Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


628 Topics in atmospheric thermodynamics
c
a
b
,T
b
a
c
,T
c
r
b
r
c
F
c
F
b
F
b
T
c
=
b
=
4
T
b
4
F
c
=
=(r
b
/r
c
)
2
σ
σ
T
b
4
σ
T
c
4
σ
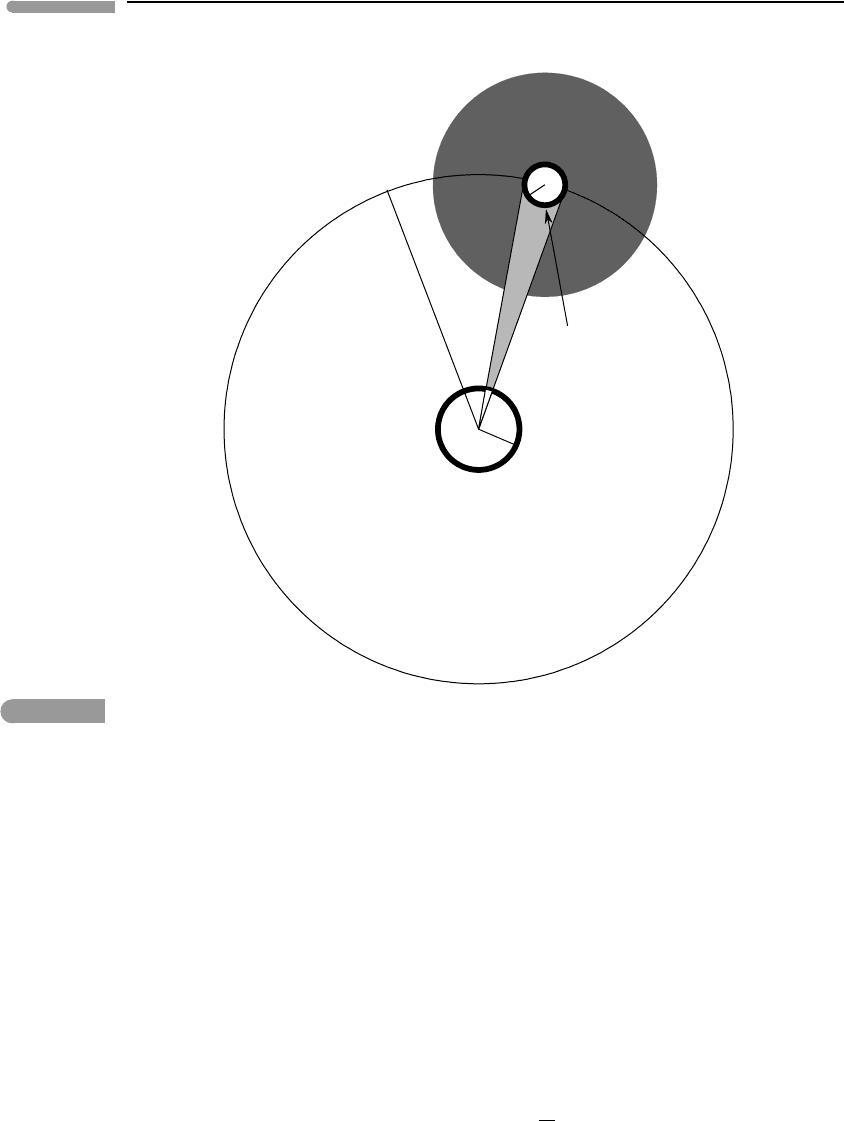
Fig. 13.4
Radiant energy exchange between a cavity at temperature T
c
and a body at temperature T
b
. Both are assumed to
behave as black bodies, and the intervening medium has unit transmittivity.
of body and cavity, the total rates of energy radiation are a
b
σ T
4
b
and a
c
σ T
c
4
, respectively.
There is an important difference between the two, though: the cavity radiates onto itself,
but the body does not. Heat transfer by radiation between the two is thus not symmetrical.
In particular, the total amount of energy radiated by the body is absorbed by the cavity, but
the converse is not true.
