Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

558 Dilute solutions
is a curve for total dissolved iron = m
Fe
3+
,total
+m
Fe
2+
,total
. Recall that the calculations
are only appropriate for a very dilute solution of iron in pure water, and that concentrations
in ocean water are 2–3 orders of magnitude higher. The qualitative behavior shown in
the figure is, however, applicable to seawater. The concentration of total dissolved ferric
iron in equilibrium with hematite is of course independent of oxygen fugacity, but that
of total dissolved ferrous iron is not. For the present day atmospheric oxygen fugacity
the concentration of total dissolved ferrous iron is vanishingly small, and only becomes
comparable to that of total ferric iron at oxygen fugacities ∼10
−45
bar. Below 10
−50
bar
f (O
2
) ferrous species become the dominant forms of dissolved iron, and iron solubility
increases rapidly with decreasing oxygen fugacity.
At 298 K magnetite becomes stable at f (O
2
) ∼10
−69
bar (Fig. 9.3). In order to see the
effect of this phase transition on iron solubility (see also Worked Example 6.7) we have to
write the corresponding saturation equations. This is accomplished by replacing each of the
first equations in (11.107) and (11.110) with the corresponding magnetite equations:
1
3
Fe
3
O
4
+
3
2
H
2
O +
1
12
O
2
Fe
(
OH
)
3aq
(III, mt) (11.113)
and:
1
3
Fe
3
O
4
+H
2
O FeOH
+
aq
+OH
−
+
1
6
O
2
(II, mt) (11.114)
with equilibrium constants:
K
III,mt
=
m
Fe(OH)
3
f
O
2
1/12
(11.115)
and:
K
II,mt
=
f
O
2
1/6
·m
FeOH
+
·m
OH
−
. (11.116)
Numerical values (with standard state data from Wagman et al., 1982) are: K
III,mt
=
1.356 × 10
−6
, K
II,mt
= 6.723 × 10
−26
. The other equations in (11.107) and (11.108)
remain unchanged, so we find the following equations for total dissolved ferric and ferrous
iron in equilibrium with magnetite:
m
3+
Fe
,total
mt sat
=K
III,mt
·
f
O
2
1/12
·
·
1 +
K
III,1
K
W
10
−pH
+
K
III,1
·K
III,2
K
2
W
10
−2pH
+
K
III,1
·K
III,2
·K
III,3
K
3
W
10
−3pH
(11.117)
and:
m
Fe
2+
, total
mt sat
=
K
II,mt
K
W
10
−pH
f
O
2
1/6
1 +
K
II,2
K
W
10
−pH
. (11.118)
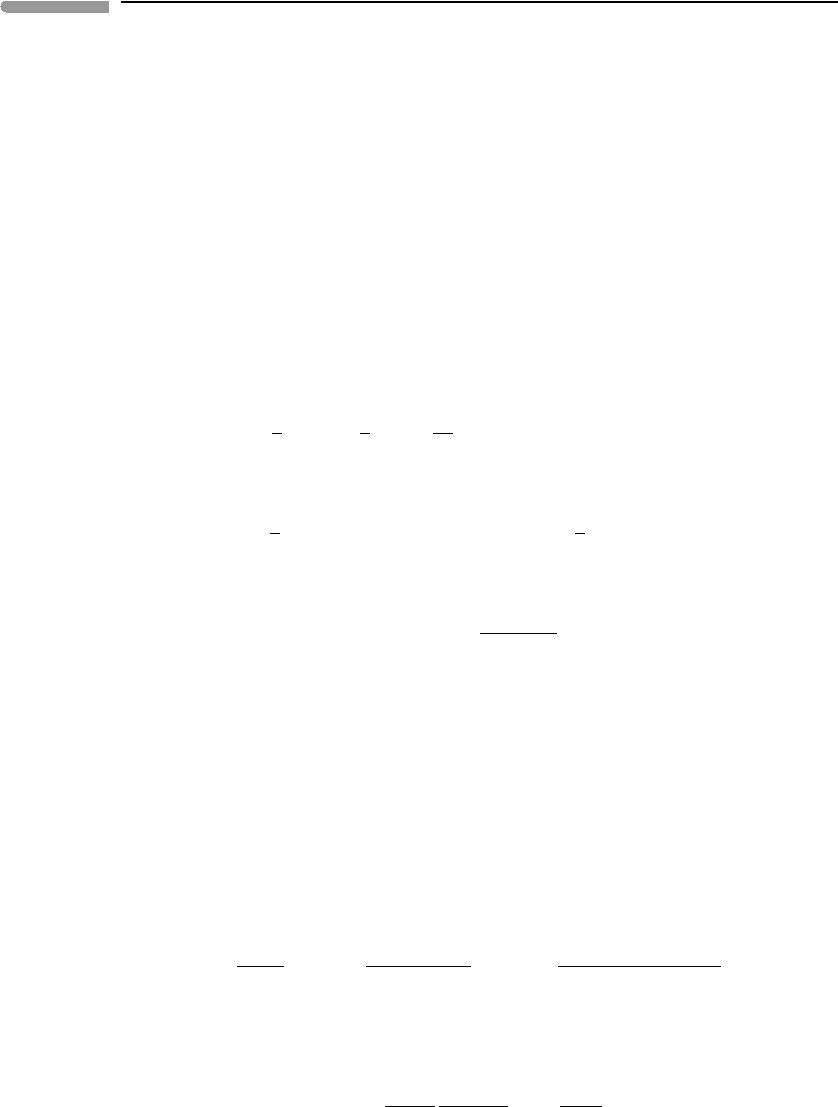
Equations (11.117) and (11.118), and the sum of both, are also shown in Fig. 11.6. The
curves for total dissolved iron in equilibrium with hematite and magnetite intersect at
559 11.5 Speciation in ionic solutions
log f (O
2
) =−68.59. This is the oxygen fugacity at which the hematite–magnetite phase
transition takes place, but the careful reader may note that there is a slight discrepancy
between this value and the one shown in Fig. 9.3. This arises from the use of standard
state properties from different data sets. I used data from Holland and Powell (1998)to
calculate the hematite-magnetite buffer in Fig. 9.3, and Wagman et al.(1982) to calculate
the equilibrium constants in this example.
