Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

568 Dilute solutions
remains unchanged. The solution must in this case be found by iteration, because in order to
calculate activity coefficients we need to know the ionic strength. A Maple procedure that
does this is described in Software Box 11.2.The calcite saturation reaction (Worked Example
11.4) is handled in an identical fashion, by adding the solubility product equation (11.106)
to the system of equations, calculating the mean ionic activity coefficient of Ca
2+
CO
2−
3
with (11.131), and using the charge balance equation (11.105).
Software Box 11.2 Calculation of carbonate speciation in aqueous solution. Non-ideal behavior
calculated with Debye–Hückel equation.
Maple worksheet aq_spec_DH.mw contains two procedures, zeco2_DH and
zecalcite_DH, that parallel the procedures in aq_spec_ideal.mw, but add
calculation of aqueous activity coefficients with the Debye–Hückel equation, (11.131)
(see Worked Example 11.5). The Debye–Hückel equations are contained in their own
procedures, at the top of the worksheet. Input for zeco2_DH and zecalcite_DH is
the same as for the equivalent procedures in aq_spec_ideal.mw. Output is also the
same, except that an additional field at the end of each line gives ionic strength.
The soda lake calculations in Worked Example 11.5 are contained in the Maple
worksheet soda_lakes_DH.mw. There are two procedures, soda_lake_Ca and
soda_lake_CO2. The first procedure calculates the composition of the soda lake as
a function of the parameter d
a
(equation (11.139)) at constant CO
2
fugacity (see Fig.
11.11). Input is f (CO
2
) in bar, high d
a
, low d
a
, number of intermediate values and
output file name. The output fields are: d
a
, pH, m
Ca
(2+)
,m
Na
+
,m
HCO
−
3
,m
CO
(2−)
3
,Na
bicarbonate ion activity product, Na carbonate ion activity product, ionic strength. The
second procedure performs the same calculations as a function of CO
2
fugacity at con-
stant d
a
(Fig. 11.12). Input is low f (CO
2
) in bar, high f (CO
2
) in bar, d
a
, number of
intermediate values and output file name. The output fields are: f (CO
2
) in bar, pH,
m
Ca
(2+)
,m
Na
+
,m
HCO
−
3
,m
CO
(2−)
3
, Na bicarbonate ion activity product, Na carbonate ion
activity product, ionic strength.
The results of the refined calculations are compared in Table 11.1 to the estimates that we
obtained assuming that the electrolyte solutions were ideal, for a constant CO
2
fugacity of
3.8 × 10
−4
bar. For rainwater in equilibrium with atmospheric CO
2
we calculate an ionic
strength of 2.4 × 10
−6
, so the Debye–Hückel equation can be expected to yield accurate
results – in fact, even the limiting law, equation (11.125), would work in this case. Calculated
electrolyte concentrations and pH are virtually indistinguishable from the ideal estimates.
The solution saturated in calcite has an ionic strength of ∼1.8 × 10
−3
, so equation (11.131)
should provide accurate results. In this case pH increases by 0.02 and the concentration
of Ca
2+
increases by about 10% relative to the ideal calculation. These values would be
appropriate for “pure” water in equilibrium which calcite, but not for seawater, which has
an ionic strength about three orders of magnitude higher than what calcite dissolution by
itself generates.
