Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

648 Thermodynamics of life
The partial derivative of the Gibbs free energy of oxygen follows equations (9.96) and
(9.100):
µ
0,O
2
1,T
RT
+lnf O
2
+2λ
2
=0 (14.6)
and the partial derivative for liquid water, ignoring the effect on pressure on the chemical
potential of liquid H
2
O, is (compare equation (9.101) for graphite):
µ
0,H
2
O
(
liquid
)
1,T
RT
+2λ
1
+λ
2
=0. (14.7)
Equations (14.2)–(14.7) constitute a system of 14 equations in the 14 unknowns:
n
H
2
O(liquid)
, n
H
2
O(vapor )
, n
H
2
, n
CH
4
, n
CO
2
, n
CO
, n
N
2
, n
NH
3
, ln(fO
2
), P , λ
1
, λ
2
, λ
3
and
λ
4
(n
t
is given by (14.1)). Numerical solution with Maple is straightforward and is briefly
discussed in Software Box 14.1. Recall that the four Lagrange multipliers are necessary in
order to solve the constrained minimum problem (Section 9.6.2), but we have no use for
their numerical values.
Let us first examine solution sets at 25
◦
C for a planet with Earth’s gravitational accel-
eration and with total volatile content adjusted so as to yield an atmospheric mass of order
10
4
kg m
−2
, comparable to the present day Earth. We start with the following values.
N
H
=1650 ×10
3
mols m
−2
N
O
=650 ×10
3
mols m
−2
N
C
=350 ×10
3
mols m
−2
N
H
=100 ×10
3
mols m
−2
The total volatile mass that results is equal to 1.765×10
4
kg m
−2
, which is almost twice the
present-day terrestrial atmospheric mass, but as we shall see some of this mass condenses
as liquid water. The relative proportions of the four components are similar to cometary
material (see Lodders & Fegley, 1998, Tables 15.3 and 15.4), but I emphasize that there is
no special significance to this starting composition, other than being plausible. The point of
this exercise is to examine how atmospheric speciation responds to changes in the model
parameters.
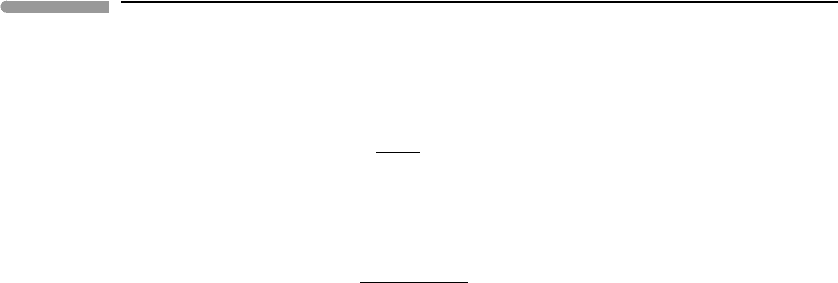
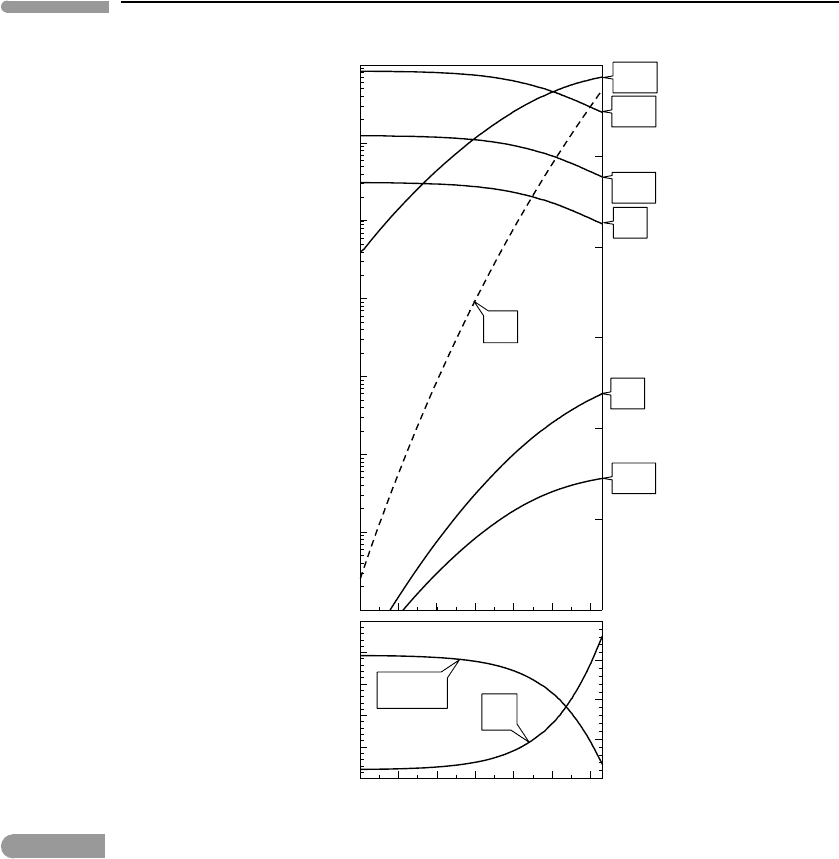
Figure 14.1 shows the effect of changing the bulk contents of H, C and O independently,
i.e. the bulk content of one each of these elements changes, while all the others remain
constant and equal to the values listed above. The independent variable in each graph is the
bulk atomic fraction of the component that varies. Temperature is kept constant at 25
◦
C,
and g = 9.8ms
−2
. The top panels show the oxygen fugacity and mol fraction of each of
the gas species except CO, which never rises above ∼ 10
−10
and which we discuss later.
