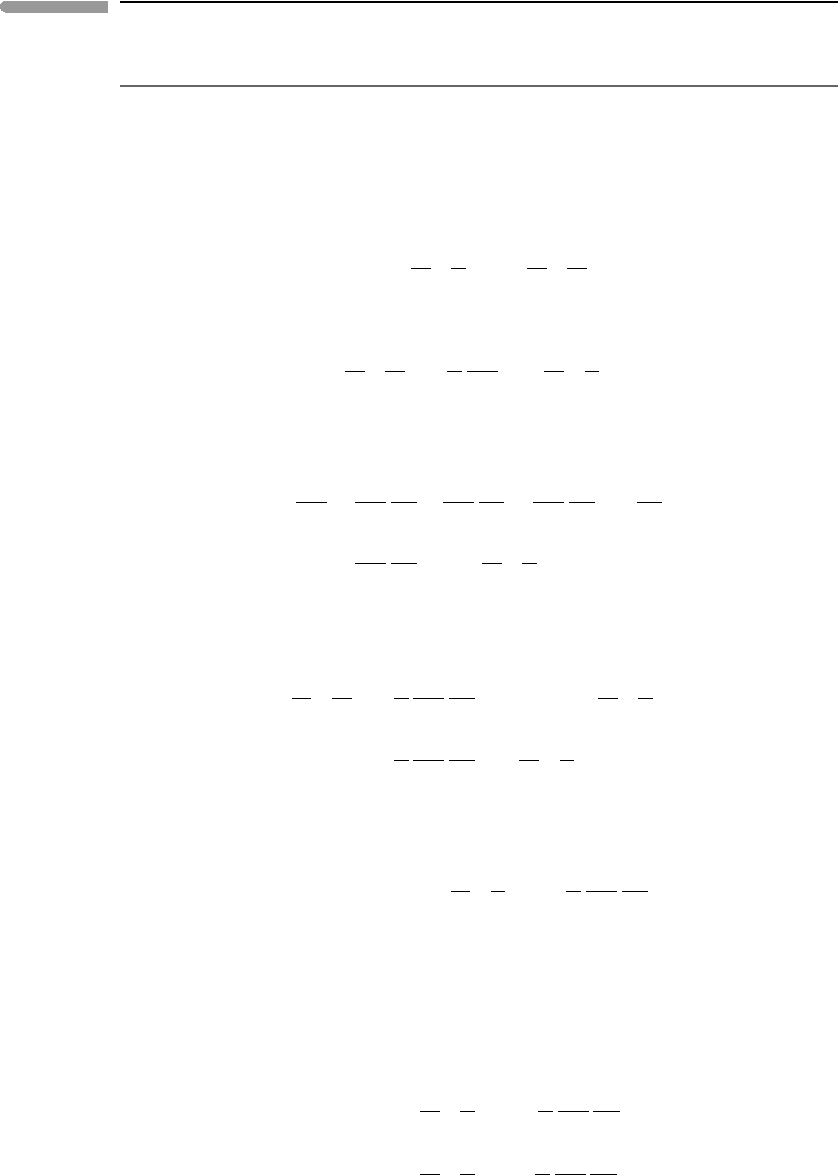
589 12.2 Chemical diffusion
12.2.4 Coupling of mass and heat transfer
Let us consider a system in which there are gradients in temperature and composition. For
simplicity we will assume that a compositional gradient exists in only one component, i.e.