An interesting extension of these calculations is to study the chemical evolution
of soda lakes. These are bodies of water that generally form in closed basins in
arid climates, subject to strong evaporation. The defining characteristic of soda lakes
is that the supply of alkali-earth cations, Ca
2+
and Mg
2+
, is severely limited (see

569 11.6 Activity coefficients in electrolyte solutions
Risacher & Fritz, 1991, 2009; Lowenstein & Risacher, 2009; Millero, 2009; Reimer et al.,
2009). This is the case, for example, if the drainage basin feeding the lake consists predomi-
nantly of felsic volcanic or plutonic rocks. Charge balance of the carbonate and bicarbonate
anions in soda lakes is in such case chiefly accomplished by the alkali cations Na
+
and
K
+
. Evaporation concentrates the solution to the point where carbonates precipitate but,
because sodium and potassium carbonates and bicarbonates are quite soluble, the crystal-
lizing carbonate assemblage typically consists of calcite, magnesite and dolomite. Because
the system is depleted in Mg
2+
and Ca
2+
, however, the pH of soda lakes is not buffered as
effectively as that of seawater, and can in fact reach fairly extreme values. We can construct
a simplified thermodynamic model of a soda lake by considering only the Na
+
and Ca
2+
cations and carbonate and bicarbonate anions, and writing the following charge balance
equation:
m
H
+
+2m
Ca
2+
+m
Na
+
−m
HCO
−
3
−2m
CO
2−
3
−m
OH
−
=0. (11.138)
We seek the values of the six ionic molalities, so we need six equations, one of which
is (11.138). The carbonate and bicarbonate dissociation reactions and the water ionization
reaction are also applicable, so we include (11.133), (11.136) and (11.137). We assume that
the soda lake is saturated in calcite, so we also use equation (11.106). These five equations
do not contain information about the degree of Ca
2+
depletion of the soda lake, yet this
is what defines them and what makes their chemistry so radically different from that of
seawater. We therefore write a final equation that describes the Ca to carbonate ratio, as
follows:
2m
Ca
2+
m
HCO
−
3
+2m
CO
2−
3
=d
a
. (11.139)
A value of d
a
= 1 means that carbonate and bicarbonate charges are exactly balanced by
Ca
2+
. In our simplified model this means that there can be no Na
+
in solution, but in nature
of course sodium (and potassium) would be present, balanced by other anions such as chlo-
ride and sulfate, as in seawater. If d
a
< 1 then the soda lake must contain Na
+
in solution.
We will assume that sodium supply to the lake is unrestricted, so that Na
+
concentration
in the solution is limited only by the charge balance constraint, equation (11.138).
The six equations: (11.106), (11.133), (11.136), (11.137), (11.138) and (11.139) contain
two free parameters: the fugacity of CO
2
and the calcium to carbonate ratio, d
a
. We study
the evolution of soda lakes as a function of these parameters. Numerical solution of the set
of equations with Maple is a straightforward extension of the procedure that we used for the
first part of this example, and is described in Software Box 11.2. Let us assume first that CO
2
fugacity is fixed by equilibrium with the atmosphere, so we make f (CO
2
) = 3.8 × 10
−4
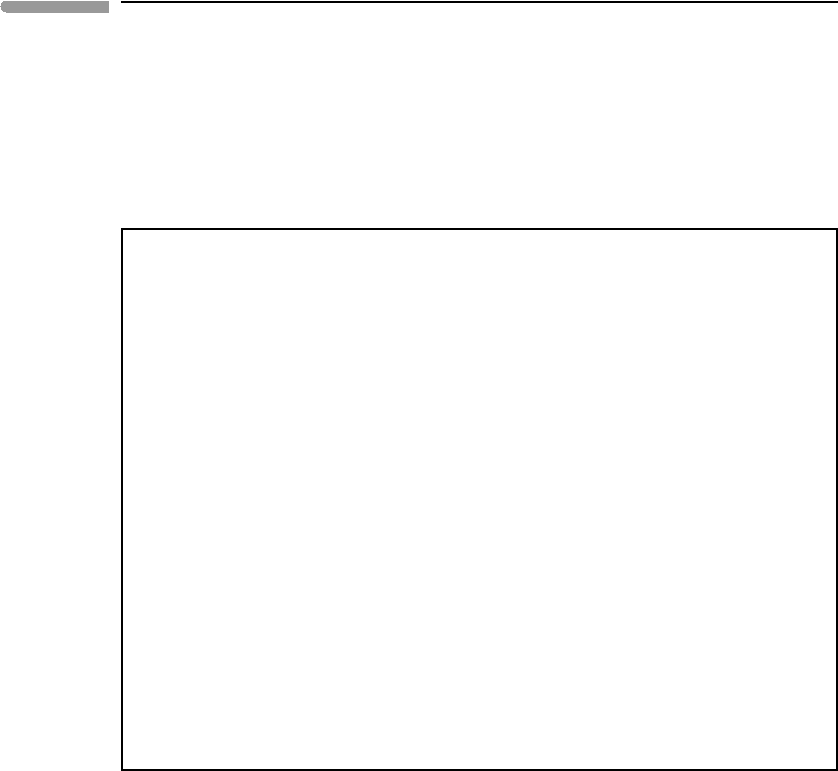
bar. Figure 11.11 shows changes in the chemical composition of the solution as a function
of the calcium to carbonate ratio, d
a