At oxygen fugacity higher than that of the magnetite–hematite equilibrium the calculated
concentrations of total dissolved ferrous and ferric species in equilibrium with magnetite
are higher than those in equilibrium with hematite. This means that magnetite is not stable,
and that iron solubility is controlled by hematite saturation. There are two equivalent ways
of seeing this: you can think that with increasing iron content the solution becomes saturated
in hematite first, or that the chemical potentials of iron species at equilibrium with hematite
are lower than those at equilibrium with magnetite. At f (O
2
) lower than that of the phase
transition the converse is true.
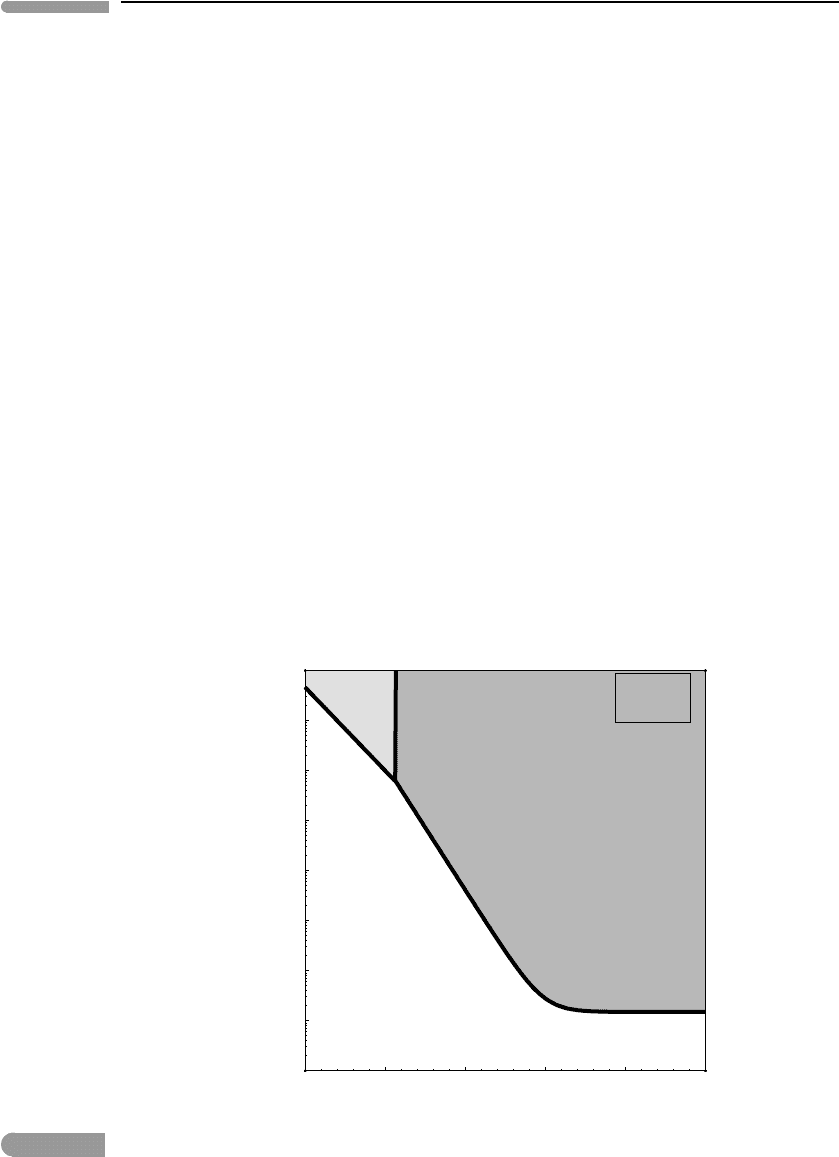
If we now “clean up” the diagram, leaving only the stable saturation curves, we get the
phase diagram shown in Fig. 11.7.The fields labeled “hematite” and “magnetite” correspond
to total dissolved iron concentrations above the solubility curves. These are “prohibited
regions”, in the sense that solution compositions inside these regions are thermodynamically
unstable relative to precipitation of the corresponding crystalline phase. Conditions below
the solubility curves correspond to solutions undersaturated in iron oxides. For oxygen
fugacities lower than ∼10
−50
bar, i.e. in the region where iron solubility varies strongly
with oxygen fugacity, the solubility is controlled by oxidation of Fe
2+
to a ferric crystalline
phase. This is so because in this region the saturation concentration of total dissolved
total dissolved iron molality
pH = 8
T= 25º C
hematite
magnetite
dissolved iron
10
–11
10
–12
10
–13
10
–10
10
–9
10
–8
10
–7
10
–6
10
–5
–80 –70 –60 –50 –40 –3
0
log f(O
2
) – bar
Fig.11.7
SameasFig.11.6,butshowingonlyconcentrationoftotaldissolvedironalongthemagnetiteandhematite
saturationcurves(thethickcurveinFig.11.6).Conditionsinsidetheshadedregionsaremetastablerelativeto
precipitation of the corresponding oxide phase.