The radiation emitted by the body gives rise to an incident energy flux F
b→c
on the
surface of the cavity given by:
F
b→c
=
a
b
σT
b
4
a
c
=
r
b
r
c
2
σT
b
4
(13.33)
which is the inverse square law of radiation, a.k.a. the equation of energy conservation. The
flux of electromagnetic radiation emitted by the cavity, σ T
c
4
, bathes its interior uniformly.
This can be demonstrated formally (Winterton, 1997) or you can accept it intuitively on
the basis of symmetry. The body interposes a surface area a
b
to this energy flux, so that the
total amount of energy emitted by the cavity that is absorbed by the body is a
b
σ T
c
4
, and
the flux of radiation incident on the body F
c→b
, is, therefore:
F
c→b
=
a
b
σT
c
4
a
b
=σT
c
4
. (13.34)

629 13.3 Radiative energy transfer
Because the body is a black body it absorbs this energy, so that the net flux of electromagnetic
energy that leaves the body, F
b,net
, equals the energy emitted minus the energy absorbed, i.e.:
F
b, net
=F
b
−F
c→b
=σ
T
b
4
−T
c
4
. (13.35)
If T
b
> T
c
then the body is losing internal energy, and must either be cooling down, if
this energy is not being replenished, or there is an internal source that supplies energy at
this rate. Conversely, if T
c
> T
b
radiant energy is being transformed to internal energy in
the body. Think of this conversion in terms of photons colliding with particles of matter,
whereupon the kinetic energy of the photons is added to the translational, vibrational or
rotational energies of the particles. As an aside, photons carry not only kinetic energy but
also momentum, and momentum conservation must be obeyed too. Transfer of momentum
from photons to particles of matter gives rise to radiation pressure.
Equation (13.35) shows that radiative heat flux varies as the difference between the fourth
powers of temperature, in contrast to diffusive and convective heat flux, which are linear
functions of temperature difference, or nearly so (e.g. equations (3.5) and (3.89)). It justifies
equation (2.16): the planet at temperature T is immersed in the solar nebula, which we can
think of as the “cavity”, at temperature T
0
.
From (13.35) we can define the equilibrium temperature for the body, T
b,eq
, as the tem-
perature at which there is no net flux of electromagnetic radiation from the body and hence
no change in its internal energy content with time. This is simply:
T
b, eq
=T
c
. (13.36)
A body at the same temperature as its environment does not exchange electromagnetic
energy with it.
Worked Example 13.1 Radiative energy balance at a planet’s surface
The Sun can be approximated as a spherical black body with an emission temperature of
∼6000 K, and planetary orbits can be thought of as circumferences on spherical cavities
centered on the Sun. Setting r
b
= r
s
= solar radius, r
c
= r
o
= orbital radius and T
b
= T
s
= Sun’s emission temperature, equation (13.33) gives the flux of solar radiation across a
planet’s orbit, which is called the solar constant (Fig. 13.5). The solar constant for the Earth
is ∼1368 W m
−2
. This is the absolute maximum rate at which energy can be extracted
from sunlight at the Earth’s surface. Even if all of this radiant energy could be converted to
mechanical energy, it represents somewhat less than 2 horse-power per square meter. The
energy density of solar power is quite low, which is a reality that economic development
of this energy source must cope with. In practice, moreover, only a fraction of the solar
energy flux can be converted to usable energy at the Earth’s surface, partly because for
the atmosphere A > 0 (see below), and also because conversion to electrical or mechanical
energy is never 100% efficient (Chapter 4).
We can calculate the equilibrium temperature of the planet, T
eq
, that we referred to in
Section 2.1. This is a temperature such that the planet emits electromagnetic radiation at the
same rate as it absorbs it from the Sun. The total amount of solar radiation that reaches a
planet is equal to the solar constant multiplied by the cross section of the planet, πr
2
p
, where
r
p
is the planet’s radius. Note that this is not the surface area of the hemisphere facing the
sun, but the cross section that intersects the solar energy flux, i.e. the stream of solar photons
(Fig. 13.5). Think of the planet as the “body” in Fig. 13.4, inside a cavity of infinite extent.