. The top panel shows pH and ionic strength. For d
a
=1 we recover the results for seawater, pH ∼8.3 and I ∼10
−3
. Both pH and ionic strength
increase with decreasing Ca
2+
content. At d
a
∼5 ×10
−5
the pH is about 9.7 and the ionic
strength is about 0.1. The results for our simplified soda lake should be fairly accurate up to
this point, but become less so as Ca
2+
content decreases further and ionic strength increases
(e.g. Fig. 11.10). Ionic strength in real soda lakes is of course much higher, so the results
are not strictly applicable to complex natural systems, but the behavior is qualitatively the
correct one.
570 Dilute solutions
10
–7
10
–6
10
–5
10
–4
10
–3
10
–2
10
–1
1
8.0
8.5
9.0
9.5
10.0
10.5
11.0
d
a
= 2 m
Ca
2+
/(m
HCO
3
- + 2 m
CO3
2-
)
pH
8.599.51010.5
10
–4
10
–3
10
–2
10
–1
1
pH
ion molality
8.599.51010.5
10
–8
10
–6
10
–4
10
–2
1
10
2
pH
ion activity product
f(CO
2
) = 380 x10
-6
bar
10
–3
10
–2
10
–1
1
10
1
Ionic strength
pH
I
CO
3
2-
HCO
3
–
Na
+
NaHCO
3
Na
2
CO
3
Na
2
CO
3
saturation
NaHCO
3
saturation
Fig. 11.11 The chemistry of an idealized soda lake in equilibrium with atmospheric CO
2
(f (CO
2
)=3.8×10
−4
bar) at 25
◦
C.
The lake is assumed to contain only Na
+
and Ca
2+
cations and HCO
−
3
and CO
2−
3
anions. Changes in pH and species
distribution are tracked as a function of the Ca
2+
deficit, given by the parameter d
a
. The pH axes of the center and
bottom diagrams are scaled to correspond approximately to the d
a
values in the top diagram. The bottom diagram
shows that the first sodium species to precipitate is nahcolite (sodium bicarbonate), at pH ≈10.5. The shaded
regions correspond to ionic strengths greater than 0.1 (shown with the dashed arrows in the top diagram),
for which Debye–Hückel theory is not accurate, so the results are only semi-quantitative.

571 11.6 Activity coefficients in electrolyte solutions
The middle panel of the figure shows the molalities of Na
+
, HCO
−
3
and CO
2−
3
ions as a
function of pH , with the pH axis scaled so as to correspond approximately to the values of
the d
a
parameter in the top panel. The shaded area on the left of the figure corresponds to
the region outside the range of validity of Debye–Hückel theory, i.e. I > 0.1. The dominant
carbonate species changes from HCO
−
3
for pH <∼10 to CO
2−
3
for pH > ∼10. All three
ions become strongly concentrated with increasing pH , so one may wonder, do sodium
carbonates precipitate? In order to answer this question we write the saturation reactions:
NaHCO
3
(
xs
)
Na
+
aq
+HCO
−
3aq
(1)
Na
2
CO
3
(
xs
)
2Na
+
aq
+CO
2−
3aq
(2) (11.140)
and the corresponding equilibrium conditions:
K
1
=m
Na
+
·m
HCO
−
3
·
γ
HCO
−
3
Na
+
aq
2
K
2
=m
2
Na
+
·m
CO
2−
3
·
γ
CO
2−
3
2Na
+
aq
3
. (11.141)
In order to determine whether the soda lake becomes saturated in sodium carbonate or bicar-
bonate we compare the values of the equilibrium constants calculated from standard state
thermodynamic properties (the left-hand side of equations (11.141)) with the ion activity
products (the right hand side of the equations) that result from the thermodynamic model
of the soda lake. The values of the equilibrium constants (calculated with standard state
properties from Wagman et al., 1982) are K
1
= 3.915 × 10
−1
(Na bicarbonate saturation)
and K
2
= 18.11 (Na carbonate saturation). As long as the ion activity products are smaller
than these numbers the lake is not saturated in sodium carbonates. The bottom panel in Fig.