we consider tracer diffusion, and we restrict the treatment to one spatial dimension. Using
J
q
for heat flux and J
i
for matter flux of component i, the rate of entropy production σ is
(see equations (12.5), (12.6) and (12.20)):
σ = J
q
∂
∂z
1
T
−J
i
∂
∂z
µ
i
T
. (12.44)
We have:
∂
∂z
µ
i
T
=
1
T
∂µ
i
∂z
+µ
i
∂
∂z
1
T
(12.45)
but chemical potential is a function of both composition and temperature, both of which
vary, so:
∂µ
i
∂z
=
∂µ
i
∂c
i
∂c
i
∂z
+
∂µ
i
∂T
∂T
∂z
=
∂µ
i
∂c
i
∂c
i
∂z
−s
i
∂T
∂z
=
∂µ
i
∂c
i
∂c
i
∂z
+s
i
T
2
∂
∂z
1
T
,
(12.46)
where s
i
is the partial molar entropy of component i, and c
i
its concentration, which we
choose to express in mols per unit volume. Substituting in (12.45):
∂
∂z
µ
i
T
=
1
T
∂µ
i
∂c
i
∂c
i
∂z
+
s
i
T +µ
i
∂
∂z
1
T
=
1
T
∂µ
i
∂c
i
∂c
i
∂z
+h
i
∂
∂z
1
T
,
(12.47)
where h
i
is the partial molar enthalpy of i. We now substitute (12.47)in(12.44) and group
terms as follows:
σ =
J
q
−h
i
J
i
∂
∂z
1
T
−J
i
1
T
∂µ
i
∂c
i
∂c
i
∂z
. (12.48)
The product h
i
J
i
, known as the heat of transport, is the thermal energy content of the matter
flow. Defining the reduced heat flux, J
q,r
as:
J
q,r
≡J
q
−h
i
J
i
(12.49)
we write the linear phenomenological relationships, (12.8), as follows:
J
q,r
=L
q
q
∂
∂z
1
T
−L
q
i
1
T
∂µ
i
∂c
i
∂c
i
∂z
J
i
=L
i
q
∂
∂z
1
T
−L
i
i
1
T
∂µ
i
∂c
i
∂c
i
∂z
(12.50)
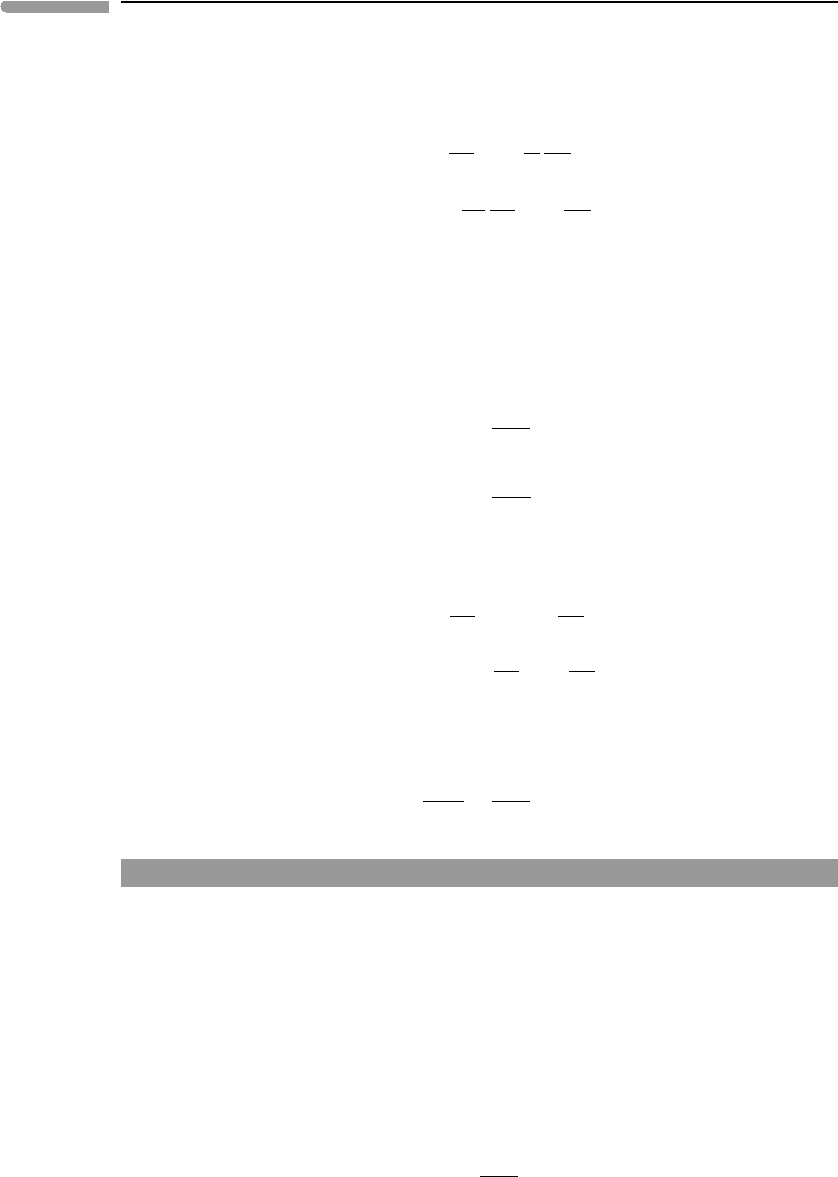
590 Non-equilibrium thermodynamics
or, using the definitions of heat conductivity k (equation (12.13)) and chemical diffusivity
D
i
(equation (12.25)), and identity (12.23):
J
q,r
=−k
∂T
∂z
−L
q
i
R
c
i
∂c
i
∂z
J
i
=−L
i
q
1
T
2
∂T
∂z
−D
i
∂c
i
∂z
.