560 Dilute solutions
ferric iron remains very low and constant, at close to 10
−12
molal, until magnetite starts
precipitating, where it decreases further with decreasing oxygen fugacity. Above ∼10
−50
bar oxygen fugacity the concentration of total dissolved ferrous iron becomes negligibly
small and iron solubility is determined by the solubility of ferric species.
The total concentration of dissolved iron in Archaean seawater in equilibrium with an
anoxic atmosphere in which f (O
2
) was less than about 10
−50
bar may have been several
orders of magnitude higher than the solubility of iron in today’s shallow oceans. Dissolved
iron in Archaean oceans would have been overwhelmingly present as ferrous species, that
could precipitate to solid ferric phases upon oxidation. Iron solubility remains essentially
unchanged over an f (O
2
) range of ∼40 orders of magnitude, from 10
−40
bar to its present-
day value. Deposition of banded iron formations by oxidation of dissolved ferrous iron
must have taken place under oxidation conditions (f (O
2
) <∼10
−50
bar) that are not even
remotely comparable to those that are needed for aerobic metabolism (Chapter 14).
The equations show that iron solubility in water is a function not only of oxygen fugacity
but also of pH, and the question arises, could the deposition of banded iron formations
primarily reflect a change in oceanic pH, rather than in oxygen fugacity? In Exercise 11.6
you can explore this and see why, although possible, this is a very unlikely explanation –
BIF deposition must be the response to oxidation of the Earth’s surficial environments.
Figure 11.7 shows a subtle detail that is not evident from the schematic phase diagrams
that we constructed in Worked Example 6.7. This is the fact that formation of magnetite
iron formations requires more concentrated iron solutions than formation of hematite iron
formations. For a total iron concentration lower than that at the magnetite–hematite transi-
tion (∼6×10
−8
molar in this simplified model) magnetite iron formations cannot form at
any oxygen fugacity, but hematite iron formations would form by oxidation.
In Worked Example 6.7 we saw that magnetite iron formations are sometimes associated
with siderite, and that some Precambrian BIFS are in fact composed chiefly of siderite. In
order to study the conditions that lead to the formation of siderite iron formations we begin
by writing the following magnetite–siderite equilibrium:
2Fe
3
O
4
+6CO
2
6FeCO
3
+O
2
. (11.119)
The oxygen fugacity at which siderite replaces magnetite as the iron solubility-limiting
phase depends on the fugacity of carbon dioxide. Using the pure gases at the temperature of
interest and 1 bar as the standard states for O
2
and CO
2
we write the equilibrium condition
for (11.119) as follows:
f
O
2
f
CO
2
6
=K
mt−sd
(11.120)
with K
mt−sd
= exp(−
r
G
0
/RT ) = 2.887 ×10
−70
at 298 K (data from Wagman et al.,
1982). As an example, let us assume that CO
2
fugacity in the Archaean atmosphere was
0.02 bar. A value considerably higher than today’s is suggested by the fact that Archaean
glaciations appear to be non-existent, despite the fact that the early Sun was fainter than
today’s (Sagan & Mullen, 1972; Kasting, 1987; Gilliland, 1989). From this CO
2
fugacity
we calculate an oxygen fugacity at the magnetite–siderite transition of ∼1.9 × 10
−80
bar,
i.e. well below the hematite phase transition. An important question is whether CO
2
is stable
at this low oxygen fugacity, relative to, for example, CO. The answer, which you are asked
to prove in Exercise 11.7, is yes.

561 11.5 Speciation in ionic solutions
It would appear that we could now simply add another phase boundary to Fig. 11.7,
showing the stability of siderite below this oxygen fugacity. The problem is that this diagram
is calculated for pH = 8, which is approximately correct for the present day atmospheric
CO
2
concentration but not for the higher concentration that we are now assuming. Under
0.02 bar of CO
2
the ocean must have been more acidic than today. The effect of pH on the
saturation boundaries is small (Exercise 11.6) but not entirely negligible. We therefore must
first calculate the pH of seawater in equilibrium with 0.02 bar of CO
2
, and then adjust Fig.
11.7 accordingly.