11.11 shows the values of the ion activity products as a function of pH , as well as the values
of the equilibrium constants. At a constant CO
2
fugacity of 3.8 × 10
−4
bar the soda lake
trends towards sodium carbonate saturation with decreasing acidity. It becomes saturated
in sodium bicarbonate (the mineral nahcolite) at pH ∼10.5, but at these conditions it is still
far from being saturated in sodium carbonate.
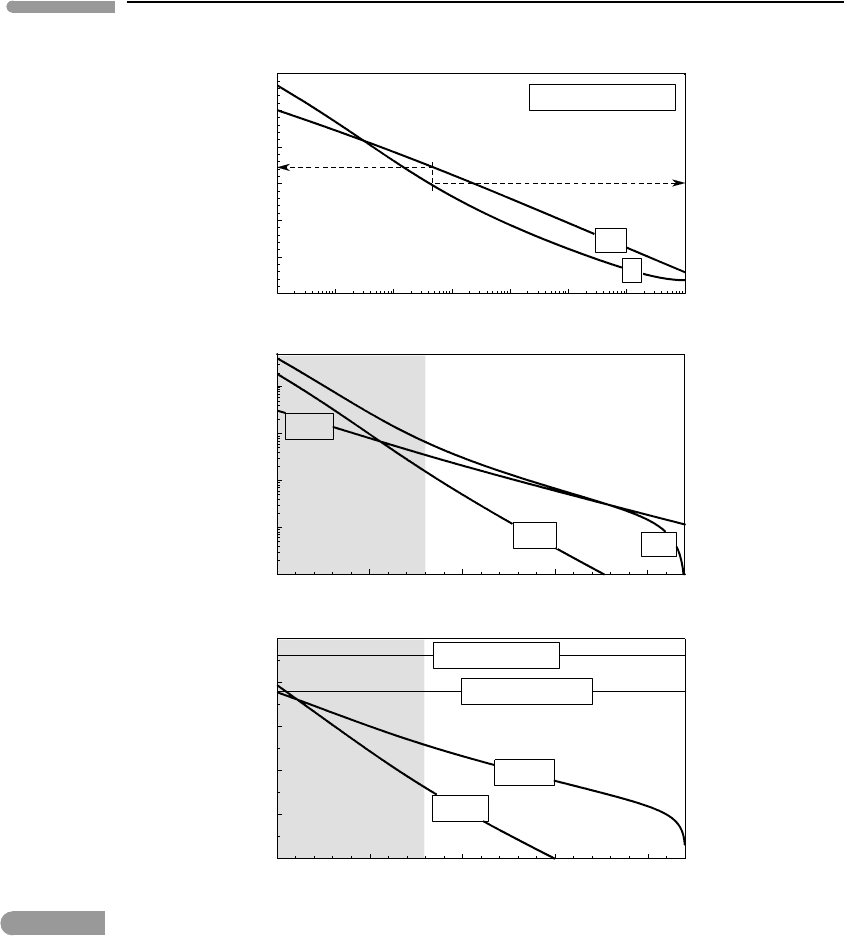
If we keep the calcium to carbonate ratio constant and vary f (CO
2
) the behavior of
the soda lake is strikingly different. Figure 11.12 shows this, for a constant value of the
parameter d
a
= 10
−3
. The solution in this case becomes more acidic as it becomes more
concentrated, in response to increasing CO
2
fugacity. This is opposite to the effectof starving
the lake of calcium at constant CO
2
fugacity. A consequence of this is that the lake is driven
towards saturation in sodium carbonates with increasing acidity, as can be seen in the middle
and bottom panels of Fig. 11.12. For d
a
= 10
−3
nahcolite precipitates at pH ∼6.5, which
corresponds to a CO
2
fugacity of about 10 bar. This result is thermodynamically correct,
but is a consequence of the constraints imposed on this simplified model, in particular the
assumption that an unlimited supply of sodium is available to balance the charge of whatever
carbonate concentration one chooses to apply. There may be few natural environments in
which conditions that allow sodium bicarbonate to precipitate from a mildly acidic solution
are realized. Environments of this kind could exist in lakes formed in craters or calderas of
active felsic and alkaline volcanoes, composed of rocks such as trachytes and pantellerites.