630 Topics in atmospheric thermodynamics
r
p
4πr
p
2
4
σT
eq
πr
p
2
2
4
(
)
r
s
/r
o
σT
s
r
o
r
s
Fig. 13.5 Radiant energy exchange between the Sun (radius r
s
) and a planet of radius r
P
in a circular orbit of radius r
o
. The
planet absorbs the solar flux contained in the light grey solid angle, defined by the cross section area πr
p
2
at the
planet’s orbital distance. Thermalized radiation is radiated back to space (a cavity of infinite extent symbolized by the
dark grey circle) over the entire surface area of the planet, 4π r
p
2
.
We see from equation (13.35) that, because during the day T
b
<T
c
, the planet absorbs
sunlight, whereas at night T
b
> T
c
and the planet emits radiation. This radiation is what
we called thermalized radiation, or thermalized sunlight, in Chapter 2. If the planet rotates
fast enough we can assume that it radiates at the same equilibrium temperature, T
eq
, over
its entire surface area, 4πr
2
p
. We will ignore the planet’s internal energy flux. The Earth’s
internal energy flux is ∼10
−5
times the solar constant, so it cannot have any noticeable effect
on its surface equilibrium temperature. The same is true of the other terrestrial planets but,
as we saw in Chapter 2, it is not the case for the fluid planets nor for Io. Assuming that
the planet behaves like a black body, T
eq
must satisfy the following energy conservation
condition:
4πr
p
2
σ
T
eq
4
=πr
p
2
r
s
r
o
2
σT
s
4
(13.37)

631 13.3 Radiative energy transfer
or:
T
eq
=
1
4
1/4
r
s
r
o
1/2
T
s
. (13.38)
At this temperature the planet radiates energy at the same (average) rate as it receives it
from the Sun. For the Earth we calculate an equilibrium temperature of ∼278 K. We can use
Planck’s law (equation (13.29)) to compare the spectrum of sunlight with that of thermalized
sunlight (Fig. 13.3). The emission peak shifts from 0.5 µm, in the visible range, to 10 µm,
in the mid infrared. Because of the very strong dependency of emitted flux on temperature
there is a difference of seven orders of magnitude between the height of the peaks at 6000 K
and 278 K.
The Earth’s equilibrium temperature is about 10 K lower than the actual mean terrestrial
surface temperature (∼288 K). Why the discrepancy? In short, because the Earth has an
atmosphere, oceans and a climate system. For example, some trace components in the tro-
posphere such as H
2
O, CO
2
and CH
4
absorb in the infrared part of the spectrum, causing
warming of the lower atmosphere (the greenhouse effect, see below). Additional complica-
tions arise from variations in solar radiation with latitude and seasons (that drive convective
heat transport) and with changes in albedo related to ground cover and cloud cover.
I stated above that at night T
b
> T
c
, but what is the “cavity temperature” at night?
Disregarding for now the planet’s atmosphere (and heterogeneities that may arise from
interplanetary and galactic energy sources) this is the temperature of the microwave back-
ground radiation, ∼2.7 K (Fig. 13.3). In terms of heat transfer, you can think of this radiation
as being emitted by an infinitely distant cavity at this uniform temperature, although this is
of course not its true physical nature (it is strongly red-shifted radiation from the early Uni-
verse). If the Earth were to be ejected from the Solar System into the depths of intergalactic
space, would it still radiate electromagnetic energy to space? Yes, at its current average
internal energy output (∼87 mW m
−2
) it would radiate as a black body at a temperature
of ∼35K (equation (13.31)) with a peak at a wavelength of ∼82 µm (equation (13.30)),
corresponding to the far infrared part of the spectrum (Fig. 13.3). Emission flux originating
from the Earth’s internal heat (at 35 K) is some five orders of magnitude lower than the flux
of thermalized solar radiation at 278 K (Fig. 13.3). This explains why it is generally not
possible to use remote sensing to determine heat flux from terrestrial planets. It is interest-
ing (and pointless) to speculate that the low surface temperature that would result from the
Earth being ejected to intergalactic space would increase (slightly) the mantle’s Rayleigh
number and hence the rate of plate tectonics.
