Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


598 Non-equilibrium thermodynamics
12.3.3 Differential and integral rate laws
We now return to the strictly kinetic question of how the concentrations of chemical species
change with time. The simplest case is that of an elementary first-order reaction A →
products. From (12.63) the rate law of a first order reaction is r =k[A], where [A] is molar
concentration per unit volume. Setting r
r
=0in(12.68), and using (12.67) and (12.66):
k
[
A
]
=
1
V
dξ
dt
(12.81)
and from (12.65):
dξ
dt
=−V
d
[
A
]
dt
(12.82)
so:
d
[
A
]
dt
=−k
[
A
]
, (12.83)
which is the first-order rate law.
Let x be the change in concentration of A (mols per unit volume), so that ξ = xV .Ifthe
initial concentration of A is [A
0
], and we make x =0att = 0, then we have:
[
A
]
=
[
A
0
]
−x (12.84)
which allows us to re-rewrite equation (12.83) as:
dx
x −
[
A
0
]
=−kdt. (12.85)
This integrates to:
x =
[
A
0
]
1 −e
−kt
(12.86)
or equivalently:
[
A
]
=
[
A
0
]
e
−kt
. (12.87)
This is the first-order rate law in integral form, and is identical to the radioactive decay
law (Section 2.9). Radioactive decay, although not a chemical reaction, is the archetypal
example of a process that follows first-order kinetics. As for radioactive decay, we can
define the half life of a chemical reaction, τ
1/2
, as the time required for the concentration of
the reactant to decay to half of its initial value. Setting [A]=1/2[A
0
] in (12.87) we find:
τ
1/2
=
ln 2
k
. (12.88)
An alternative estimate of the characteristic rate of a process, that is commonly used for
chemical reactions, is the reaction time scale, which is the time requiredfor the concentration
of the reactants to become “exponentially close” to the equilibrium concentration. The
definition of the reaction time scale τ , is given by:
[
A
τ
]
−
A
eq
=
1
e
[
A
0
]
−
A
eq
, (12.89)
599 12.3 Rate of chemical reactions
where [A
eq
] is the equilibrium concentration of A and [A
τ
] is its concentration at time τ .
Equivalently:
x
τ
=x
eq
1 −
1
e
. (12.90)
Substituting in (12.86), and noting that the equilibrium concentration is attained as t →∞,
we find for a first-order elementary reaction:
τ =
1
k
. (12.91)
The definitions of reaction half life and reaction time scale are always the same, but the
specific equations (12.88) and (12.91) are valid only for reactions that follow first-order
kinetics.
When we consider higher order reactions the number of possible rate laws multiplies.
Equation (12.83) is the only possible first-order rate law, but for a second-order reaction we
have two possibilities: A +B →products and 2A →products. For third-order reactions
there are three possibilities. As an example we will look at the second-order reaction A +
B → products, others are left as exercises. We can write the rate law for this reaction as
follows:
d
[
A
]
dt
=−k
[
A
][
B
]
(12.92)
or, in terms of the progress variable x and the initial concentrations [A
0
] and [B
0
]:
dx
x −
A
0
B
0
−x
=−kdt. (12.93)
The integral is messier than that for a first-order reaction, but, thanks to Maple:
ln
[
B
0
]
−x
[
B
0
]
−ln
[
A
0
]
−x
[
A
0
]
=−
[
A
0
]
−
[
B
0
]
kt (12.94)
or, with a bit of rearrangement:
x =
[
A
0
][
B
0
]
(
1 −ϑ
)
[
B
0
]
−ϑ
[
A
0
]
(12.95)
where:
ϑ = e
[
A
0
]
−
[
B
0
]
kt
. (12.96)
The result is more informative if we express it in terms of the concentrations of the reactants:
[
A
]
[
B
]
=ϑ
[
A
0
]
[
B
0
]
. (12.97)
Equations 12.96 and 12.97 show that, as we should expect, the system becomes enriched
in the reactant that is present in excess. Consider the limiting case in which [B
0
]![A
0
],
so that even when all of reactant A has reacted it is [B]≈[B
0
]. Then equation (12.97)
simplifies to:
[
A
]
=
[
A
0
]
e
−
[
B
0
]
kt
. (12.98)

600 Non-equilibrium thermodynamics
This is known as pseudo-first-order kinetics. It differs from true first-order behavior
(equation (12.87)) in that the exponential factor is multiplied by the concentration of
the abundant species, B. Calculation of reaction half life and time scale for second- and
third-order reactions is left as an exercise.