These rocks are rich in alkalis and relatively poor in calcium and magnesium, and volcanic
gases could provide a high CO
2
flux.
572 Dilute solutions
8.0
7.0
9.0
pH
pH
f(CO
2
) bar
10.0
11.0
10
–4
10
–3
10
–2
10
–1
10
–8
10
–6
10
–4
10
–2
1
1
10
2
10
–6
10
–5
10
–4
2 m
ca
2+
/ (m
HCO
3
- + 2 m
co
3
2–
) = 10
–3
10
–3
10
–2
10
–1
10
–1
1
I
10
–2
1
Ionic strength
ion molalityion activity product
NaHCO
3
Na
2
CO
3
Na
2
CO
3
saturation
NaHCO
3
saturation
98710
98 7
10
pH
pH
CO
3
2-
HCO
3
–
Na
+
Fig. 11.12 Same as Fig. 11.11, but tracking changes in the soda lake as a function of CO
2
fugacity, for a constant value of d
a
=
0.001. The behavior of the soda lake is remarkably different. Precipitation of nahcolite takes place in this case in
response to increasing acidity. This may occur in response to influx of CO
2
-rich volcanic gases.
11.6.2 Activity coefficients in concentrated electrolyte solutions
That the complete Debye–Hückel equation, (11.131), is not accurate for solutions with
ionic strengths greater than ∼0.1 has been known since the equation was proposed. Debye–
Hückel theory, however, performs well for dilute solutions, in which key assumptions,
such as treating the solvent as a continuum (i.e. assuming that the macroscopic dielectric
constant is valid) and ignoring all characteristics of the ions beyond their charge and radius,

573 11.6 Activity coefficients in electrolyte solutions
can be expected to be better approximations than in concentrated solutions. This suggests
that equation (11.131) encapsulates important aspects of the behavior of real electrolytes,
and that concentrated solutions may be amenable to be described by extensions of Debye–
Hückel’s theory. Two such theories are widely used to study natural systems. One of them,
due to Pitzer and co-workers, is largely based on calibrating non-ideal empirical interaction
parameters among individual ions. The other one, proposed by Helgeson and collaborators,
seeks to preserve the Debye–Hückel philosophy as much as possible, by identifying the
species that form by ion association, defining their standard state properties as a function
of temperature and pressure, and accounting for the effects of temperature and pressure on
the dielectric properties of the solvent. Both formulations lead to equations of significant
operational complexity (as opposed to mathematical complexity), the full development of
which is beyond the space available here. Excellent summaries of the contrasting Pitzer
and Helgeson approaches, as well as of a few other simpler alternatives, can be found in
the textbooks by Anderson (2005) and Nordstrom and Munoz (1986).