The bottom panels display atmospheric pressure at the planet’s surface and the amount of
H
2
O that condenses as liquid, converted to thickness of the water column (55.56×10
3
mols
m
−2
=1 m of water depth).
The calculations show a steep jump in species distribution, between a H
2
–NH
3
–CH
4
atmosphere with virtually no CO
2
, and a CH
4
–CO
2
atmosphere with small but non-zero
contents of NH
3
and H
2
. The atmosphere becomes less reduced in response to decreasing
bulk H content or increasing bulk O or C contents, but in every case there is a sudden jump
in species abundances over very narrow bulk composition intervals. The behavior is in some
649 14.1 Chemical evolution of post-nebular atmospheres
10
–6
10
–5
10
–4
10
–3
10
–2
10
–1
CO
2
CO
2
CO
2
CO
2
CO
2
CH
4
CH
4
CH
4
H
2
H
2
H
2
H
2
H
2
–72
–72
–74
–76
–78
–80
–72
–74
–76
–78
–80
–76
–80
–84
1.5
0.5
1
1.5
0.5
1
1.5
0.5
1
H
2
NH
3
NH
3
NH
3
NH
3
NH
3
NH
3
CO
2
P
P
P
Water
0.2 0.2 0.3 0.02 0.04 0.06 0.08 0.10.10.4 0.6 0.8
Water
Water
10
5
0
10
5
0
10
5
0
f
O
2
f
O
2
f
O
2
N
2
N
2
N
2
N
2
H
2
O
H
2
O
H
2
O
N/C+O) = 0.1
C/O = 0.54
N/(C+H) = 0.05
C/H = 0.21
N/(O+H) = 0.43
C/H = 0.39
1
10
–7
10
–6
10
–5
10
–4
10
–3
10
–1
1
10
–7
10
–6
10
–5
10
–4
10
–3
10
–1
1
10
–7
Species mol fraction
Species mol fraction
Pressure (bar)
Pressure (bar)
Pressure (bar)
log f(O
2
)
log f(O
2
)
log f(O
2
)
Water depth (m)
Water depth (m)
Species mol fractionWater depth (m)
Bulk H/(C+H+O+N) – atoms
Bulk O/(C+H+O+N) – atoms
Bulk C/(C+H+O+N) – atoms
Fig. 14.1
Species distribution in a C–H–O–N atmosphere calculated by Gibbs free energy minimization at 25
◦
C. The top
diagrams show mol fractions in the gas phase (solid curves, port axes) and oxygen fugacity (dashed curves, starboard
axes). Bottom diagrams show thickness of the liquid water column averaged over the planet’s surface, and
atmospheric pressure at the planet’s surface. Bulk content of H, O or C is varied in each plot, while keeping bulk
contents of all other components constant. Note the sharp transition between H
2
–NH
3
atmospheres and CH
4
–CO
2
atmospheres.
ways analogous to a phase transition (Chapter 7), in the sense that there are two possible
“phases”: a CO
2
-bearing atmosphere with very little ammonia and molecular hydrogen, or
aCO
2
-free atmosphere dominated by methane, ammonia and molecular hydrogen. Atmo-
spheres in which CO
2
,NH
3
and H
2
are all significantly abundant are not thermodynamically
stable. Over a very narrow compositional interval spanning the transition the equilibrium
species distribution consists chiefly of CH
4
+H
2
O ±N
2
.Atmospheres with this composition
are unlikely to be common, however, as they require very fine-tuned conditions (Fig. 14.1).