(12.51)
The two cross terms are the heat flux carried by matter flow, which is known as the
Dufour effect, and the flow of matter driven by the temperature gradient, which is known
as the Soret effect, or also as thermal diffusion. Defining the Dufour coefficient, D
D
, and
the coefficient of thermal diffusion, D
T
, as follows (do not confuse D
T
with the thermal
diffusivity, κ!):
D
D,i
≡
L
q
i
R
c
2
i
D
T ,i
≡
L
i
q
c
i
T
2
(12.52)
equations (12.51) become:
J
q,r
=−k
∂T
∂z
−c
i
D
D,i
∂c
i
∂z
J
i
=−c
i
D
T ,i
∂T
∂z
−D
i
∂c
i
∂z
(12.53)
in which we have from the Onsager reciprocal relationship, L
q
i
=L
i
q
:
D
D,i
D
T ,i
=
RT
2
c
i
. (12.54)
Worked Example 12.2 Soret and Dufour diffusion in planetary processes
The derivations in this section make for some rather elegant, if elementary, algebra (even
more so in three dimensions). They lead to equation (12.54), which suggests an experimental
test of the Onsager reciprocal relation. But how important are these effects in planetary
processes? During the 1980s it was thought that Soret diffusion could be an important,
perhaps even dominant, mechanism of igneous differentiation (see Walker & DeLong,
1982; Lesher, 1986; Lesher & Walker, 1991). This is now known not to be the case, except
perhaps in very specific and localized situations, and then only over minuscule lengthscales.
Let us see why.
It is convenient to define the Soret coefficient, s
T
, as the ratio of thermal to chemical
diffusivity:
s
T
≡
D
T ,i
D
i
. (12.55)

591 12.2 Chemical diffusion
We note that s
T
has dimension of [T ]
−1
, i.e. K
−1
. The matter flux equation becomes:
J
i
=−s
T
c
i
D
i
∂T
∂z
−D
i
∂c
i
∂z
. (12.56)
The condition of no matter flow, i.e. J
i
= 0, results if the thermal and chemical gradients
have opposite signs and their effects exactly balance each other out. Thus, by setting J
i
=0
we get an estimate of the magnitude of the compositional gradient that can be caused by
a thermal gradient. It is convenient to do this in terms of mol fraction rather than molar
concentration, so using the relationship X
i
=c
i
V we write:
−
s
T
X
i
D
i
V
∂T
∂z
−
D
i
V
∂X
i
∂z
=0 (12.57)
which simplifies to:
dX
i
dT
=−s
T
X
i
. (12.58)
Thus, approximately:
δX
i
∼−s
T
X
i
δT . (12.59)
The key is, of course, the value of s
T
. Soret coefficients are poorly understood functions of
composition, temperature and temperature gradient (Lesher & Walker, 1991; Huang et al.,
2010). For a wide range of materials, including silicate melts, aqueous electrolyte solutions
and gases, s
T
is of order 10
−2
to 10
−3
K
−1
. This means that we can expect Soret diffusion
to be a significant effect only in settings in which there are strong temperature differences,
of order 100 K or more. Such temperature differences could exist, for example, across the
thermal boundary layer of a convective magma chamber. Soret diffusion could be important
in that instance (Sonnenthal, 2004).