The formulation of Pitzer and co-workers starts with the limiting law of Debye and Hückel
and expands the excess Gibbs free energy of mixing, beyond the amount that is accounted
for by Debye–Hückel theory, as a virial-like, but largely empirical, series in composition.
Philosophically this is the same approach used in the Pitzer and Sterner equation of state
(Section 9.4.4). The earliest attempt along these lines is due to Guggenheim (1935; 1967,
p. 286), who suggested a characteristically simple and clever approach. His idea was based
on the fact that, because the Debye–Hückel equation is chiefly (or only, for the limiting law)
a function of solvent properties and ionic strength, different solutes of the same charge type
(and approximately the same ionic size) should have the same excess chemical potential in
any solution of the same ionic strength. This is not what is observed. Guggenheim proposed
that some arbitrarily defined and well-characterized electrolyte, for example NaCl, be used
as a reference, and that one then measure the difference in the excess Gibbs free energy
of mixing of other electrolytes relative to that of the standard. He then suggested that
the difference in excess Gibbs free energy of mixing between an arbitrary solution and a
solution of pure standard electrolyte be expanded as a sum of terms, each of which has the
form β
ca
m
c
m
a
, where m
c
and m
a
are cation and anion molalities, and β
ca
is an empirical
interaction parameter that characterizes each cation–anion pair. Guggenheim did not include
terms for interactions between ions with the same charge. This is known as the principle
of specific ion interactions and was first stated by Brønsted (1922) who hypothesized that
ions of the same charge never come close enough together to have an effect on the free
energy of the solution beyond that which arises from their charge (i.e. their identity does not
matter). This may be true in dilute solutions, but not in concentrated ones. By differentiating
Guggenheim’s expression for excess Gibbs free energy of mixing one obtains expressions
for the osmotic coefficient (equation (11.36)) and activity coefficients (equation (11.13)),
relative to those of the standard. The properties of the standard electrolyte are given by the
Debye–Hückel theory. These will not be accurate in absolute terms, but since the activity
coefficients for all electrolytes are referred to this same standard the differences among
them can be expected to reproduce the observed behavior fairly well.
Pitzer’s approach is an extension of these ideas, differing in three important ways: (i) the
binary interaction terms contain more than one empirical parameter, which are functions of
ionic strength (Guggenheim’s β
ca
is a constant, except perhaps for an unknown temperature
dependency), (ii) interactions between ions of the same sign are included, and (iii) triple
particle interactions, which may be negligible in dilute solutions but not at high concentra-
tions, are also included. Pitzer’s general equation for the excess Gibbs free energy of the

574 Dilute solutions
solution has the form:
G
ex
RT
=w
s
f
(
I
)
+
1
w
s
ij
λ
ij
(
I
)
n
i
n
j
+
1
w
2
s
ij k
µ
ij k
n
i
n
j
n
k
, (11.142)
where w
s
is the mass of solvent (in kg), f (I ) is a function of ionic strength and solvent
properties that is similar, but not identical, to the Debye–Hückel equation (11.131), and
the n
i
, n
j
, n
k
are number of mols of ionic species. The interaction parameters λ
ij
(I) are a
function of ionic strength, they include more than one adjustable parameter, and they apply
to interactions among same charge as well as opposite charge ions, and neutral species
too if they exist. The ternary interaction parameters µ
ij k
, on the other hand, are not a
function of ionic strength and are set to zero if all three ions are of the same charge.
Equation (11.142) is manipulated so as to express the interaction parameters as functions
of measurable electrolyte properties, and the resulting expression differentiated relative to
w
s
or m
i
in order to obtain the osmotic coefficient of the solvent or the activity coefficient
of each solute (equations (11.13) and (11.36)). The details, which are rather ponderous,
canbefound,forexample,inPitzer(e.g.1987,1995),andthefinalequationsarealso
summarized by Anderson (2005). Important applications of Pitzer’s equations include to
terrestrial evaporites (see Harvie & Weare, 1980; Harvie et al., 1984) and ocean water (see
Millero, 2004, 2009, and references therein), Martian brines and evaporites (see Chevrier
& Altheide, 2008; Chevrier et al., 2009; Marion et al., 2003, 2008, 2009), and icy-satellite
cryomagmas (Marion et al., 2005).