To conclude this section we derive a fundamental relationship between emissivity and
absorptivity.Assume that the body in Fig. 13.4 is not a black body. Then, for each wavelength
λ it has an emissivity /
λ
=1 and an absorptivity A
λ
=1. The cavity is a black body that emits
an energy flux F
∗
c, λ
, given by equation (13.29). From equation (13.34) and the definition
of absorptivity, the energy flux absorbed by the body at a given wavelength is:
F
c→b,λ
=A
λ
F
∗
c, λ
. (13.39)
From the definition of emissivity, the emission from the body is given by:
F
b, λ
=/
λ
F
∗
b, λ
, (13.40)
632 Topics in atmospheric thermodynamics
where F
∗
b, λ
is the black body emission at the body’s temperature. The net radiant flux
leaving the body is then given by:
F
b, net, λ
=F
b, λ
−F
c→b,λ
=/
λ
F
∗
b, λ
−A
λ
F
∗
c, λ
. (13.41)
If the temperatures of the body and the cavity are the same then F
∗
c, λ
= F
∗
b, λ
and also,
from equation 13.35, F
b, net, λ
= 0. The following result, known as Kirchoff’s law, follows:
A
λ
=/
λ
. (13.42)
In other words, absorptivity and emissivity at a given wavelength are equal. There are
some important caveats. First, if a body absorbs radiation that was emitted at a temperature
different from that of the body itself then the integrated absorptivity and emissivity are not
equal. For example (see Fig. 13.3), because sunlight peaks at ∼0.5 µm whereas thermalized
sunlight on Earth peaks at ∼10.4 µm, the absorptivity and emissivity of the Earth’s surface
integrated over all wavelengths are not equal. Second, it is in general the case that A = /
even if emitted and absorbed radiation correspond to the same black body temperature. The
reasons for this have to do with the fact that, in contrast with a black body, radiation from
real bodies is in general not isotropic and may also be polarized differently from absorbed
radiation. Discussion of these effects is beyond the scope of this book.
13.3.3 Molecular absorption and emission of electromagnetic radiation
Electromagnetic radiation transports heat because photons can interact and exchange energy
with particles of matter. Heat transport by radiation is relatively minor in liquids and gener-
ally negligible in solids, but absorption and emission of radiant energy by planetary liquids
and solids is important. In contrast, gases are important in terms of radiant energy trans-
ported as well as absorbed and emitted. We saw in Chapter 1 that molecules in gases carry
energy as translational, rotational and vibrational kinetic energy, and that, as temperature
increases, all three modes come to participate in the heat capacity of gases. Molecules
also store energy in their electronic configurations. This mode does not show up in the
heat capacity of gases at “normal” planetary conditions because at these conditions there
is not enough energy to bring about changes in the electronic configuration of a molecule
or atom, or, in the terminology of quantum mechanics, to excite the electronic energy
modes.
In order to discuss emission, absorption and transport of radiant energy in gases we must
take a closer look at the various modes in which energy is stored in molecules. The rotational,
vibrational and electronic modes are all quantized, which means that they can carry energy
only in specific levels that are separated by discrete energy differences. Molecules can
absorb or emit energy only in discrete packages that correspond to the differences between
quantum energy levels. Photons carry discrete amounts of energy too, given by (hc)/λ.A
photon can interact with a molecule only if its energy corresponds to some of the possible
energy transitions in the molecule. Only photons of the appropriate wavelength (= energy)
can be absorbed and when they are, one of the molecular energy modes is excited to a higher
energy level. Conversely, when the molecule “drops” from an excited state to the ground
state it emits a photon of a wavelength that corresponds to this energy difference.
It is perhaps intuitively apparent that it takes relatively little energy to change the state
of rotation of a molecule, more energy to change the vibrations of the atomic bonds, and
even more energy to alter the electronic configuration of the molecule. This is formally

633 13.3 Radiative energy transfer
proved in quantum mechanics and is important for our purposes. The energies required
to excite electronic modes are carried by photons with wavelengths of order 0.1–0.5 µm,
within the ultraviolet and visible part of the spectrum (see Fig. 13.3). Electronic excitation
corresponds to breaking or forming of chemical bonds, i.e. chemical reactions. Vibrational
energy transitions are associated with lower energies, corresponding to infrared photons
with wavelengths of 1–10 µm. Rotational transitions occur in response to even lower
energy photons, in the microwave region of the spectrum (10
2
− 10
4
µm). Excitations
of rotational modes are generally unimportant in planetary processes, as they correspond
to exceedingly low temperatures, but are important in astrophysics, where non-thermal
mechanisms for the emission of microwave electromagnetic radiation exist (they are also
what makes microwave ovens work).