This is not the whole story, however, as the rate of mass transfer is still determined by the
chemical diffusivity, regardless of whether the driving force is a gradient in composition
or temperature. Given that thermal diffusivity, κ ∼10
−6
m
2
s
−1
is typically six orders of
magnitude greater than chemical diffusivity in silicate melts, D
i
∼ 10
−12
m
2
s
−1
(see
Worked Example 12.1), thermal gradients decay much faster than chemical gradients. The
question is, then, how far can Soret diffusion extend in the time that it takes for a thermal
gradient to dissipate? Using λ
q
for the thermal diffusion lengthscale and λ
i
for the chemical
diffusion lengthscale we have, from equations 3.16 and 12.30:
λ
i
λ
q
∼
D
i
κ
1/2
∼10
−3
. (12.60)
Suppose that a thermal gradient of ∼100 K exists across a 10-m thick thermal boundary
layer of a magma chamber. Soret diffusion would cause a significant compositional change
over a thickness of only ∼1 cm. An essentially identical result was obtained by Cygan and
Carrigan (1992) by means of a rigorous numerical solution of the mass flow equations (i.e.
(12.56)). Soret diffusion is not important in nature, although it could have noticeable effects
on isotopic fractionation during high-temperature experiments (Richter et al., 2008, 2009),
and also has technical applications.
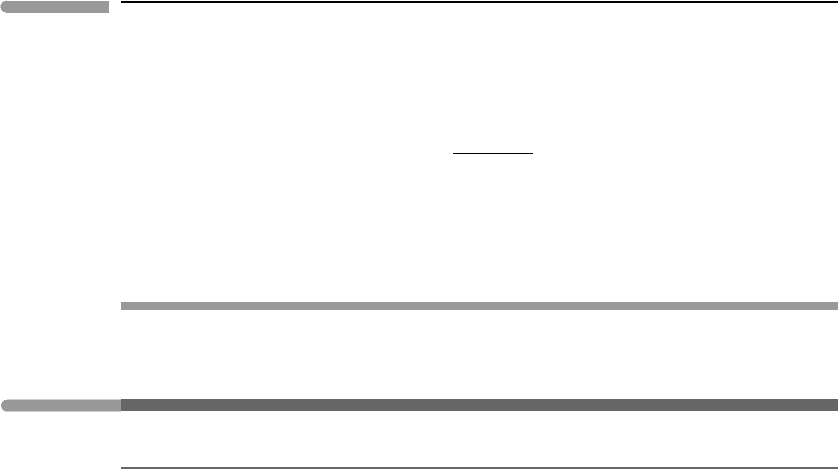
592 Non-equilibrium thermodynamics
The Dufour effect is smaller than the Soret effect. Using (12.54) and (12.55) in the heat
flow equation (12.53), and setting J
q,r
=0, we find:
δT ∼−
s
T
D
i
RT
2
kV
δX
i
. (12.61)
Substituting typical values we find δT ∼ 10
−4
δX
i
. We can interpret this result as meaning
that the Dufour effect is equivalent to the heat flow driven by a temperature difference
of order 10
−4
K times the mol fraction difference. It is unlikely that there are natural
environments where this is a significant effect.
12.3 Rate of chemical reactions
12.3.1 Fundamental concepts
Chemical reactions displace systems towards equilibrium. Therefore, chemical reactions
take place while a system is not at equilibrium. The study of the rate of chemical reactions is
a huge field and we can only cover some of the basic concepts. In particular, we will focus
only on chemical reactions in homogeneous systems, e.g. a gas phase or a liquid solution.
We begin by considering elementary reactions. These can be defined as reactions that go
from reactants to products without any intermediate steps. At a molecular level, elementary
reactions take place by collisions between the actual reactant molecules. Most chemical
reactions are not elementary. Rather, they are combinations of elementary steps. Here we
focus on elementary reactions in homogeneous systems only, whether or not I state this
explicitly.