Pitzer’s model is calibrated at 298 K and 1 bar. Extrapolation to other conditions is accom-
plished by expressing the interaction parameters as polynomial functions of temperature
and pressure, although at present these functions are calibrated for only a relatively small
set of electrolytes of geological importance (see, for example, Marion et al., 2008). An
important aspect of the model, which is expressed in equation (11.142), is that it assumes
that interactions occur among individual ions. If a specific ionic association (i.e. species
formation) takes place this is not considered explicitly but is rather reflected in the values
of the interaction parameters, which generate a lower activity coefficient for the affected
ions. The model is therefore purely thermodynamic, in the sense that it focuses only on
macroscopic properties, regardless of what the microscopic mechanism for these properties
may be.
Helgeson’s approach (see Helgeson, 1969; Helgeson et al., 1981; Tanger & Helgeson,
1988) differs significantly from Pitzer’s in this last respect. Ion association and complex
formation are explicitly accounted for. Infinite dilution standard state properties are gen-
erated for all species, together with heat capacity and volumetric data. This allows a more
straightforward extrapolation to high temperatures and pressures. In fact, at high tempera-
ture Helgeson’s model is physically more satisfactory than Pitzer’s, because the decrease in
the dielectric constant of water with increasing temperature, that limits electrolyte dissocia-
tion, is explicitly accounted for (all electrolytes at high temperature tend to behave as weak
electrolytes). Speciation calculations in Helgeson’s model are accomplished by writing
chemical reactions among the species and solving for the species concentrations that make
the Gibbs free energy change of all the homogeneous equilibrium reactions vanish (this
is equivalent to the chemical equilibrium approach to fluid speciation, see Section 9.6.1).
Activity coefficients are based on the Debye–Hückel model, complemented by empirical
species-specific terms. The computational details are intricate and are nicely summarized
by Anderson (2005).

575 Exercises for Chapter 11
Exercises for Chapter 11
11.1 Integrate (11.41) in two different ways, so as to obtain (11.42) and (11.43).
11.2 Study various pathways for the formation of ClO
2
(chlorine peroxide gas), e.g. from
various combinations of the elements in their molecular and atomic states, as well as
from the respective hydrides (HCl and H
2
O). What are likely pathways for formation
of perchlorates in planetary atmospheres? What makes these reactions possible (i.e.
where is the “missing free energy” coming from?) What are likely constraints on the
types of natural environments where perchlorates can form? Necessary thermody-
namic properties can be obtained from Wagman et al. (1982) or NIST’s Chemistry
WebBook.
11.3 Show that the equations derived in Section 11.2.3, that lead to the relations between
activity and osmotic coefficients, equations (11.41) through (11.43), are also valid
for electrolyte solutions, using bulk electrolyte properties.
11.4 Modify the Maple procedure used to calculate carbonate speciation in rainwater (Fig.
11.5) to include calcite saturation equation (11.106).
11.5 Study the distribution of ferric species in aqueous solution as a function of pH
at some constant value of oxygen fugacity that is high enough to make concen-
trations of ferrous species negligible (e.g. the present day terrestrial atmosphere).
Find the conditions under which Fe
3+
becomes the dominant aqueous species.
Discuss how analytical Fe
3+
concentration varies with pH at constant oxygen
fugacity. How would your conclusions be affected by changes in oxygen fugac-
ity, as long as its value is oxidizing enough to suppress ferrous species? What is
the likely significance of primary hematite deposits, i.e. hematite that precipitated
from aqueous solution directly, without formation of intermediate metastable ferric
hydroxides?
11.6 Discuss why, although possible, it is unlikely that most terrestrial BIF’s formed in
response to a change in oceanic pH, and that a change in oxidation conditions is the
simplest and most probable explanation.