The CO
2
/CH
4
ratio increases with the oxidation state. CO
2
becomes the dominant atmo-
spheric species for bulk compositions sufficiently rich in O or poor in H. Oxygen fugacity,
however, remains < ∼10
−70
bar even in atmospheres with ∼90 mol% CO
2
. This oxygen
fugacity is below the stability limit of hematite (e.g. Fig. 11.7 and 11.8). Thus, Fe
2+
-rich
Archaean oceans are consistent with a CO
2
-dominated atmosphere (Section 11.5). Increas-
ing oxidation of a CH
4
–CO
2
atmosphere always raises atmospheric pressure, as the light
carbon species, CH
4
, is replaced by the much heavier CO
2
. The thickness of the water col-
umn, however, responds differently depending on whether oxidation is driven by hydrogen
loss, which produces CO
2
at the expense of liquid water and CH
4
, or by an increase in bulk
650 Thermodynamics of life
oxygen content, which produces liquid water and CO
2
at the expense of CH
4
. Note that the
models in Fig. 14.1 yield relatively thin water columns, less than ∼10 m, which are orders
of magnitude thinner than the terrestrial oceans. This is a function of the total volatile mass
that I arbitrarily chose as input for the calculations. The thickness of the water column can
be varied without changing atmospheric pressure nor composition by adding H and O to
the bulk composition in a proportion of 2 to 1 (Exercise 14.1).
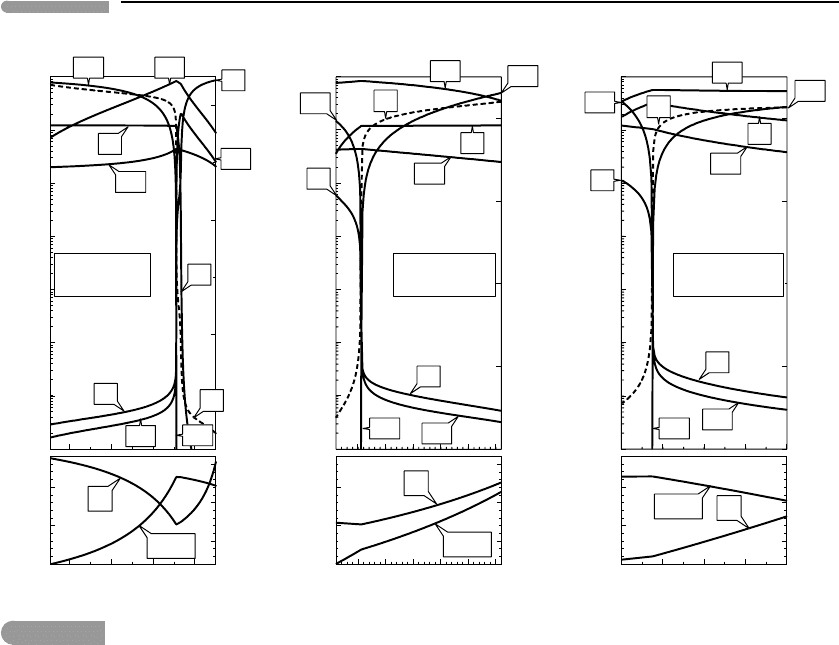
These trends are largely unaffected by changes in bulk nitrogen content. The left panel
in Fig. 14.2 shows the effect of varying bulk N content while keeping bulk H, O and C
contents fixed at the starting values chosen above. Nitrogen-bearing species become more
abundant with increasing N content, and atmospheric pressure increases, but the relative
proportions of C–H–O species, oxygen fugacity and the depth of the liquid water column
remain constant. An ammonia-dominated atmosphere forms if the bulk composition is
sufficiently rich in both nitrogen and hydrogen. This is shown in the right panel of Fig. 14.2,
in which gas speciation is tracked as a function of variable bulk H content, for a bulk
N content ten times greater than in Fig. 14.1 (atomic ratio N/(C +O) = 1, compared to
0.2 0.4 0.6
0
2
4
6
0.5
1
1.5
P
Water
NH
3
H
2
H
2
O
N
2
f
O
2
NH
3
H
2
CO
2
N/(C+O) =1
C/O=0.54
H/(C+O) =1.65
C/O=0.54
10
–6
10
–5
10
–4
10
–3
10
–2
10
–1
1
CH
4
NH
3
H
2
3
2.5
2
1.5
0.1 0.2
Bulk N/(C+H+O+N) – atoms Bulk H/(C+H+O+N) – atoms
0.3
1
Water
P
N
2
CO
2
H
2
O
f
O
2
10
–7
10
5
0
Species mol fraction
10
–6
10
–5
10
–4
10
–3
10
–2
10
–1
1
10
–7
Species mol fraction
Pressure (bar)
Pressure (bar)
–72
–72
–74
–76
–78
–71
log f(O
2
)
log f(O
2
)
CO
2
CH
4
Water depth (m)
Water depth (m)
Fig. 14.2
Same as Fig. 14.1, focusing on the effects of nitrogen content. Formation of an ammonia–methane atmosphere
requires a bulk composition very rich in N and H (right panel).