We can expect on physical grounds that there are likely to be some simple constraints
on the nature of elementary reactions, in particular with regards to the number of partic-
ipating molecules. This number is known as the molecularity of the reaction. Elementary
reactions are known to have molecularities of 1, 2 or 3 only, and are designated unimolecu-
lar, bimolecular or termolecular, respectively. Of these, bimolecular reactions are the most
straightforward, as they simply require a collision between two reactant molecules, for
example A and B:
A +B →products.
In contrast to this simple case, unimolecular and termolecular reactions each present prob-
lems of their own, and it is arguable whether they are rigorously elementary. Let us focus
on termolecular reactions first, which we can schematize as:
A +B +C → products.
The probability that three different molecules will converge on the exact same spot at exactly
the same time is very low, and in fact termolecular elementary reactions are much rarer than
bimolecular ones. The simplest picture of how termolecular reactions take place is that they
consist of two collisions in rapid succession, such that one of the collisions produces a

593 12.3 Rate of chemical reactions
temporary “agglutination” of two of the reactant molecules, for example:
A +B →AB,
AB +C → products.
An important point is that A and B remain stuck for only a very short period of time. If they
collide with C within this time then the products form; if not A and B separate. The fact that
termolecular reactions become more common with increasing pressure is consistent with
this model, as higher molecular density makes it more likely that C will collide with the AB
cluster before it has time to break down. We can expect that reactions with molecularities
higher than three must be exceedingly rare, and indeed there is no experimental evidence
for such reactions taking place.
Unimolecular reactions correspond to:
A →products
and they present a different problem. If a reaction is truly unimolecular, then what makes
a molecule react? Some fraction of the reactant molecules must acquire higher energy,
sufficient to cause the energized molecules to break apart into the product molecules (if
all reactant molecules were energized then the reactant would disappear instantaneously
and we would not have to worry about chemical kinetics). One way in which this can
happen is by irradiation with photons of the appropriate wavelength – this is known as
photodissociation (Section 12.4.2). But unimolecular reactions that are not photo-activated
also exist. The path of unimolecular decomposition in this case is known as the Lindemann
mechanism and can be schematized as follows:
A +M → A
∗
+M
A
∗
+M → A +M
A
∗
→products.
The first step, known as the activation step, involves a collision with another molecule,
M, known as a collision partner. M could be another molecule of A, it could be a product
molecule, or it could be a molecule of some other species. The important point is that as
a result of the collision some of the energy of M is transferred to A, which becomes the
activated molecule A
∗
. This molecule can lose its energy excess either by colliding with
another collision partner in a deactivation step (second step) or by breaking apart into the
products (third step). The reaction is not truly unimolecular, but if we add up the first and
third steps it looks unimolecular.
One can legitimately argue whether a unimolecular reaction of this kind is an elementary
reaction, and the same is true of termolecular reactions which consist of two collisions
in rapid succession. Such arguments may not be all that important, however, as what we
observe macroscopically is not the molecularity but a related quantity, known as the order
of the reaction. In the simple examples discussed above the molecules that participate in
elementary reactions were labeled A, B and C, but they do not necessarily have to be
different. We can therefore write a generalized elementary reaction as follows:
i
ν
i
Y
i
→products, (12.62)

594 Non-equilibrium thermodynamics
where ν
i
is the stoichiometric coefficient of reactant species Y
i
. We can expect that the rate of
an elementary reaction must be proportional to the number of molecules that are available to
react, which in the simplest case means the concentration of each of the reactant molecules.
A thermodynamically more rigorous choice would be their activities (see Section 12.3.2),
but concentration is universally used in kinetic studies, because it is a directly observable
quantity, whereas activity is not. We therefore write the rate law of reaction (12.62) as:
r =k
i
[
Y
i
]
ν
i
, (12.63)
where r is the reaction rate expressed in units of number of (reacting) mols per unit of
volume per unit of time, and [Y
i
] is the concentration of reactant Y
i
in mols per unit
volume. This notation for concentration is different from what we have used so far, but
is standard in the literature of chemical kinetics, and convenient too. The parameter k is
called the rate constant for the reaction. It is important to understand that the rate constant is
not a thermodynamic quantity, and that it in fact encapsulates aspects of chemical kinetics
that cannot be addressed by thermodynamics. We shall have more to say about this in later
sections, but we note at this point that the rate constant is a measure of the fraction of
molecules that are reactive. Owing to the statistical distribution of molecular energies (e.g.