12.3.4 Some simple composite reactions
We will look at two examples, sequential reactions and parallel reactions, of how the rate
laws of elementary reactions combine to yield more complex behaviors. Consider first the
case in which a reactant A goes to a product C with formation of an intermediate compound,
B. For simplicity let all of the reactions be first-order:
A →B, rate constant = k
1
B → C, rate constant = k
2
.
The concentrations of the three species vary as:
d
[
A
]
dt
=−k
1
[
A
]
d
[
B
]
dt
=k
1
[
A
]
−k
2
[
B
]
d
[
C
]
dt
=k
2
[
B
]
.
(12.99)
The behavior of this reaction depends on the stability of the intermediate species, B. This
must be reflected in the relative values of the two rate constants. If B is relatively unstable
then it is likely to be short-lived, which requires that k
2
be much larger than k
1
, so that the
concentration of B always stays close to zero. Conversely, if k
2
is much smaller than k
1
then B must be relatively long-lived and its concentration may build to the point where it
becomes the dominant species in the system. We seek an equation for the change of [B]
with time.
Let us assume that the initial concentrations are [A
0
]= 0 and [B
0
]=[C
0
]=0. The con-
centration of A as a function of time is given by equation (12.87), with k =k
1
. Substituting
(12.87)in(12.99):
d
[
B
]
dt
=k
1
[
A
0
]
e
−k
1
t
−k
2
[
B
]
. (12.100)
Solution of this differential equation by hand is not immediate, but Maple’s differential
equation solver does it in a single step. The final result is:
[
B
]
=
[
A
0
]
k
1
k
2
−k
1
e
−k
1
t
−e
−k
2
t
(12.101)
from which we can also calculate:
d
[
B
]
dt
=
[
A
0
]
k
1
k
2
−k
1
−k
1
e
−k
1
t
+k
2
e
−k
2
t
. (12.102)

601 12.3 Rate of chemical reactions
If k
1
!k
2
then these equations become, approximately:
[
B
]
=
[
A
0
]
e
−k
2
t
−e
−k
1
t
d
[
B
]
dt
=
[
A
0
]
k
1
e
−k
1
t
−k
2
e
−k
2
t
(12.103)
whereas for k
2
!k
1
we have:
[
B
]
=
[
A
0
]
k
1
k
2
e
−k
1
t
−e
−k
2
t
d
[
B
]
dt
=
[
A
0
]
k
1
k
2
k
2
e
−k
2
t
−k
1
e
−k
1
t
.