Molecular gases absorb and emit radiation of wavelengths extending from the ultraviolet
to the infrared, by exciting electronic and vibrational energy modes. The mechanisms are
different for the two types of energy transitions. Absorption of ultraviolet radiation by
electronic transitions is associated to photodissociation reactions. Oxygen–ozone reactions
in the Earth’s stratosphere are a good example (Worked Example 12.3). The first and third
reactions in the Chapman cycle (equation (12.144)) absorb photons with wavelengths in
the 0.2–0.25 µm range. In both cases absorption of a photon excites an electronic transition
which results in breakage of an atomic bond. Absorption of ultraviolet radiation by these
reactions in the Earth’s stratosphere has two important effects. First, because complex
organic molecules such as proteins and DNA can also break up by absorbing photons in this
energy range, photoactivated reactions that produce and destroy ozone allow us to be here
discussing these things. Second, absorption of ultraviolet radiation heats the stratosphere
and inverts the temperature gradient that drives convection in the troposphere.
Infrared photons are not energetic enough to break atomic bonds and facilitate chemical
reactions. They are absorbed by exciting vibrational modes. There is an additional restriction
in this case, that arises from quantum mechanics selection rules. The selection rule for
vibrational excitations is that they can only happen in molecules in which the electrostatic
dipole moment (i.e. the distribution of electric charge across the molecule) is asymmetric.
What this means is that homonuclear diatomic molecules such as O
2
and N
2
cannot absorb
infrared radiation, because the electric charge of both atoms is identical. In contrast, diatomic
molecules made up of different atoms (e.g. CO or HCl) and polyatomic molecules have
dipole moments that are not symmetric relative to the molecular structure. By the selection
rule they can absorb infrared photons as long as they have the correct energy to excite one
of the possible vibrational transitions. A well-known example of this is the capability of
molecules such as CO
2
,H
2
O and CH
4
to absorb infrared radiation at wavelengths that are
close to the emission peak of thermalized solar radiation (see below).
Gases emit and absorb photons of specific wavelengths only, that correspond to allowed
energy transitions in the molecules. Emission and absorption of electromagnetic radia-
tion by molecular gases gives rise to line spectra. The molecules and atoms in solids
and liquids, in contrast, are close enough that the quantum states of individual atoms are
not independent of one another and the discrete energy transitions become smoothed out.
The result is that absorption and emission of electromagnetic radiation in solids and liq-
uids extend over continuous regions of the spectrum. This is also true of gases in which
electrons are free, because in such case electrons do not have set energy levels, and it
is the reason why the outer envelopes of stars, made up of ionized gas, radiate as black
bodies.
634 Topics in atmospheric thermodynamics
13.3.4 Absorption and emission of electromagnetic radiation:
the macroscopic description
Consider a layer of material of infinitesimal thickness, dx (Fig. 13.6) and a beam of electro-
magnetic radiation of wavelength λ incident on one of its sides. Recall that the irradiance,
F
λ
, is the total flux of radiation traveling in all directions. We will consider only the simplest
case of changes in radiation intensity in a single direction. The intensity of the radiation,
I
λ
, is defined as the flux of radiation traveling in a single direction, which in this case is
perpendicular to the layer of material. As in all radiation problems, a complete analysis
requires that we consider the full geometry of the problem and how radiation intensity
changes with direction, but the physical principles involved are easier to see if we ignore
these complications.