Section 1.14) we can expect that there will always be some fraction of the total ensemble of
molecules that do not carry enough energy for the molecular bonds to be broken during a
collision. Those molecules that are energetic enough are the reactive molecules. The fraction
of reactive molecules, and thus the rate constant, increases with temperature in the case of
thermally activated reactions (Section 12.4.1), but molecules can become activated by non-
thermal processes as well, such as absorption of photons within a specific wavelength. This
is the basis of photochemical reactions (Section 12.4.2).
Reaction (12.63) is said to be of order ν
i
in species Y
i
, and the order of the reaction as a
whole is the sum
i
ν
i
. At first sight the order of an elementary reaction is the same as its
molecularity, and indeed elementary reactions can be of first, second or third order only, but
the detailed microscopic picture is a bit different. It is clear that bimolecular reactions must
be of order two. Regardless of whether an elementary termolecular reaction occurs by a
single triple collision or by two collisions in rapid succession, we may expect it to be of order
three, because in either case the rate should vary with the product of the concentrations of the
three species. The point is that what is actually measured is the order of the reaction, which
is a macroscopic variable, and the molecularity is one possible microscopic interpretation
of this observation. Finally, it must be noted that the overall order of a compound reaction,
composed of an assemblage of elementary steps, is defined by equation (12.63), but in such
case there is no connection with any simple microscopic picture, and the order need not be
1, 2, or 3, in fact not even an integer (Logan, 1996; Houston, 2006).
An important part of the study of chemical kinetics focuses on the rate laws for reactions
of the various orders, and on how these laws combine in non-elementary reactions. We
discuss these topics below, but before doing that it is important to clarify some of the
relationships between thermodynamics and kinetics.
12.3.2 Thermodynamics, kinetics and entropy production by chemical reactions
All of the arguments that we made in the preceding section are strictly kinetic. By writ-
ing an elementary reaction, for example as A +B → products, we are eschewing any
thermodynamic content, as we are implying that the reaction proceeds in only one direction,

595 12.3 Rate of chemical reactions
and that there is no possibility of equilibrium between reactants and products. Thermody-
namics tells us that this is not the case, and that in fact there is always the possibility, at least
in principle, that the free energies of products and reactants will be the same, and equilib-
rium will be attained. The key to understanding the relationship between thermodynamics
and kinetics can be found by focusing on equilibrium situations, and more specifically on
how a chemical reaction approaches equilibrium.
Let us write a somewhat different version of reaction (12.62) as follows:
i
ν
i
Y
i
→
←
j
ν
j
Z
j
, (12.64)
where now ν
i
is the stoichiometric coefficient of reactant species Y
i
, and ν
j
is the stoi-
chiometric coefficient of product species Z
j
. The implication of equation (12.64) is that
there are two elementary reactions taking place simultaneously: the forward reaction (Y s
going to Zs) and the reverse reaction (Zs going to Y s). At equilibrium the rates of the two
reactions are the same, and in fact this is one possible definition of chemical equilibrium,
but in general this need not be so. We also define a variable, ξ , called the extent of reaction
or progress variable, as follows:
dξ ≡
dn
Z
j
ν
j
=−
dn
Y
i
ν
i
, all i, j , (12.65)
where dn
Zj
is the number of mols of species Zj that are produced, dn
Yi
is the number
of mols of species Yi that are consumed, and ν
j
, ν
i
are the corresponding stoichiometric
coefficients. By defining the progress variable in this way we avoid any ambiguities about
the extent of reaction that might arise from the variable stoichiometric coefficients. Note
that the progress variable is always a positive quantity. We also define the net rate of reaction
(12.64), r
n
, as follows:
r
n
≡
1
V
dξ
dt
(12.66)
with dimension of mols per unit volume per unit time. If the reaction rates of the forward
and reverse reactions in (12.64) are r
f
and r
r
, respectively, then the net rate of the reaction,
r
n
, must be equal to the difference between the forward and reverse rates, i.e.:
r
n
=r
f
−r
r
. (12.67)
We now seek to relate kinetics to thermodynamics, so we will write the rate laws
(equation (12.63)) in terms of activity rather than concentration. One way to think about
this is that in (12.63) the activity coefficients are subsumed in the rate constants, whereas
now we include them in the activities. We therefore write the forward and reverse reaction
rates as follows:
r
f
=k
f
i
a
Y
i
ν
i
r
r
=k
r
j
a
Z
j
ν
j
,
(12.68)
where k
f
, k
r
are the rate constants for the forward and reverse reactions.