(12.104)
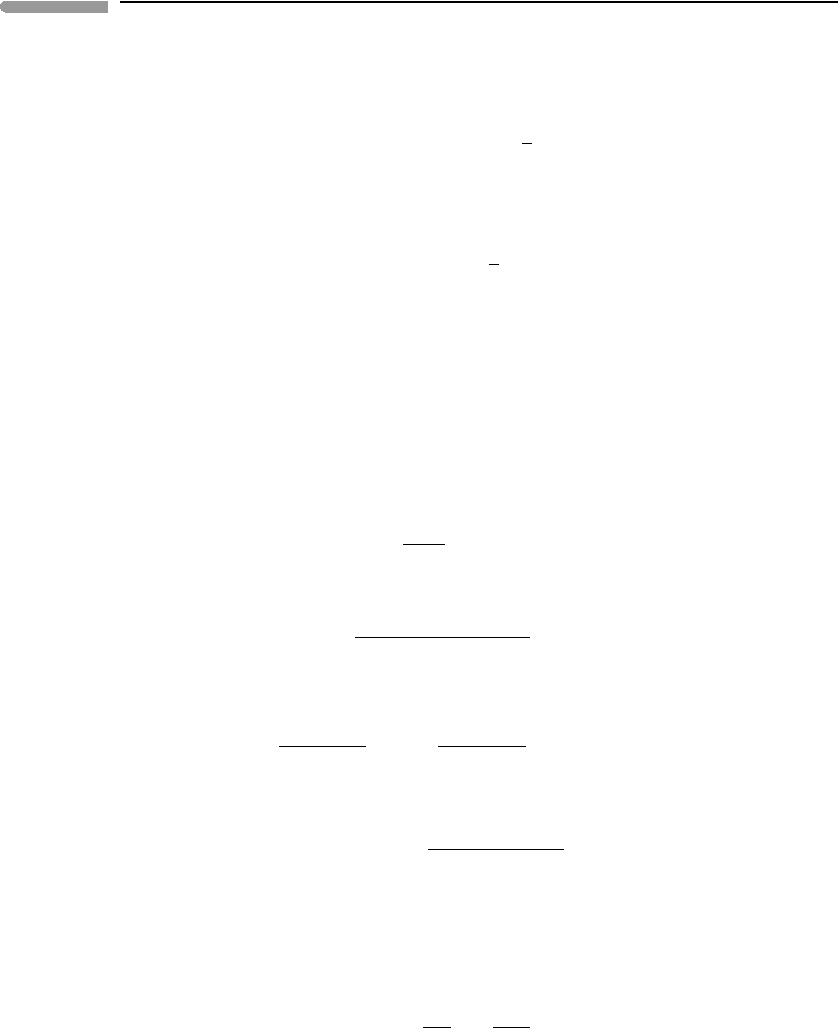
These two distinct behaviors are plotted in Fig. 12.4.Ifk
1
!k
2
then A quickly decays to B.
The concentration of B builds up to a value comparable to [A
0
], and then decays as B reacts
to C. The derivative d[B]/dt (bottom panel) varies strongly and approaches zero only after
a long time, when most of B has decayed. In this case B is a long-lived intermediate species.
In contrast, if k
2
! k
1
[B] reaches a maximum value that is only a small fraction of [A
0
]
and then remains approximately constant. This is emphasized by the graph of d[B]/dt ,
which after the initial “build-up” period stays close to zero. B is in this case a short-lived,
or relatively unstable, intermediate species, and its concentration after the initial “build-
up” period can be considered to be approximately constant. This leads to the steady-state
approximation, from which it is possible to calculate the behavior of the entire system in
a relatively straightforward fashion, by converting the differential equations into algebraic
equations. Setting d[B]/dt = 0 we get, from (12.99) and (12.87):
[
B
]
=
k
1
k
2
[
A
0
]
e
−k
1
t
(12.105)
and, from mass balance:
[
C
]
=
[
A
0
]
−
[
A
]
−
[
B
]
=
[
A
0
]
1 −
k
1
e
−k
1
t
−k
2
e
−k
2
t
k
2
. (12.106)
This approach yields a tremendously simplified solution. In this particular case, which
consists of only two first-order reactions, the exact solution is easy to obtain, but this is
not the case in general, and the steady state approximation, if it can be justified from the
relative values of the decay constants, is a powerful shortcut.
A different case is that of parallel reactions, in which the same species follow different
reaction pathways. These could be either the same reactants giving rise to different product
assemblages, or different reactant assemblages generating the same products. Consider the
case of the following two competing first-order reactions:
A →B, rate constant =k
B
A →C, rate constant = k
C
.
602 Non-equilibrium thermodynamics
0 0.5 1 1.5 2
0
0.2
0.4
0.6
t (arbitrary units)
t (arbitrary units)
[B] (arbitrary units)
d[B]/dt (arbitrary units)
0 0.5 1 1.5
2
–0.6
–0.4
–0.2
0
0.2
0.4
0.6
k
1
=1,k
2
=10
k
1
= 10, k
2
=1
k
1
=1,k
2
=10
k
1
= 10, k
2
=1
Fig. 12.4
Concentration (top) and rate of change of concentration with time (bottom) of the intermediate species, B,
in a sequential reaction. If B is a short-lived species (k
1
k
2
) d[B]/dt approaches zero after a relatively short initial
build-up period and [B] remains approximately constant after this. If B is a long-lived species (k
1
!k
2
) d[B]/dt does
not approach zero until the reaction is almost complete and its concentration cannot be considered to remain constant.