Suppose that the Archaean ocean was saturated in both calcite and siderite. We can then
add two more equations to the system of equations that we solved in Worked Example 11.4.
One of them is the solubility product of siderite:
FeCO
3
Fe
2+
aq
+CO
2−
3aq
(11.121)
for which K
sp,sd
=3.13 ×10
−11
. The other is the Fe
2+
hydrolysis reaction:
Fe
2+
aq
+H
2
O FeOH
+
aq
+H
+
aq
, (11.122)
which can be converted to the second dissociation reaction in (11.110) using the ionization
product of water (equation (11.91)). We must also modify the charge balance equation
(11.105) to include the molalities of Fe
2+
and FeOH
+
. Modifying the Maple routine to do
the calculations is easy, and is left as an exercise for the reader (Exercise 11.8).
ForaCO
2
fugacity of 0.02 bar we calculate the pH of our simplified ocean to be 7.13.
The solution set of the system of equations for siderite saturation (Exercise 11.8) also yields
the total concentration of dissolved ferrous iron, which in this case is 4.11 × 10
−5
molal.
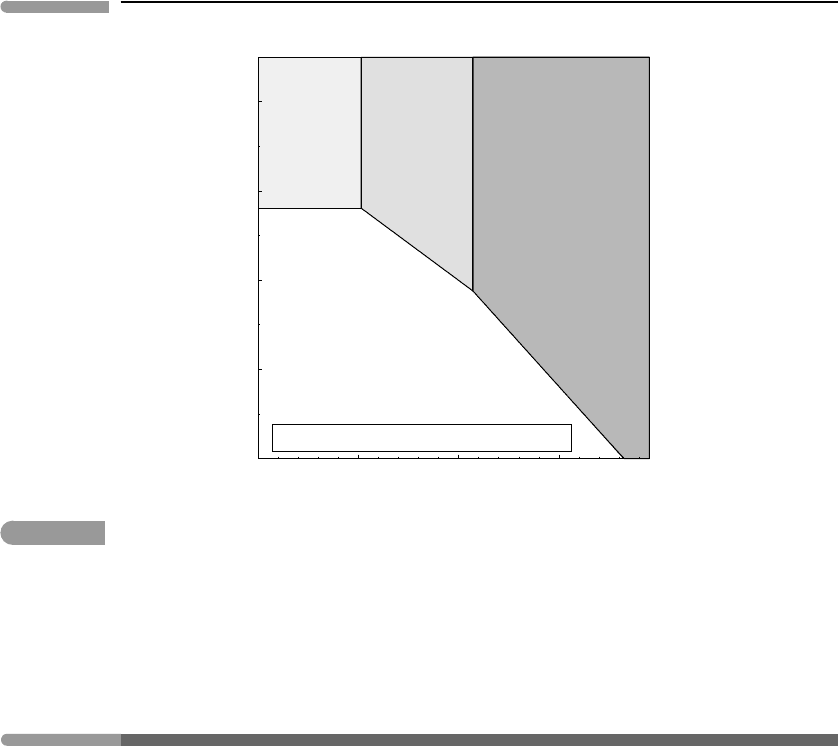
Figure 11.8 shows the hematite and magnetite saturation boundaries redrawn for pH =7.13
(compare Fig. 11.7). The concentration of ferrous aqueous species in equilibrium with
siderite is independent of oxygen fugacity (equation (11.121)), so the siderite saturation
boundary is parallel to the f (O
2
) axis, at a total ferrous iron molality of 4.11 × 10
−5
. This
line intersects the magnetite saturation boundary at f (O
2
) = 1.9 × 10
−80
bar, which is
the same oxygen fugacity that we calculated for the magnetite–siderite equilibrium from
equation (11.120). Consistency between two sets of calculations is always reassuring.
The resulting diagram shows the stability fields of the three most common phases in
banded iron formations. Reaction (11.119) determines the maximum oxygen fugacity that
allows siderite precipitation, which varies as the sixth power of CO
2
fugacity. An increase in
CO
2
fugacity expands the siderite field both towards the right (at the expense of magnetite)
and downwards, by lowering the concentration of Fe
2+
required for siderite crystallization
(solubility product for reaction (11.121)). We can calculate with equation (11.120) the
CO
2
fugacity needed to eliminate the magnetite field altogether, i.e. so that the siderite
field adjoins the hematite field. Making log f (O
2
) =−68.59 (the oxygen fugacity at
the magnetite–hematite transition) we get f (CO
2
) = 1.44 bar. The rarity of hematite–
siderite iron formations suggests that this is a reasonable upper bound for CO
2
fugacity in
the Archaean atmosphere at the time at which banded iron formations precipitated.
All of these calculations ignore the activity coefficients of aqueous species, which are
not negligible in a solution as concentrated as seawater. The rest of this chapter is focused
on this problem.