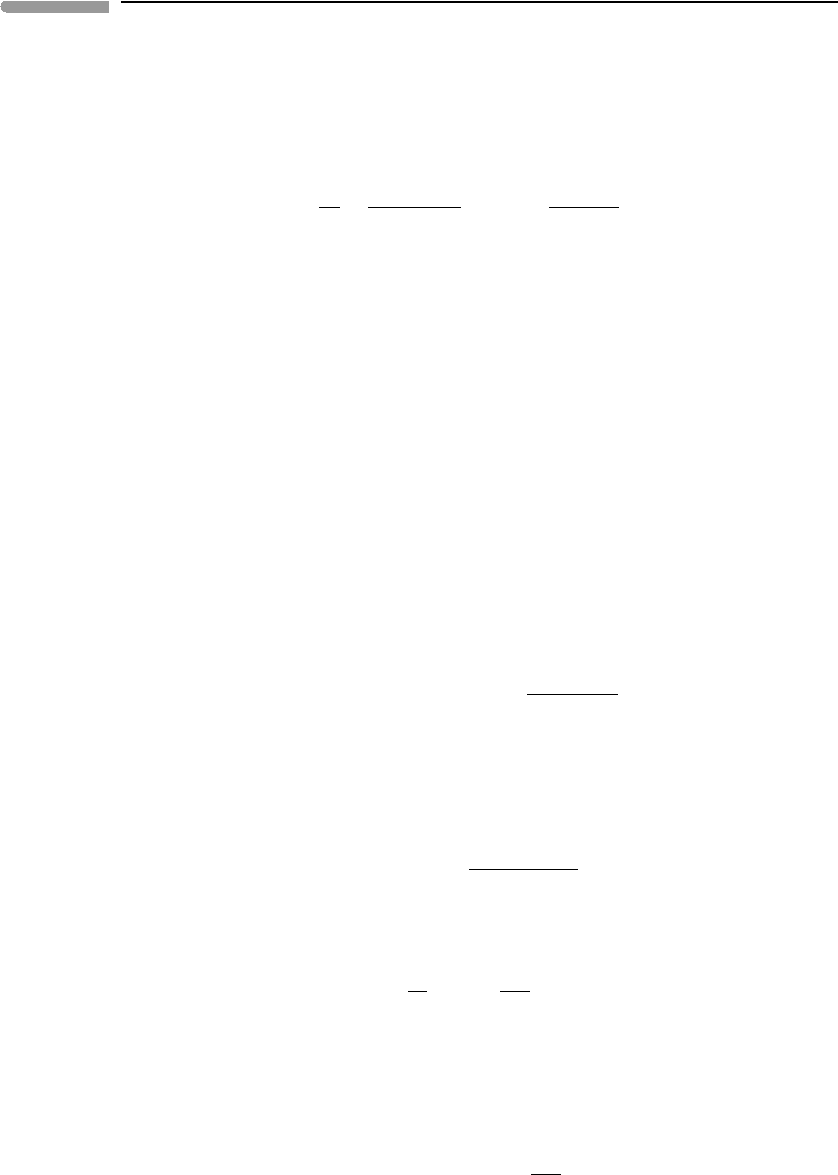
596 Non-equilibrium thermodynamics
The double arrow in equation (12.64) is always true, but we commonly think of a reaction
as “proceeding to the right” or “proceeding to the left”. What these statement refer to is
to the relative magnitudes of the forward and reverse rates. In particular, we can define
chemical equilibrium as the condition for which r
n
=0. From (12.68) this implies:
k
f
k
r
=
j
a
Z
j
ν
j
eq
i
a
Y
i
ν
i
eq
=exp
−
r
G
0
P ,T
RT
, (12.69)
where I have added the subscript eq to specify that these are the activities at equilibrium. Up
until this point we have only dealt with equilibrium situations, so that this notation was not
necessary, but this is no longer the case. If the rate laws given by equations (12.68) are valid,
then equation (12.69) says that the ratio between the forward and reverse rate constants is
determined by equilibrium thermodynamics, and is in fact equal to the equilibrium constant.