We assume that [A
0
]=0, [B
0
]=[C
0
]=0. The rate of decay of A is given by:
d
[
A
]
dt
=−k
B
[
A
]
−k
C
[
A
]
(12.107)
so that, from equation (12.87):
[
A
]
=
[
A
0
]
e
−
(
k
B
+k
C
)
t
(12.108)

603 12.3 Rate of chemical reactions
Because A is consumed by both reactions, the total rate constant is the sum of the individual
rate constants. For product B we have:
d
[
B
]
dt
=k
B
[
A
]
=k
B
[
A
0
]
e
−
(
k
B
+k
C
)
t
(12.109)
which integrates to:
[
B
]
=
[
A
0
]
k
B
k
B
+k
C
1 −e
−
(
k
B
+k
C
)
t
(12.110)
and similarly for C:
[
C
]
=
[
A
0
]
k
C
k
B
+k
C
1 −e
−
(
k
B
+k
C
)
t
. (12.111)
We see that the ratio between the concentrations of B and C is a constant, known as the
branching ratio, and given by:
[
B
]
[
C
]
=
k
B
k
C
. (12.112)
As usual, the mathematics become considerably more complicated as soon as we consider
anything but first-order kinetics. In many cases closed analytical solutions do not exist, and
the problems must be solved numerically.
Worked Example 12.3 Ozone in planetary atmospheres
Ozone is a minor but essential component in the terrestrial stratosphere (Chapters 13 and 14),
and is also found in trace amounts in the Martian atmosphere. Its atmospheric concentration
is not in equilibrium with oxygen, as can be seen from the homogeneous gas phase reaction:
3O
2
→
←
2O
3
. (12.113)
Atmospheric pressure in Earth at an elevation of 30 km is approximately 20 mbar
(Chapter 13), so we can estimate a characteristic partial pressure of O
2
in the stratosphere
of ∼4 mbar. The standard state Gibbs free energy change for reaction (12.113)at298Kis
r
G
0
1,298
=326.4 kJ, from which we calculate an equilibrium ozone partial pressure in the
stratosphere of ∼6×10
−33
bar. Measured values are in the range 10
−8
–10
−7
bar, i.e., some
25 orders of magnitude higher. This huge ozone excess is the result of a dynamic process
sustained by a constant supply of energy, which was first proposed by Chapman (1930a, b).
In its simplest form the Chapman cycle begins with photodissociation of molecular oxygen
according to:
O
2
+hν
uv
→O +O,
where hν
uv
represents an ultraviolet photon (Chapter 13). Atomic oxygen can be consumed
by any of the following three reactions:
O +O → O
2
O +O +M → O
2
+M
O +O
2
+M →O
3
+M,

604 Non-equilibrium thermodynamics
where M is a collision partner, which in the terrestrial atmosphere is typically N
2
or O
2
(i.e.
one of the dominant species). An intuitive argument based on the fact that [M] >>> [O] and
[O
2
] >>> [O] suggests that the dominant reaction is likely to be the third one. A rigorous
analysis using the values of the corresponding rate constants confirms this expectation (see,
for example, de Pater & Lissauer, 2001, p. 114). Ozone is produced by a sequential reaction
with a short-lived intermediate product, atomic oxygen, and is in turn consumed by two
reactions, photochemical dissociation:
O
3
+hν
uv
→O +O
2
and chemical recombination with atomic oxygen:
O +O
3
+M → 2O
2
+M.