562 Dilute solutions
–90 –80 –70 –60 –5
0
10
–10
10
–8
10
–6
10
–4
10
–2
log f(O
2
) – bar
total dissolved iron molality
f(CO
2
) = 0.02 bar, pH = 7.13, T = 25º C
siderite magnetite hematite
dissolved iron
Fig. 11.8
Same as Fig. 11.7, but at pH =7.13, corresponding to a calcite-saturated solution at f(CO
2
) =0.02 bar. Increasing
CO
2
fugacity expands the siderite field downwards and rightwards, along the magnetite saturation curve. The
magnetite field disappears at f (CO
2
) ≈1.44 bar. This represents the minimum chemical potential of CO
2
at which
siderite and hematite can precipitate at equilibrium. The rarity of this assemblage in terrestrial banded iron
formations places an upper bound on Archaean atmospheric CO
2
fugacity.
11.6 Activity coefficients in electrolyte solutions
The key difficulty when calculating chemical equilibrium in electrolyte solutions is in
estimating reliable values for the activity coefficients of the many charged and neutral
species that exist in solution. The preceding section shows that even in a highly idealized
solution of Fe in water one must deal with almost ten species, and the number of species
grows rapidly with the number of components in the solution. The problem is not unlike that
of calculating speciation in a gas phase (Chapter 9), but it is more complicated because of (i)
the greater number of species, (ii) the existence of a solvent whose properties change with
temperature and (iii) the nature of the interactions among charged particles, and between
ions and solvent molecules.
The fact that electrolyte solutions behave differently from solutions of neutral species was
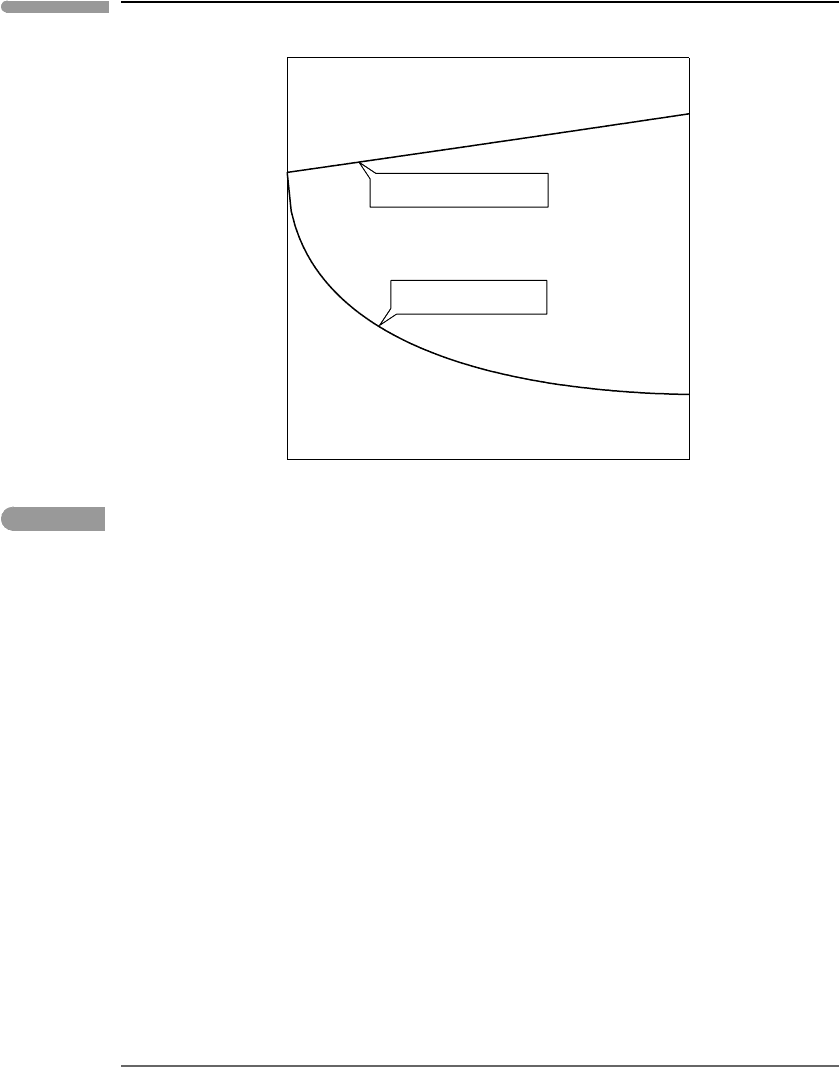
recognized early in the twentieth century. The different behaviors are compared schemat-
ically in Fig. 11.9, which shows the logarithm of the activity coefficient as a function of
molality for very dilute solutions of a neutral species (lnγ ) and an electrolyte (lnγ
±
). In
both cases the activity coefficient becomes unity at m =0. It is important to recall that this is
conventional, and that it arises from choosing to define the standard state at infinite dilution.