In general, the material may both absorb and emit radiation of a given wavelength, so
that the total change in the intensity of the beam over the thickness dx is given by:
dI
λ
=dI
λ,absorbed
+dI
λ,emitted
. (13.43)
From the definition of absorptivity we see that:
dI
λ,absorbed
=−A
λ
I
λ
. (13.44)
It is reasonable to assume that the absorptivity is a function of some intrinsic material
property, on how much matter there is, and of the thickness of the layer, so we write:
A
λ
=k
λ
ρdx, (13.45)
where ρ is density and k
λ
is called the mass absorption coefficient for radiation of wave-
length λ. Because absorptivity is a non-dimensional number, the mass absorption coefficient
must have dimensions [L]
2
[M]
−1
, e.g. m
2
kg
−1
. In this derivation we will consider only
absorption and emission by molecular mechanisms such as those that we discussed in the
previous section. The intensity of electromagnetic radiation can also change as a result of
scattering, as when photons interact with solid or liquid particles suspended in the atmo-
sphere. This process will not be considered here, but it is straightforward to add the effects
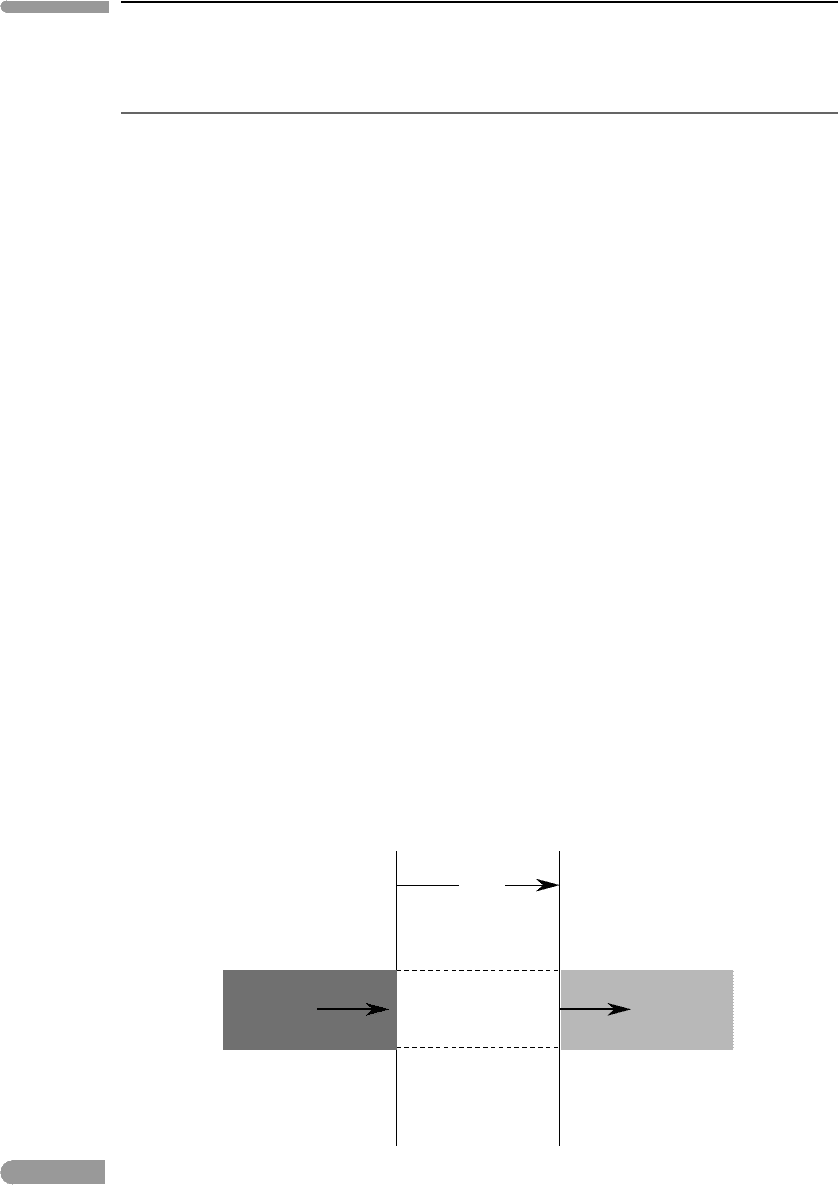
dx
dτ
λ
= k
λ
ρ dx
dl
λ
= (–l
λ
+ S
λ
) dτ
λ
l
λ
+ dl
λ
l
λ
Fig. 13.6
Interaction between a monochromatic beam of electromagnetic radiation of intensity I
λ
and a slab of material of
thickness dx.

635 13.3 Radiative energy transfer
of scattering to the absorption coefficient, in which case the name changes to extinction
coefficient to account for the combined effects of absorption and scattering.