The simplest version of the Chapman cycle, in an atmosphere in which all components other
than oxygen can be considered to be inert, can then be summarized as follows, where the
symbols following the reactions are the corresponding rate constants:
O
2
+hν →O +O, j
1
O +O
2
+M → O
3
+M, k
2
O
3
+hν →O +O
2
, j
3
O +O
3
→O
2
+O
2
, k
4
(12.114)
The rate constants for the two photochemical reactions, symbolized by j
1
and j
3
for reasons
that we discuss later, are functions of the solar energy flux and of the absorption cross
sections of oxygen and ozone molecules at ultraviolet wavelengths (Section 12.4.2 and
Chapter 13). They vary in a complex fashion with latitude, season, time of day and elevation.
For this example we will take characteristic values for the terrestrial stratosphere, averaged
over time, altitude and latitude: j
1
∼10
−12
s
−1
and j
3
∼10
−4
s
−1
(de Pater & Lissauer,2001,
p. 112). The other two reactions are thermally activated and follow Arrhenius-like behavior
(Section 12.4.1). Characteristic values for the corresponding rate constants at stratospheric
conditions are: k
2
∼10
−33
cm
6
molecule
−2
s
−1
and k
4
∼10
−15
cm
3
molecule
−1
s
−1
. Note
that the units of the rate constants are determined by the order of the reaction. For example,
because reaction 2 is third order its concentration product has units of [molecules]
3
×
[volume]
−3
, so that the rate constant must have units of [molecules]
−2
× [volume]
2
×
[time]
−1
, in order to yield a reaction rate in the proper units: [molecules] × [volume]
−1
×
[time]
−1
. The use of molecules (rather than mols) and cm (rather than m) is common in
chemical kinetics.
In the Chapman cycle there are two species that are likely to have short half lives: atomic
oxygen and ozone. We can expect this on thermodynamic grounds. As we saw, ozone is not
stable relative to molecular oxygen, and a similar calculation shows the same to be true for
atomic oxygen. We can then assume that the concentrations of these two species are in a
steady state, i.e.:
d[O]
dt
=2j
1
[O
2
]−k
2
[O][O
2
][M]+j
3
[O
3
]−k
4
[O][O
3
]=0 (12.115)

605 12.3 Rate of chemical reactions
and
d[O
3
]
dt
=k
2
[O][O
2
][M]−j
3
[O
3
]−k
4
[O][O
3
]=0. (12.116)
Adding the two equations and rearranging:
[O]=
j
1
k
4
[O
2
]
[O
3
]
(12.117)
substituting in (12.116) and rearranging:
[O
3
]
2
+
j
1
j
3
[O
2
][O
3
]−
j
1
k
2
j
3
k
4
[O
2
]
2
[M]=0. (12.118)
For a pressure of 20 mbar we calculate from the ideal gas EOS that [M] ≈5×10
17
molecules
cm
−3
, so that [O
2
] ≈ 10
17
molecules cm
−3
. Using the values for the rate constants listed
above we also find:
k
2
k
4
[M]∼0.5. (12.119)
We therefore note that it must be:
j
1
j
3
[O
2
][O
3
]
j
1
k
2
j
3
k
4
[O
2
]
2
[M]
=
[O
3
]
k
2
k
4
[O
2
][M]
1 (12.120)
so that we can drop the linear term in (12.118) and solve for [O
3
] as follows:
[O
3
]≈[O
2
]
j
1
k
2
j
3
k
4
[M]
1/2
. (12.121)
Substituting numerical values we find that [O
3
] ≈ 7 × 10
12
molecules cm
−3
, which
corresponds to an ozone partial pressure of ∼3 ×10
−7
bar.
This calculation yields an ozone concentration that is close to the measured value, but
not quite right. It overestimates the actual atmospheric concentration by up to one order
of magnitude. The reason for this discrepancy is that other species that are present in the
terrestrial atmosphere are not inert with respect to ozone destruction, but they do not affect
ozone formation, which relies exclusively on the photochemical dissociation of oxygen, i.e.
the first reaction in (12.114). Three such species are the radicals OH and NO, and atomic Cl.