In other words, and as we discussed in Section 11.1.2, any excess Gibbs free energy that
may exist at infinite dilution remains unknown and is lumped into the standard state Gibbs
free energy. What matters now is that, whereas at very great dilution the activity coefficient
563 11.6 Activity coefficients in electrolyte solutions
solute concentration
ln γ
0
0
neutral species (ln γ)
electrolyte (ln γ ±)
m << 1
Fig. 11.9
Schematic diagram showing the contrasting behaviors of dilute solutions of a neutral species and an electrolyte. The
key difference is that, whereas in a dilute solution of a neutral species lnγ varies linearly with concentration, the
variation in a dilute electrolyte solution is of the form ln γ =−αI
β
, with α>0 and 0 <β<1.
of neutral species varies linearly with molality, the same is not true for electrolytes. The
activity coefficient of electrolytes in dilute solutions varies in a strongly non-linear fashion
with concentration, and approaches the limiting value at infinite dilution with an infinite
slope. For neutral species the activity coefficient is generally greater than 1, whereas for
electrolytes activity coefficients in dilute solutions are less than one, but may become greater
than one at higher concentrations.
The distinct behavior of electrolytes can be thought of as arising from the formation
of “ionic atmospheres”. These are regions around each ion that, owing to electrostatic
attraction, carry an excess of charge of a sign opposite to that of the central ion. As long as
the individual ionic atmospheres remain distant, i.e. at low concentration, they shield the
central ions and make it less likely that they will interact with other ions. This is expressed
macroscopically as a decrease in their chemical potential. As the solution becomes less
dilute the ionic atmospheres interfere with one another and shielding becomes less effective.
Particles of a neutral solute, in contrast, are not surrounded by ionic atmospheres so one
would expect that any energetic effect arising from interactions among them would vary
more or less linearly, or at least monotonically, with concentration.
11.6.1 Debye–Hückel theory
The first theory capable of predicting activity coefficients for electrolytes on the basis of
a rigorous physical description of the microscopic structure of electrolyte solutions was
proposed by Debye and Hückel in 1923. The place of Debye–Hückel theory in the ther-
modynamics of electrolytes is not unlike that of the van der Waals equation of state in the
thermodynamics of fluids. The theory is quantitatively accurate only for dilute solutions,

564 Dilute solutions
however. For example, it is already grossly inaccurate for seawater, in which the total con-
centration of dissolved electrolytes is less than 1 mol kg
−1
. It is, however, the basis for many
of the more elaborate formulations that are used to calculate activity coefficients in concen-
trated electrolyte solutions. In contrast to most of the latter formulations, which are empirical
to varying extents, the Debye–Hückel approach has a strong theoretical foundation.
A rigorous derivation of the remarkably simple final equation of Debye and Hückel for
the mean ionic activity coefficient of an electrolyte is beyond the scope of this book, but
it is instructive to construct a semi-formal a posteriori justification of their equation. We
begin by defining the ionic strength of a solution, symbolized by I , as:
I =
1
2
i
z
2
i
m
i
, (11.123)
where z
i
is the charge of ion i, m
i
its molality, and the sum is over all of the ionic species
present in the solution. The ionic strength is a measure of the total concentration of dissolved
electrolytes. If we apply (11.123) to a solution of a single electrolyte we see that the behavior
of an electrolyte solution as it approaches the infinite dilution limit (Fig. 11.9) can be
represented by a function of the form:
ln γ =−αI
β
, α>0, 0 <β<1. (11.124)
The derivative of this function relative to I diverges as I goes to zero, as required by the
observed behavior of very dilute electrolyte solutions schematized in Fig. 11.9. Debye and
Hückel proved rigorously that the actual equation valid as the solution approaches infinite
dilution is:
log
10
γ
i
=−A|z
i+
z
i−
|I
1/2
, (11.125)
where z
i+
and z
i−
are the charges of the cation and anion in electrolyte i, and A is a constant
that depends on the solvent only, but varies with temperature and pressure. I is the ionic
strength, given by (11.123), and includes the concentration of i as well as of any other ions
present in the solution. Equation (11.125) is known as Debye–Hückel’s limiting law. It is
only accurate for ionic strengths lower than ∼10
−3
, but at such low concentrations it is
accurate enough that it is routinely used to extrapolate measurements of thermodynamic
functions done at very low dilutions to the infinite dilution limit. Its main use for our
purposes will be to understand the meaning of the parameter A and, with it, some of the
physical aspects of Debye–Hückel theory. Their theory shows that A is given by:
A =
2πNρs
3
1/2
, (11.126)
where N is Avogadro’s number, ρ is the density of the solvent, and s is a length given by:
s =
e
2
4π/
0
ε
r
k
B
T
(11.127)
with e the elementary unit of charge, /
0
the permittivity of free space, ε
r
the dielectric
constant of the solvent and k
B
Boltzmann’s constant. The meaning of the length s becomes
clear if we re-write (11.127) as follows:
k
B
T =
e
2
4π/
0
ε
r
s
(11.128)

565 11.6 Activity coefficients in electrolyte solutions
and note that the equation now has units of energy. The length s is the distance between
two units of charge at which their electrostatic energy (see also equations (11.65)) equals
their thermal energy. The higher the temperature, or the dielectric constant, the closer the
charges have to be in order for the electrostatic force to overcome thermal agitation. We
next note that we can re-write the product N ρ as:
Nρ =
M
V/N
≈
M
λ
3
, (11.129)
where M is the molecular weight of the solvent, V its molar volume and λ is a length of
the order of the intermolecular distance in the solvent. The parameter A is a function of the
ratio (s/λ), i.e.:
A ∼
2πM
s
λ
3
1/2
. (11.130)
If (s/λ) is small then the distance over which electrostatic forces are effective is small com-
pared to the distance between solvent molecules, and formation of an ionic atmosphere
would tend to be restricted: the value of the parameter A decreases and the activity coef-
ficient approaches unity (equation (11.125)). On the other hand, a large value of the ratio
(s/λ) implies that electrostatic forces are effective over distances greater than intermolec-
ular separation in the solvent, allowing the formation of ionic atmospheres that lower the
chemical potential of the ion.