651 14.1 Chemical evolution of post-nebular atmospheres
10
–6
10
–5
10
–4
10
–3
10
–2
10
–1
1
10
–7
10
–6
10
–5
10
–4
10
–3
10
–2
10
–1
1
10
–7
Species mol fraction
Species mol fraction
0 5*104 105 1.5*105
0
10
20
30
40
50
Total volatile mass – kgm
–2
Water depth (m)
2
4
6
8
10
Pressure (bar)
–72
–71
log f(O
2
)
P
Water
NH3
H
2
H
2
O
N
2
CO
2
CH
4
f
O
2
246810
0
2
4
6
8
Gravitational acceleration – ms
–2
Water depth (m)
0
0.5
1
Pressure (bar)
–72
–71
log f(O
2
)
P
Water
NH
3
H
2
H
2
O
N
2
CO
2
CH
4
f
O
2
Fig. 14.3
Effects of total volatile mass and gravitational acceleration on atmospheric speciation. Atmospheric pressure varies
directly and almost linearly with both variables. Decrease in atmospheric pressure is accompanied by evaporation of
liquid water, and increase in X(H
2
O) in the gas phase. The effect of gravitational acceleration is strongly non-linear for
small planets, accounting at least in part for Mars’s predicament.
N/(C +O) =0.1 in the equivalent plot in Fig. 14.1). In this case too there is a rapid transition
from a strongly reducing NH
3
–CH
4
atmosphere to a N
2
–CO
2
atmosphere, in response to
hydrogen loss.
The effects of changing total volatile mass and gravitational acceleration are shown in
Fig. 14.3. Increasing both of these variables raises atmospheric pressure almost linearly
but has negligible effects on atmospheric composition, except at very low pressure, or for
H
2
O vapor content, at all pressures. The effect on X(H
2
O) is a consequence of saturation in
liquid water: the chemical potential, and hence partial pressure, of H
2
O vapor is fixed, so
its concentration must decrease as pressure increases. Decreasing pressure is accompanied
by an increase in the concentration of molecular hydrogen because the methane oxidation
reactions:
CH
4
+H
2
O CO +3H
2

652 Thermodynamics of life
and
CH
4
+2H
2
O CO
2
+4H
2
both have large and positive
r
V , so they are favored by low pressure. As the concentra-
tion of H
2
increases with decreasing pressure so does the ammonia concentration. Note in
passing that although all the calculations are performed at constant pressure, assumed to be
the pressure at the planet’s surface given by the atmospheric mass, the results in Fig. 14.3
can also be used to infer that the equilibrium species distribution does not change signifi-
cantly with elevation. The emphasis is on equilibrium because, as we saw in Chapter 12,
photoactivated processes affect non-equilibrium species distribution with elevation.
The depth of the water column varies linearly with total volatile mass, but it responds in a
non-linear fashion to changes in gravitational acceleration at constant volatile mass. In the
latter case evaporation of water (required to preserve the chemical potential of H
2
Ointhe
atmosphere) depletes a liquid reservoir of definite size. Of course gravitational acceleration
does not change with time once a planet has accreted. Rather, this result should be seen as
a demonstration of the difficulty that a small planet such as Mars may have had in holding
on to its oceans and lakes.
The concentrations of minor species can be calculated a posteriori, because they have a
negligible effect on the Gibbs free energy of the system and on the mass balance constraints
(equations (14.3)). For example, we can calculate the concentrations of hydrogen cyanide
and formaldehyde from the equilibria:
NH
3
+CH
4
HCN +3H
2
(i)
and:
H
2
O +CH
4
CH
2
O +2H
2
(ii)
for which we have:
X
HCN
=
X
NH
3
·X
CH
4
X
H
2
3
·P
2
exp
−
r
G
0,(i)
RT
X
CH
2
O
=
X
H
2
O
·X
CH
4
X
H
2
2
·P
exp
−
r
G
0,(ii)
RT
.