What thermodynamics cannot do is provide the values of the individual rate constants.
We now seek a thermodynamic function that gives the distance of a chemical reaction
from equilibrium. This function is called the affinity, which I will represent with E .Itis
defined as the difference between the sum of the chemical potentials of the reactants and
those of the products. Using the notation of equation (12.64) we have:
E ≡
i
ν
i
µ
Y
i
−
j
ν
j
µ
Z
j
. (12.70)
If the affinity is positive then reaction (12.64) proceeds from left to right, whereas E = 0
corresponds to equilibrium. We can also write (12.70) as follows:
E =−
r
G
0
P ,T
+RT ln
i
a
Y
i
ν
i
j
a
Z
j
ν
j
, (12.71)
where in this case the activities are the actual values, i.e. the values as the chemical reaction
is taking place, and not necessarily the equilibrium values. Using (12.69) we see that the
affinity is also given by:
E = RT ln
k
f
i
a
Y
i
ν
i
k
r
j
a
p
j
ν
j
(12.72)
or, from (12.68):
r
f
r
r
=exp
E
RT
, (12.73)
which should be compared to (12.69): the ratio between the rate constants is a constant (the
equilibrium constant), but the ratio between the reaction rates varies with the affinity, or,
in other words, with the progress of the reaction. Using (12.73) we can write (12.67)as
follows:
r
n
=r
f
1 −exp
−
E
RT
, (12.74)

597 12.3 Rate of chemical reactions
which shows that the net rate of the reaction approaches a maximum value, equal to the
rate of the forward reaction, when E →∞, i.e. when the products are infinitely dilute.
The net reaction rate decreases exponentially as equilibrium is approached and E → 0,
but equation (12.74) yields a value for the net reaction rate only if r
f
is known. This in
turn requires that we know the value of the rate constant k
f
(equation (12.68)), which
is something that thermodynamics cannot supply. Equation (12.74) does say that the net
reaction rate increases exponentially with distance from the equilibrium state, but it does
not say that the rates of different reactions can be compared on the basis of their affinities,
as the rate constants for different reactions are generally different, and not predictable
from thermodynamics. Equations (12.69) and (12.74) encapsulate the relationship between
thermodynamics and kinetics.
We can calculate the rate of entropy production by a chemical reaction. Using
equation (12.65) we expand (12.16) as follows:
d
i
S =−
1
T
j
ν
j
µ
Z
j
−
i
ν
i
µ
Y
i
dξ (12.75)
or:
d
i
S =
E
T
dξ (12.76)
from which the rate of entropy production per unit volume (equation (12.4)) is:
σ =
E
T
1
V
dξ
dt
. (12.77)
Note that this is always positive, i.e. chemical reactions always produce entropy. Comparing
with equations (12.5) and (12.66) we identify the thermodynamic flow with the net reaction
rate, i.e.:
J =
1
V
dξ
dt
=r
n
. (12.78)
We can think of a chemical reaction as a mass transfer process in which chemical components
migrate from the reactant species to the product species, and in which the net reaction rate
is the mass transfer rate. The thermodynamic potential gradient is given by:
F =
E
T
(12.79)
so that the linear phenomenological relationship (12.7) implies:
r
n
=L
E
T
. (12.80)
This appears to be at odds with (12.74), in which we found a non-linear relationship between
the thermodynamic flow and the potential gradient. The apparent contradiction arises from
the fact that (12.74) is valid in general, whereas the linear relation (12.80) is valid only
close to equilibrium. This is explored further in Exercise 12.2.