These species form by photodissociation of H
2
O, N
2
O and halomethanes such as CCl
2
F
2
,
respectively. The latter are exclusively of anthropogenic origin, whereas nitrogen oxide has
both natural and anthropogenic sources (e.g., jet engine exhaust). Each of these species
gives rise to an ozone-consuming sequence of reactions, that can be written as follows:
OH +O
3
→HO
2
+O
2
HO
2
+O → OH +O
2
606 Non-equilibrium thermodynamics
and:
NO +O
3
→NO
2
+O
2
NO
2
+O → NO +O
2
and:
Cl +O
3
→ClO +O
2
ClO +O → Cl +O
2
.
The net result of all three reaction sequences is the same, namely:
O +O
3
→2O
2
with regeneration of the active species. For this last reason these are known as cat-
alytic cycles. If we use X to designate a catalyst in general then all of these reactions
correspond to:
X +O
3
→XO +O
2
, k
5
XO +O → X +O
2
, k
6
.
(12.122)
We can modify (12.115) and (12.116) as follows:
d[O]
dt
=2j
1
[O
2
]−k
2
[O][O
2
][M]+j
3
[O
3
]−k
4
[O][O
3
]−k
6
[XO][O]=0 (12.123)
and
d[O
3
]
dt
=k
2
[O][O
2
][M]−j
3
[O
3
]−k
4
[O][O
3
]−k
5
[X][O
3
]=0 (12.124)
and add a rate law for any one of the two X-bearing species (they don’t vary independently),
for example:
d[XO]
dt
=k
5
[X][O
3
]−k
6
[XO][O]=0. (12.125)
Eliminating [O] between (12.123), (12.124) and (12.125) we get a quadratic equation
in [O
3
]:
[O
3
]
2
+
k
2
k
5
j
3
k
4
[O
2
][M][X]+
j
1
j
3
[O
2
]
[O
3
]−
j
1
k
2
j
3
k
4
[O
2
]
2
[M]=0. (12.126)
Comparing the two contributions to the coefficient of the linear term, and using the same
values for the rate constants and concentration of the collision partner as before, we find:
k
2
k
5
j
3
k
4
[O
2
][M][X]
j
1
j
3
[O
2
]
=
j
1
k
4
k
2
[M]
1
k
5
[X]
∼
10
−12
k
5
[X]
1, (12.127)
607 12.3 Rate of chemical reactions
where the last inequality is true unless the rate constant k
5
is very small – this will be
justified further a posteriori. We can then simplify (12.126) as follows:
[O
3
]
2
+
k
2
k
5
j
3
k
4
[O
2
][M][X][O
3
]−
j
1
k
2
j
3
k
4
[O
2
]
2
[M]=0 (12.128)
which, using the quadratic formula and noting that the only physical root is the positive
one, yields:
[O
3
]=
k
2
k
5
2j
3
k
4
[O
2
][M][X]
2
+
j
1