(14.8)
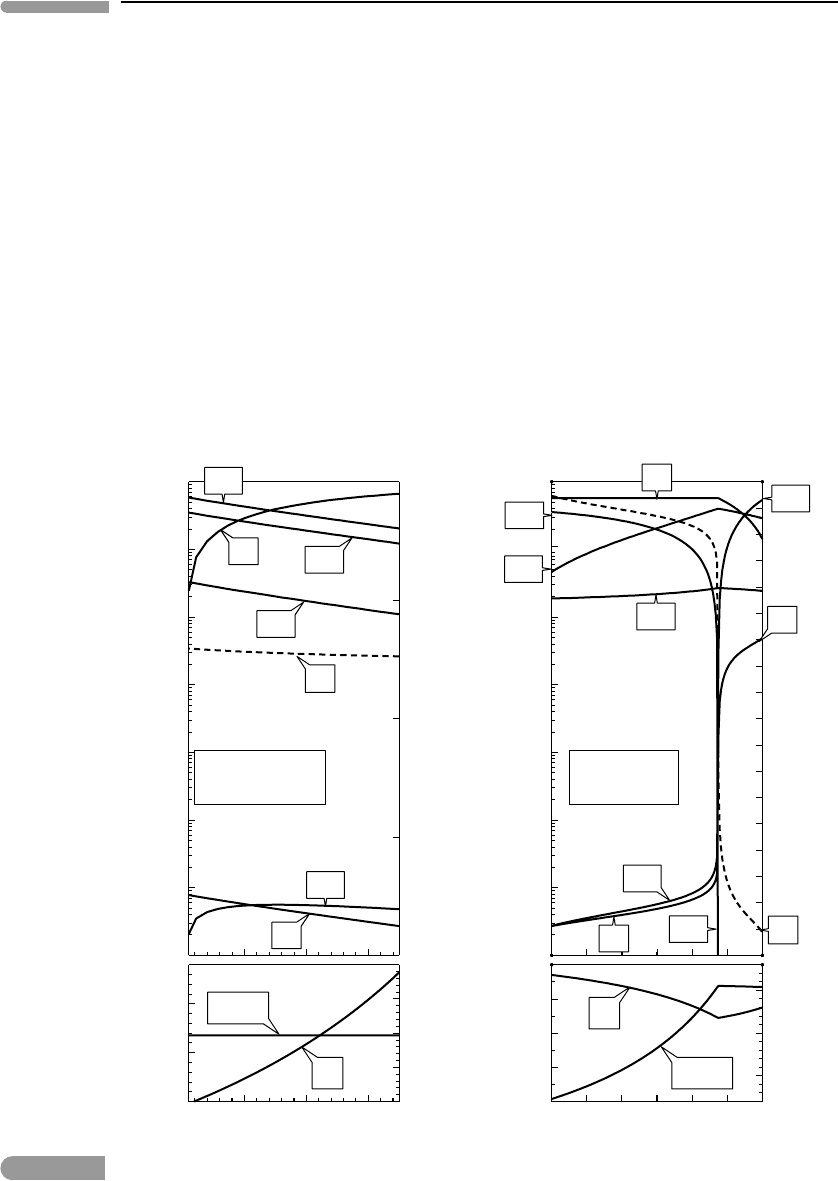
Representative results are shown in Fig. 14.4 (left panel), together with CO concentrations,
which were calculated as part of the Gibbs free energy minimization procedure, but could
also have been calculated as in equations (14.8). Formaldehyde and other organic molecules
in which carbon is present in the same oxidation state (CH
2
O) are essential biological
building blocks (Section 14.2.1). These results suggest that their equilibrium atmospheric
concentrations are unlikely to ever have been significant, even in reducing atmospheres.
The synthesis of these molecules must have been the outcome of other processes that took
place at the inception of life on Earth.
For simplicity sulfur is not included in the model calculations, because it is likely to have
always been less abundant than C, H, O and N. Even if we ignore the absolute abundances of
sulfur species, it is important to constrain their oxidation state, partly for biological reasons.

653 14.1 Chemical evolution of post-nebular atmospheres
10
–10
10
–9
10
–8
10
–7
10
–6
10
–5
Species mol fraction
–84
–80
–76
–72
log f(O
2
)
NH
3
H
2
CO
HCN
0.2 0.4 0.6 0.8
10
–25
10
–24
10
–23
10
–22
10
–21
Species mol fraction
–84
–80
–76
–72
log f(O
2
)
f
O
2
f
O
2
CO
CH
2
O
10
–6
10
–5
10
–4
10
–3
10
–2
10
–1
1
10
1
X(CO
2
) / X (CH
4
)
–84
–80
–76
–72
log f(O
2
)
0.2 0.4 0.6 0.8
10
–40
10
–35
10
–30
10
–25
10
–20
10
–15
X(SO
2
) / X(H
2
S)
–84
–80
–76
–72
Bulk H/(C+H+O+N)–atoms Bulk H/(C+H+O+N)–atoms
log f(O
2
)
Fig. 14.4
Left: atmospheric concentrations of the minor species CO, CH
2
O and HCN (note scale break between both diagrams).
Ammonia and molecular hydrogen shown for comparison. Right: SO
2
/H
2
S concentration ratio, compared to CO
2
/CH
4
ratio. Sulfur is present as a reduced species even in atmospheres that have ten times more CO
2
than CH
4
.