We define a non-dimensional variable, called the optical thickness, τ
λ
, as follows:
τ
λ
=k
λ
ρx, (13.46)
and from equations (13.44), (13.45) and (13.46), we write the change in beam intensity due
to absorption as follows:
dI
λ,absorbed
=−k
λ
ρI
λ
dx =−I
λ
dτ
λ
. (13.47)
We can also write an expression for dI
λ,emitted
similar to (13.47) by defining a variable S
λ
,
called the source term, which, as I
λ
, has units of energy flux. Thus:
dI
λ,emitted
=S
λ
dτ
λ
. (13.48)
The source term is a measure of how much radiation the layer emits per unit of non-
dimensional optical thickness. Using equations (13.47) and (13.48), (13.43) becomes:
dI
λ
=−I
λ
dτ
λ
+S
λ
dτ
λ
(13.49)
The solution of this differential equation is straightforward if S
λ
is a constant (see Exercise
Problem 13.12, or differentiate (13.50) and substitute in (13.49) to verify that it is a solution):
I
λ
=
I
λ
(
0
)
−S
λ
e
−τ
λ
+S
λ
, (13.50)
where I
λ(0)
is the intensity of the incident beam and I
λ
is the intensity at optical
thickness τ
λ
.
The meaning of the optical thickness becomes apparent if we consider the case in which S
λ
=0. Equation (13.50) then simplifies to the following, which is known as the Beer–Lambert
law (see also equation (12.137)):
I
λ
=I
λ
(
0
)
e
−τ
λ
. (13.51)
The optical thickness is the non-dimensional absorption length. About 2/3 of the incident
radiation is absorbed at τ
λ
=1, and at five optical thicknesses more than 99% of the incident
radiation has been absorbed.As an example, consider transmission of solar radiation through
the terrestrial atmosphere. Solar radiation peaks at visible and ultraviolet wavelengths. The
temperature of the atmosphere is of the order of 250 K. We can see from Planck’s and
Wien’s laws that the atmosphere does not emit any significant amount of radiation in this
region of the spectrum, so that S
λ
≈0. From our discussion in Section 13.3.3 it follows that
atmospheric oxygen has a large value of k
λ
for λ ∼0.2 µm (ultraviolet photons). For the
Earth’s atmosphere τ
λ
! 1 for ultraviolet radiation. We say that the atmosphere is optically
thick in the ultraviolet, or, equivalently that the atmosphere is nearly opaque to ultraviolet
radiation.
Returning to the full absorption–emission equation, (13.50), we consider what happens
if the temperature of the medium is such that emission at the wavelength of the incident
radiation (the term S
λ
) cannot be ignored (this can be the case for infrared radiation in
CO
2
-rich planetary atmospheres). In this case, for small optical thicknesses the source term
cancels out and the radiation flux is dominated by the incident flux, whereas for large optical
thicknesses radiation flux is dominated by thermal emission in the layer.

636 Topics in atmospheric thermodynamics
13.3.5 Absorption cross section and mean free path
The mass absorption coefficient,k
λ
, is a macroscopic parameter that describesthe interaction
between electromagnetic radiation and matter. We can give it a microscopic interpretation,
as follows. From the definition of optical thickness, equation 13.46, we see that the product
k
λ
ρ has dimension of length
−1
, so that (k
λ
ρ)
−1
is the natural lengthscale for absorption
of radiation of wavelength λ. We now search for a product of two microscopic variables
with the same dimension. We define the absorption cross section at wavelength λ, σ
λ
,as
the effective target area that each molecule offers to photons of this wavelength. Thus, if
a substance is inert to a particular wavelength, such as molecular oxygen to infrared, σ
λ
vanishes. In contrast, if a substance absorbs radiation of a given wavelength (e.g. CO
2
in
the infrared) its absorption cross section for that wavelength is a finite and potentially large
number. The dimension of σ
λ
is length
2
molecule
−1
. If we multiply the absorption cross
section by the number density of molecules, N , defined as the number of molecules per unit
volume, we get a product with dimension of length
−1
. This product is the total absorption
cross section per unit volume, so we can suggest the following relationship between the
macroscopic absorption coefficient and the microscopic absorption cross section:
k
λ
ρ =σ
λ
N. (13.52)
Imagine now that we have a slab of absorbing substance of cross section a
2
and thickness
ι
λ
, such that ι
λ
is the characteristic distance that a photon of wavelength λ can penetrate into
the substance before it becomes certain that it will be absorbed. This distance is called the
photon’s mean free path. The mean free path must be of the same order as the distance into
the layer at which the sum of the absorption cross sections of all of the molecules equals the
actual cross section of the slab. Since σ
λ
N is the absorption cross section per unit volume,
multiplying this by the volume of the slab we get the total absorption cross section of the
slab, which we require to be equal to the physical cross section of the slab, i.e.:
σ
λ
Na
2
ι
λ
=a
2
, (13.53)
which shows that the mean free path is given by:
ι
λ
=
1
σ
λ
N
=
1
k
λ
ρ
. (13.54)
The mean free path thus equals the lengthscale for absorption, (k
λ
ρ)
−1
. From equations
(13.46) and (13.54) we have:
τ
λ
=
x
ι
λ
. (13.55)
An optical thickness of 1 is reached, and most of the incident radiation is absorbed, when
the distance traveled by the electromagnetic radiation is of the order of the mean free path.