11.7 Show that in an atmosphere as reducing as f (O
2
) ∼10
−70
bar the concentration of
carbon monoxide is negligible relative to that of carbon dioxide, so that CO
2
is the
dominant oxidized carbon species. If the atmosphere was also saturated in H
2
O, could
CH
4
concentration be significant at this oxygen fugacity? A review of Chapter 9 can
be useful. We will discuss these calculations in greater detail in Chapter 14.
11.8 Modify the Maple procedures discussed in Software Box 11.1 to include saturation in
both calcite and siderite. The calculation must include the Fe
2+
hydrolysis reaction,
equation (11.122). Assume an ideal aqueous solution. Plot the concentrations of the
various aqueous ferrous species, and total Fe
2+
analytical concentration as a function
of pH . Assume that oxygen fugacity is low enough to make Fe
3+
concentration
negligible.
11.9 Write a Maple procedure that calculates Debye–Hückel osmotic coefficients, by
integrating (11.131) according to (11.43).
11.10 Study the phase relations of saturation of calcite, dolomite and magnesite from aque-
ous solution as a function of dissolved Mg
2+
and Ca
2+
concentrations, atmospheric
CO
2
concentration and temperature. Write the necessary Maple procedures, using
Debye–Hückel activity coefficients. Your calculations are not applicable to seawater

576 Dilute solutions
(why?), but are the results likely to be reasonably accurate for a simple solution like
the one you are modeling? Molalities of Mg
2+
and Ca
2+
in present-day ocean water
are ∼0.053 and 0.010, respectively (from Millero, 2004). What carbonate should
one expect to precipitate according to the simplified model? How does this compare
with carbonate precipitation from seawater? Discuss your findings.
12
Non-equilibrium thermodynamics and rates of
natural processes
With the exceptions of Chapters 2 and 3, our discussions have largely focused on equi-
librium conditions, and chiefly on the equilibrium of systems in which the only kind of
mechanical work that takes place is expansion work. Even with these severe restrictions
thermodynamics can provide a wealth of information about the nature and evolution of
planetary bodies. But these are by no means the limits of thermodynamics. In fact, one
could argue that thermodynamics only begins to get interesting when these restrictions are
lifted.An in-depth discussion of the possibilities that open up would demand an entire book,
at least as long as this one. That is a fight for another day. The goal of this chapter is to
lift a corner of the proverbial veil. I will introduce some of the principles of linear non-
equilibrium thermodynamics and use them to examine chemical diffusion and chemical
reaction mechanisms and rates. These are two classes of processes that are responsible for
displacing systems towards equilibrium in a wide range of situations.
12.1 Non-equilibrium thermodynamics
By definition, equilibrium thermodynamics concerns itself with static systems and with
“quasi-static” transformations between equilibrium states. These are abstractions, but if
our discussions so far are any indication, they are very useful ones. Throughout the pre-
vious chapters we have also come across non-equilibrium processes, however. Examples
include catastrophic planetesimal collisions (Chapter 2), heat transfer (Chapter 3) and non-
isentropic melting (Chapter 10). Processes such as these are the purview of non-equilibrium
thermodynamics. I will present here a short introduction to non-equilibrium thermodynam-
ics of systems close to equilibrium (the precise meaning of this will become clear in an
instant), along the lines initiated by de Donder, Onsager, Mazur, Prigogine, de Groot and
collaborators (see, for example, de Donder, 1936; de Groot, 1959; de Groot & Mazur, 1984;
Prigogine, 1961, 1962, 1967; Prigogine & Defay, 1965). This topic is not only intellectually
exciting, but it also provides the framework that underlies processes as apparently distinct
as heat and mass diffusion, the progress and rate of chemical reactions, viscous deformation
and electrical currents.
12.1.1 Fundamental concepts
We begin by revisiting our definition of entropy (Chapter 4). Let us split the entropy change
of a system into an external part, d
e
S and internal part, d
i
S:
dS = d
e
S +d
i
S. (12.1)
577