We do this by means of the equilibrium:
H
2
S +O
2
SO
2
+H
2
(iii)
which yields:
X
SO
2
X
H
2
S
=
f
O
2
X
H
2
·P
exp
−
r
G
0,(iii)
RT
. (14.9)
Representative results are shown in the right panel of Fig. 14.4, where they are compared to
the CO
2
/CH
4
ratios for the same range of atmospheric compositions. An important conclu-
sion is that carbon oxidizes more readily than sulfur, so that a CO
2
-dominated atmosphere
can still be reducing enough to contain H
2
S and virtually no SO
2
. We will return to this
later in this chapter.
Photodissociation of CH
4
,NH
3
and H
2
O molecules produces free hydrogen which, as we
saw in Chapter 13, can be lost by thermal escape. This is an irreversible process, for the same
relationship between kinetic energy and gravitational binding energy (equation (13.1)) that
makes hydrogen loss possible makes it impossible for a planet to capture hydrogen atoms.

654 Thermodynamics of life
If surface conditions are such that the atmosphere contains significant H
2
O vapor, then
hydrogen loss leads inevitably to a dry planet with an atmosphere dominated by carbon
dioxide and nitrogen, regardless of how reduced the initial atmospheric composition might
have been (Fig. 14.1 left panel, and Fig. 14.2 right panel). In the absence of other processes a
CO
2
-rich atmosphere is a terminal state, as carbon–oxygen gas species are not readily lost
by atmospheric escape processes. Protracted hydrogen loss is the most likely explanation for
the nature of the present-day atmospheres of Venus and Mars. Neither the Earth nor Titan fit
this picture, however. Titan’s atmosphere consists chiefly of nitrogen, with minor amounts
of CH
4
,H
2
and other reduced carbon species. Thus, it must be located on the reduced side of
the speciation transition (Fig. 14.1 and 14.2). Yet according to equation (13.1) and Fig. 13.1
hydrogen loss from Titan’s atmosphere must be at least as efficient as in Venus and Mars.
Oxidation of Titan’s atmosphere is prevented by its very low surface temperature, which
keeps the partial pressure of H
2
O(∼ the saturation vapor pressure over ice at very low
temperature) virtually equal to 0. The example of Titan emphasizes the importance of H
2
O
vapor as the oxygen source for atmospheric oxidation. It is the only abundant molecule that
contains both H and O, so that photodissociation followed by hydrogen loss makes oxygen
available. If there is no H
2
O in the atmosphere, for instance because it is sequestered in
low-temperature ice, then there is simply no source of oxygen.
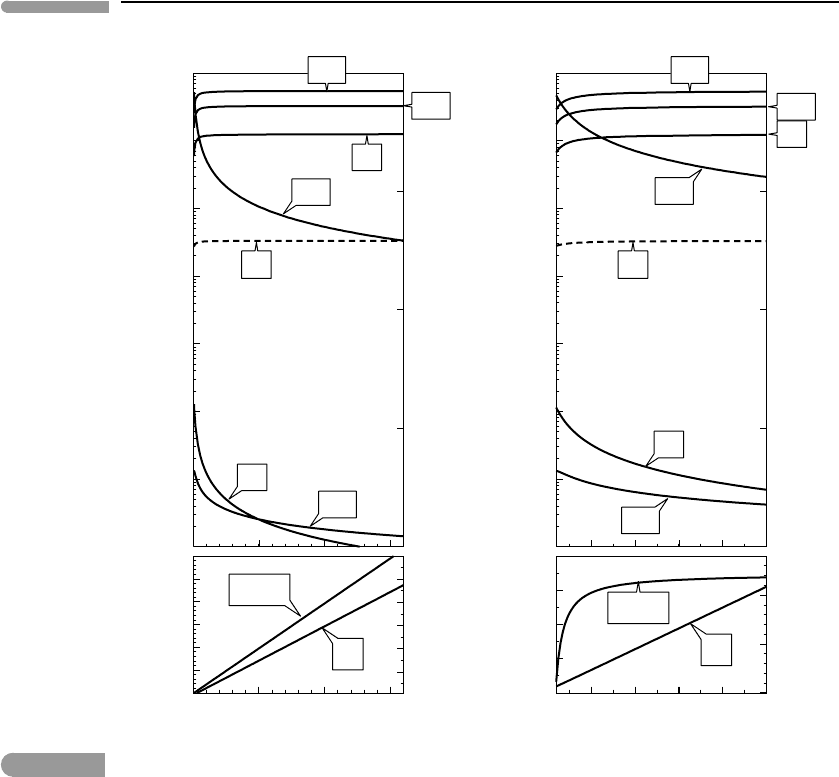
The Gibbs free energy minimization model also allows us to examine the effect of tem-
perature on the equilibrium species distribution. Representative results are shown in Fig.