Other things being equal, the mean free path varies inversely with the number density, N
(equation (13.54)), explaining in part why solids tend to be more opaque (absorptivity = 1)
than gases.
In a mixture of ideal gases, the number density N of a gas species at constant temperature is
proportional to the partial pressure of the species. For dilute gases at low pressure, therefore,
mean free path varies inversely with the partial pressure of the absorbing species (equation
(13.54)) and optical thickness can be approximated with a linear function of partial pressure
(equations (13.54) and (13.55)).

637 13.3 Radiative energy transfer
13.3.6 A radiative toy model of greenhouse warming
We close our discussion of radiative heat transfer by constructing a “toy model” of green-
house warming. The goal of this section is not to develop a complete and quantitatively
accurate model of the process, which is far beyond the scope of this book. Rather, I sim-
ply want to highlight the aspects of radiative heat transport that underlie the planetary
greenhouse effect. With this caveat, we proceed as follows (see Fig. 13.7).
Consider a planet such that the solar constant at its orbit is F
s
. The planet has an atmo-
spheric layer in which radiative heat exchanges take place. The elevation and thickness of
this active layer are unspecified, except for the fact that it is close enough to the planet’s
solid surface, and thin enough, that the radius of the planet, r
p
, and the mean radius of the
active atmospheric layer can be considered to be equal. We will simplify the mathematical
treatment by assuming that we can describe the interactions between electromagnetic radi-
ation, the atmosphere and the surface on the basis of only two sets of A, Θ,Rand /.As
we saw, all of these parameters depend on wavelength but here we will assume that we can
++
=1 (downwards)
= 1 (upwards)
= 1 (both directions)
(both directions)
Active atmospheric
layer, temperature = T
a
Planetary surface
temperature = T
g
= 1
F
a
=
π r
p
F
s
2
π r
p
2
R
a,λs
π r
p
2
2
4
(Θ
a,λs
)
π r
p
2
Θ
a,λs
4π r
p
2
4π r
p
2
Θ
a,λt
Θ
a,λt
F
s
R
g,λs
F
s
π r
p
2
Θ
a,λs
A
g,λs
A
g,λs
F
s
π r
p
2
Θ
a,λs
R
g,λs
R
g,λs
F
s
F
s
4π r
p
2
F
a
4π r
p
2
F
g
4π r
p
2
A
g,λt
F
g
F
a
F
a
4π r
p
2
R
g,λt
R
a,λs
A
a,λs
+
+
= 1
A
g,λt
R
g,λt
+
A
a,λs
Θ
a,λs
Θ
a,λs
+
A
a,λt
Θ
a,λt
=
A
a,λt
a,λt
σT
a
F
g
=
4
g,λt
σT
g
F
a
4π r
p
2
R
g,λt
F
a
g,λt
=
A
g,λt
a,λt
Fig. 13.7
Energy balance for a simple radiative model of greenhouse warming of a planet of radius r
P
and solar constant F
s
. The
planet has an atmospheric layer that interacts with solar and thermal radiation, and attains an equilibrium
temperature T
a
. The equilibrium temperature of the planet’s surface is T
g
. The arrows illustrate the direction of the
various energy fluxes, but not their relative intensities.