Before we proceed note two formal aspects of equation (11.125). First, it is customary
to write it in terms of decimal logarithm, rather than natural logarithm. Second, there is the
usual problem with units that arises when expressing concentration as molality. As written,
equation (11.126) implies thatA has units of (kg mol
−1
)
1
2
. If we express molality in mol kg
−1
then the units of I
1/2
are (mol kg
−1
)
1
2
and the logarithm is dimensionless, as it must be.
Alternatively, if we choose to use dimensionless molality (Section 11.1.1) then the term
in parentheses in equation (11.126) must be multiplied by 1 mol kg
−1
(see also equation
(11.4)) and A becomes a dimensionless parameter. This is the usual convention. Numerically
it makes no difference, but mathematical rigor requires that we worry about this.
Debye–Hückel’s parameter A is a property of the solvent only (and temperature, both
directly, see equation (11.127), and via the effect of T on the dielectric constant and density
of the solvent). The limiting law, equation (11.125), does not contain any solute properties
besides their charges. In particular, it ignores ionic radius. We can expect this to be a
reasonable model at very high dilution, where ions are separated by distances that are large
enough compared to their radii that they can be thought of as point charges.As concentration
increases the radius of the ions becomes significant relative to their separation and we must
expect the activity coefficient to become a function of ionic radius. This leads to the full
Debye–Hückel equation:
log
10
γ
i
=
−A|z
i+
z
i−
|I
1/2
1 +å
i
BI
1/2
, (11.131)
whereå
i
,knownasthedistance of maximumapproach,isafunction oftheeffectiveionicradii
of the ions that constitute electrolyte i, and B is a parameter that depends only on the identity
of the solvent and temperature. The product BI
1/2
has dimension of length
−1
(necessarily,
as å
i
has dimension of length) and can be thought of as the inverse of the effective radius of
the ionic atmosphere. If the distance of maximum approach is much smaller than this radius
then the denominator of (11.131) approaches 1 and we recover the limiting law, equation
566 Dilute solutions
(11.125). Note that the radius of the ionic atmosphere is a function both of a solvent property
(B) and of the ionic strength of the solution. For historical reasons, the unit that is universally
used for å
i
and B is the Ångstrom = 10
−10
m. Values of the å parameter for many common
ions were tabulated by Kielland (1937) and this is still the standard reference more than
seven decades later. The values of the A and B parameters for water have been calculated
over a large temperature range by Helgeson and Kirkham (1974).
Although equation (11.131) has theoretical justification it does not reproduce the observed
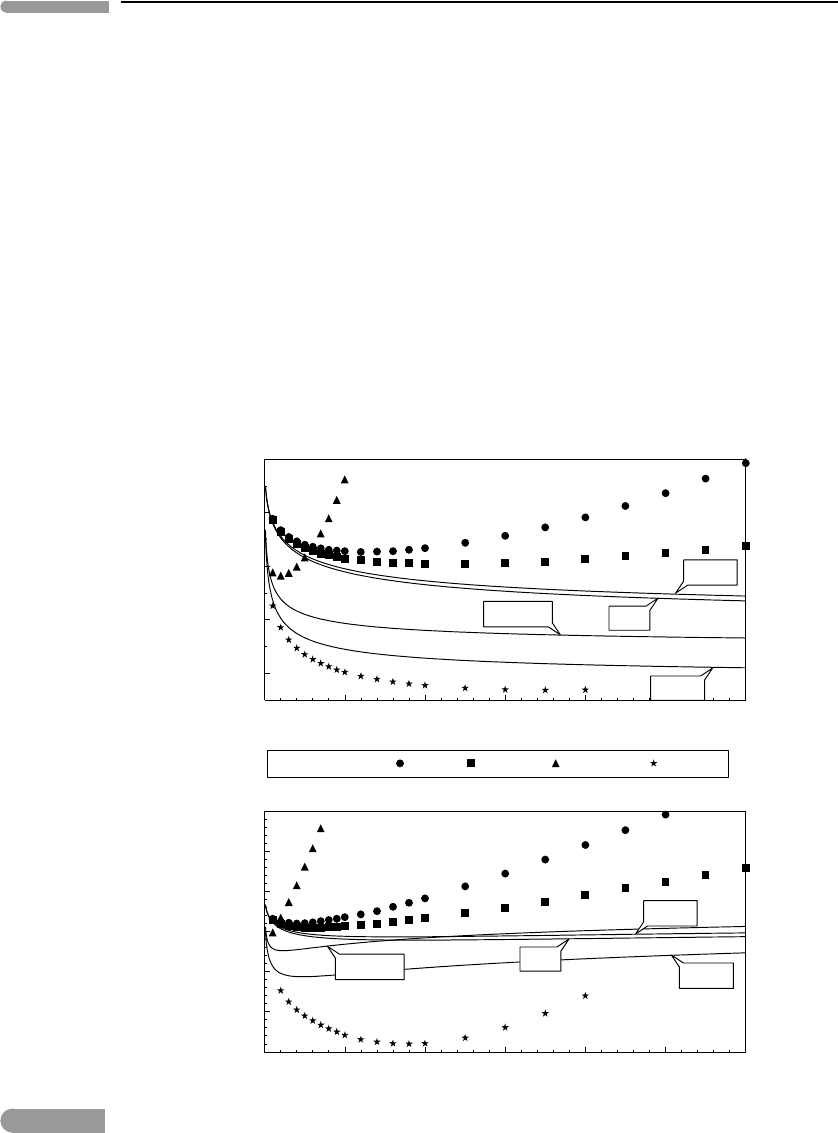
behavior of electrolytes at ionic strengths greater than ∼0.1. Figure 11.10 shows measured
activity and osmotic coefficients for four strong electrolytes at 298 K (data from Robinson
& Stokes, 1959). Also shown are activity coefficients calculated with the Debye–Hückel