14.5, in which I have adjusted the bulk composition so that there are ∼100 m of liquid water
in equilibrium with a CO
2
-rich atmosphere at 25
◦
C and ∼1.5 bar pressure. As tempera-
ture increases water must evaporate in order to preserve the equilibrium saturation vapor
pressure. This raises the concentration of H
2
O vapor in the atmosphere, and also atmo-
spheric mass and hence atmospheric pressure. At temperatures approaching 100
◦
C, H
2
O
becomes the dominant atmospheric component, even if water may be kept from boiling
by the high atmospheric pressure. This condition, known as a steam atmosphere, greatly
accelerates the rate of hydrogen loss because it increases both the concentration of H
2
O
in the atmosphere, and hence the rate of photodissociation (equation (12.83)), and its tem-
perature (equation (13.1)). Temperature increase also raises the equilibrium H
2
and NH
3
concentrations by several orders of magnitude.
Buildup of a steam atmosphere is thought to be a self-reinforcing process, by virtue of
the strong infrared absorption of H
2
O molecules (Section 13.3.6). It is possible that once
the concentration of H
2
O vapor exceeds certain threshold it causes a runaway temperature
increase that results in complete dessication of the planet’s surface and CO
2
accumulation
in the atmosphere. As long as there is liquid water CO
2
may be scavenged by carbonate
precipitation at a rate that is largely controlled by the rate at which silicate weathering
supplies cations such as Ca
2+
,Mg
2+
and Fe
2+
in aqueous solution. This process is known
as the Urey reaction (Urey, 1952). Once liquid water disappears this scrubbing mechanism is
no longer possible, and CO
2
atmospheric concentration cannot decrease. Hydrogen escape
rates are likely to have always been lower in Earth than in Venus and Mars (e.g. Fig. 13.1).
Whether or not Venus ever had a steam atmosphere, it may have lost its water early on,
and with it the capability of controlling its atmospheric CO
2
concentration. Mars probably
underwent slower dessication and oxidation, and eventual freezing of its remaining surface
water. Hydrogen loss from Earth has been slow enough to allow much of its surface water
to persist over the age of the solar system.
If atmospheric methane concentration decreases to trace levels then the atmosphere loses
its ability to buffer oxygen fugacity. As long as there is water vapor available that can
655 14.1 Chemical evolution of post-nebular atmospheres
10
–7
10
–6
10
–5
10
–4
10
–3
10
–2
10
–1
1
Species mol fraction
0 20 40 60 80 100 120
160
170
180
Temperature - ºC
Water depth (m)
1.5
2
2.5
3
Pressure (bar)
–80
–75
–70
–65
–60
–55
log f(O
2
)
P
Water
NH
3
H
2
H
2
O
N
2
CH
4
f
O
2
CO
2
Fig. 14.5
Response of atmospheric speciation to temperature changes. The bulk composition was adjusted so as to generate
∼180 m of liquid water in equilibrium with a CO
2
-dominated atmosphere at 25
◦
C. Increasing temperature raises the
mol fraction of H
2
O vapor in the atmosphere and atmospheric pressure. For this bulk composition H
2
O becomes the
dominant atmospheric component at ∼100
◦
C, giving rise to a steam atmosphere.
undergo photodissociation oxygen concentration could in principle increase freely, but this
is generally not the case. Under equilibrium conditions atmospheric oxidation is limited
by the availability of reduced species at the planet’s surface, chiefly ferrous iron, sulfides
and reduced carbon. Figure 11.8 shows that oxidation of iron to its terminal ferric state
buffers equilibrium oxygen fugacity to log(f O
2
) ∼−68.6 (at 25
◦
C). Sulfide oxidation can
be modeled by means of the following reaction, that produces anhydrite and hematite at the
expense of pyrite and calcite:
4FeS
2
+8CaCO
3
+15O
2
8CaSO
4
+2Fe
2
O
3
+8CO
2
,

656 Thermodynamics of life
–6 –4 –20
2
–100
–90
–80
–70
–60
log f(O
2
)
log f(CO
2
)
pyrite + calcite
hematite + anhydrite
hematite
magnetite
CH
2
O + O
2
CO
2
+ H
2
O
T = 25º C
Fig. 14.6
Equilibrium oxygen fugacities buffered by organic matter, ferrous–ferric equilibrium, and sulfide–sulfate equilibrium.