k
2
j
3
k
4
[O
2
]
2
[M]
1/2
−
k
2
k
5
2j
3
k
4
[O
2
][M][X]. (12.129)
Comparing the second term in the square root in (12.129)to(12.121) we see that this is the
square of the ozone concentration in the absence of an active species, which we can call
[O
3
]
0
, so that we have:
[O
3
]=
k
2
k
5
2j
3
k
4
[O
2
][M][X]
2
+
(
[O
3
]
0
)
2
1/2
−
k
2
k
5
2j
3
k
4
[O
2
][M][X] (12.130)
or:
[O
3
]
[O
3
]
0
=
k
2
k
5
2j
3
k
4
[O
2
][M][X]
[O
3
]
0
2
+1
1/2
−
k
2
k
5
2j
3
k
4
[O
2
][M][X]
[O
3
]
0
. (12.131)
The ratio is equal to one for [X]=0, as we should expect, and it is less than one for any
value of [X] > 0. Thus, equation (12.131) confirms a decrease in the ozone concentration
when active species are added to the atmosphere. This behavior is general, but in order
to study it in more detail it is necessary to substitute numerical values. Using once more
the rate constants and gas concentrations in the terrestrial stratosphere discussed earlier we
find:
k
2
2j
3
k
4
[O
2
][M]∼10
20
(12.132)
so that, for these particular parameter values, (12.131) becomes:
[O
3
]
[O
3
]
0
=
k
5
×10
20
[X]
[O
3
]
0
2
+1
1/2
−k
5
×10
20
[X]
[O
3
]
0
. (12.133)
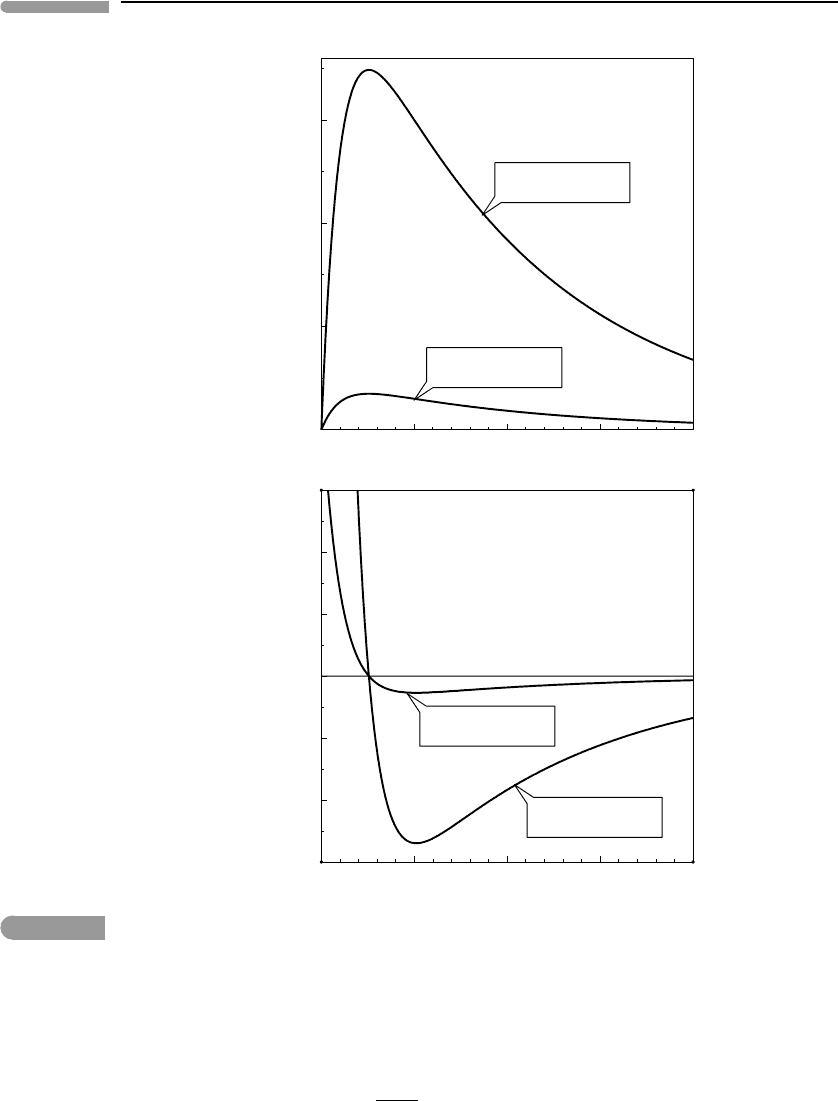
The behavior of this function is shown in Fig. 12.5, for values of the rate constant k
5
ranging
from 10
−8
to 10
−14
cm
3
molecule
−1
s
−1
, which ensure the validity of (12.127). We see that
a sufficiently small concentration of the active catalytic species has a negligible effect on
ozone concentration, but that there is a threshold value beyond which ozone concentration
becomes quite sensitive to increased concentration of the active species and declines steeply.
The value of this concentration threshold depends on the magnitude of the rate constant k
5
.
For example, k
5
for the Cl-initiated cycle is of the order of 10
−11
cm
3
molecule
−1
s
−1
, which
means that significant ozone depletion begins when the concentration of Cl atoms is as low
as ∼10
−10
times the initial ozone concentration, and that a Cl concentration of only 10
−8
[O
3
]
0
is sufficient to virtually deplete stratospheric ozone. The ozone-depleting catalytic