equation (11.131), and osmotic coefficients calculated by integrating (11.131) according to
(11.43) (Exercise 11.9). The discrepancy above I ∼0.1 is very large. Note that, as equation
(11.131) does not have a minimum for any value of I, it is incapable of reproducing the
behavior of actual electrolytes, which generally show a minimum in γ at moderate ionic
strengths.
012345
6
0.2
0.4
0.6
γφ
0.8
1
solute molality
012 3 4 5
6
solute molality
Measured values NaCl NaClO
4
Mg(ClO
4
)
2
Na
2
SO
4
0.6
0.7
0.8
0.9
1
1.1
1.2
NaClO
4
NaCl
Mg(ClO
4
)
2
Na
2
SO
4
Na
2
SO
4
Mg(ClO
4
)
2
NaCl
NaClO
4
Fig. 11.10
Measured activity and osmotic coefficients (symbols) in aqueous solutions of sodium chloride, sulfate and perchlorate,
andmagnesiumperchlorate(datafromRobinson&Stokes,1959),comparedtothevaluesoftheactivitycoefficients
calculatedwithDebye–Hückelequation(11.131),andosmoticcoefficientscalculatedbyintegrating(11.131)
accordingto(11.43).
567 11.6 Activity coefficients in electrolyte solutions
Worked Example 11.5 The pH of natural waters revisited and a foray into soda lakes
We can refine the calculations in Worked Examples 11.3 and 11.4 by incorporating activity
coefficients calculated with the full Debye–Hückel equation, (11.131). In order to calculate
the pH of rainwater we re-write the first dissociation reaction, (11.98) as follows:
K
ds,I
=
m
HCO
−
3
m
H
+
f
CO
2
·γ
HCO
−
3
aq
·γ
H
+
aq
(11.132)
or, using the definition of mean ionic activity coefficient, equation (11.75)
K
ds,I
=
m
HCO
−
3
m
H
+
f
CO
2
·
γ
HCO
3
−H
+
aq
2
(11.133)
where:
log
10
γ
HCO
3
−H
+
aq
=
−A|z
H
+
z
HCO
−
3
|I
1/2
1 +
1
2
å
H
+
+å
HCO
−
3
BI
1/2
. (11.134)
The distance of maximum approach between two ions equals the sum of their radii, but
Kielland (1937) and all subsequent writers use ionic diameters – hence the division by 2 in
the denominator of (11.134). The second dissociation reaction, (11.97), becomes
K
ds, II
=
m
CO
2−
3
m
H
+
m
HCO
−
3
·
γ
CO
2−
3
aq
·γ
H
+
aq
γ
HCO
−
3
aq
. (11.135)
In contrast to (11.132), the individual ion activity coefficients in (11.135) do not work out
to a mean ionic activity coefficient. We have two options to deal with this situation. We can
choose to calculate single ion activity coefficients by applying (11.131) to each ion, so that
the product of charges in the numerator becomes the square of the charge of the ion, and
the distance of maximum approach is the ionic diameter. Or we can multiply the numerator
and denominator in (11.135)byγ
H+
aq
and re-write this equation as:
K
ds,II
=
m
CO
2−
3
m
H
+
m
HCO
−
3
·
γ
CO
2−
3
2H
+
aq
3
γ
HCO
3
−H
+
aq
2
. (11.136)
Mathematically the two approaches are identical. Physically, however, equation (11.136)is
preferable because single ion activity coefficients cannot be measured, whereas mean ionic
activity coefficients can. Finally, we include activity coefficients in the water ionization
reaction, (11.91), which becomes:
K
W
=m
H
+
·m
OH
−
·
γ
H
+
OH
−
aq
2
(11.137)
and we calculate pH with equation (11.94).
We are now ready to re-do the calculation in Worked Example 11.3, by using equations
(11.133), (11.136) and (11.137), plus the charge balance equation (11.100), which of course