and that is at equilibrium for: log(f O
2
) = (8/15) log(f CO
2
) −64.7. These two oxidation
boundaries are shown in Fig. 14.6. If a planet has internal activity and is able to resupply its
surface with reduced species such as pyrite, olivine, pyroxene and other iron silicates via
volcanism and tectonism then its equilibrium atmospheric oxygen fugacity cannot exceed
these boundaries. Venus is still active today, so that the oxygen liberated by photodissoci-
ation of H
2
O and hydrogen escape in the geologic past must be present in the lithosphere,
combined with iron, sulfur and other elements of variable oxidation state. There is no
H
2
O left to supply oxygen, and there is almost certainly an excess of reduced species of
internal origin. The atmosphere of Venus is a thermodynamic dead end. The case of Mars
is somewhat different. First, it still has frozen H
2
O on its surface, and perhaps liquid or
frozen H
2
O in its shallow subsurface. Second, Mars may have largely lost its capability to
transport reduced species from the planet’s interior to its surface, and the surface is already
thoroughly oxidized. The possibility therefore exists that oxygen fugacity in the Martian
atmosphere exceeds the iron and sulfur oxidation boundaries (Fig. 14.6), as attested by the
existence of perchlorates on its surface (Chapter 11).
The Earth is of course different, because its atmosphere is very far from thermodynamic
equilibrium with its surface, and in particular with the biosphere. Carbon in organic mat-
ter typically has the same oxidation state as in formaldehyde, CH
2
O, so that equilibrium
between organic matter and atmospheric oxygen can be modeled by the reaction:
CH
2
O +O
2
CO
2
+H
2
O.
which results in: log(f O
2
) = log(f CO
2
) −92.67. This reaction, also shown in Fig. 14.6,
is located more than 90 orders of magnitude (!) below the actual oxygen fugacity in the
terrestrial atmosphere. In a planet covered with organic matter it would not be even possible
for ferrous iron or sulfide to oxidize, if the atmosphere was in thermodynamic equilibrium
with the biosphere. Upkeep of the oxygen content of the terrestrial atmosphere is a non-
equilibrium process driven by supply of solar energy.

657 14.2 Thermodynamics of metabolic processes
14.2 Thermodynamics of metabolic processes
14.2.1 Carbon fixing and respiration of reduced organic carbon
Chemical interactions between living organisms and their substrate are of two principal
types. One of these consists of reactions that reduce oxidized carbon absorbed from the
environment, typically CO
2
, in which C has an oxidation state of +4. The products of these
reactions are organic molecules in which the oxidation state of C is characteristically 0.
The simplest such compound is formaldehyde: CH
2
O. Reactions of this type are called
carbon-fixing reactions. The reduced carbon species generated by these reactions fulfill
two distinct biological roles. Firstly, they are used by living matter to synthesize all of the
complex organic molecules needed for life – we will not discuss this role any further but
we will keep in mind that this process, which is the essence of anabolic metabolism, is the
reason why carbon fixation is essential for life as we know it. Secondly, reduced carbon
species can serve as reactants for metabolic reactions that transfer energy from the substrate
to the living organism (i.e. catabolic metabolism). These reactions are called respiration
reactions and are the other type of chemical interactions that take place between living
organisms and their substrate. Some respiration reactions use reduced carbon as a reactant.
This is most commonly organic carbon that was fixed by reactions of the first type, although
reduced carbon of inorganic origin (e.g. CH
4
in volcanic gases) can also be used. However,
many respiration reactions exist in which no carbon species are involved (more on this in
the next section).
Because their role is to transfer energy from the substrate to energy-carrier molecules
within living cells, a necessary condition of respiration reactions is that they must be thermo-
dynamically spontaneous. Any combination of chemical species with
r
G<0 (or E > 0)
could, in principle, constitute the basis for respiration. In contrast, with one important excep-
tion that we will discuss in detail below, carbon-fixing reactions have
r
G>0. They must
therefore be sustained by a constant energy flux from the environment. Perhaps the most
familiar carbon-fixing reaction is oxygenic photosynthesis, which we can model as follows:
CO
2(g)
+H
2
O
(l)
+hν →CH
2
O
(g)
+O
2(g)
, (14.10)
where hν represents electromagnetic radiation in the visible part of the spectrum and
CH
2
O
(g)
is formaldehyde gas. Actual photosynthesis produces more complex molecules
with the same oxidation state as formaldehyde, such as glucose (C
6
H
12
O
6
), but the
thermodynamic relations are qualitatively the same as those that can be derived from
equation (14.10). The standard state Gibbs free energy change for this reaction is
528.96 kJ per mol of formaldehyde. At equilibrium we thus have:
f
CH
2
O
·f
O
2
f
CO
2
≈10
−93
(14.11)
which, as we saw in the previous section, means that the terrestrial atmosphere (f
O
2
≈
0.2 bar) is very far from being in thermodynamic equilibrium with reduced organic matter.
Reaction (14.10) is a photochemical reaction that fixes carbon and liberates molecular
oxygen against a huge chemical potential gradient by converting an electromagnetic energy
flux to chemical bonding energy (see also Worked Example 12.3). Photosynthesis based
on other elements that can change oxidation state also exists. Two important examples are
