Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


608 Non-equilibrium thermodynamics
10
–14
10
–12
10
–10
[X] / [O
3
]
0
[O
3
] / [O
3
]
0
10
–8
10
–6
10
–
4
0
0.2
0.4
0.6
0.8
1
K
5
=10
–8
K
5
=10
–10
K
5
=10
–12
K
5
=10
–14
Fig. 12.5 Atmospheric ozone depletion caused by a catalytic cycle initiated by an active species X (X could be, for instance, OH,
NO or Cl). Numbers in boxes are values of the rate constant k
5
, for the reaction: X +O
3
→XO +O
2
,incm
3
molecule
−1
s
−1
.[O
3
]
0
is the concentration of ozone in the absence of species X. Note that minuscule concentrations
of the active species X can wipe-out atmospheric ozone, and that there is a threshold value of [X] above which ozone
destruction increases rapidly.
cycles are in reality considerably more complex that the examples that we considered here
but the behavior depicted in Fig. 12.5 is qualitatively correct.
It is important to point out that the laws of thermodynamics are not “violated” by the
fact that ozone concentration in the atmosphere is not at equilibrium with that of oxygen.
This non-equilibrium ozone concentration is kept at a (more or less) constant level by a
continuous supply of energy, in the form of solar photons that split oxygen atoms in the first
reaction of the Chapman cycle (equation (12.114)). Production of ozone, and its persistence
in a concentration far greater than the equilibrium concentration, are dynamic processes that
depend on the continuous operation of this photochemical reaction. If ultraviolet radiation
were to be turned off permanently then ozone, and atomic oxygen, would convert to the
stable species O
2
, at rates dependent on the specific reaction mechanism.
The two first reactions of the Chapman cycle can be combined to yield reaction (12.113).
The enthalpy change for this reaction is 285.4 kJ. We can calculate the total energy of the
photons consumed by this reaction with the formula:
U
ν
=
hcA
λ
, (12.134)
where h is Planck’s constant, c is the speed of light, A is Avogadro’s number, and λ is the
wavelength of the photon. It is found experimentally that photodissociation of O
2
occurs for
photons of λ<240 nm (see also Chapter 13), from which we calculate U
ν
> 498 kJ mol
−1
.
There is more than enough energy to account for the higher enthalpy of ozone relative to
oxygen. Where does the energy excess go? Because of conservation of momentum, the
photons’ momentum (= hν/c) must be transferred to the oxygen atoms, increasing their
translational kinetic energy. This energy influx is then distributed throughout the gas by
molecular collisions, increasing its temperature and therefore its entropy. We return to this
discussion in the next chapter.
609 12.4 Controls on rate constants
12.4 Controls on rate constants
Much of this chapter has focused on dynamic processes, by which we mean that the
thermodynamic state of the system changes with time. Rates are quantified by means of
“constants”, such as chemical diffusivities and reaction rate constants. But what determines
the values of these “constants”? We shall briefly discuss two important classes of processes:
thermally activated processes and photoactivated processes.
12.4.1 Thermally activated processes. Arrhenius law
The rates of many natural processes, such as chemical diffusion, viscous flow (which we
discussed in Chapter 3 and which, as we saw there, corresponds to diffusion of momen-
tum) and thermally activated chemical reactions, vary exponentially with temperature
according to:
Ψ =A exp
−
E
a
RT
, (12.135)
where A and E
a
are positive constants and Ψ is a parameter that describes the charac-
teristic rate of the process. For example, Ψ can be the chemical diffusion coefficient D
(Section 12.2.2), the rate constant of a chemical reaction, k (Section 12.3.1), or the viscous
flow shear rate du/dx (Section 3.7.4). In the latter case the parameter Ψ is the rate of
shearing, which at constant shear stress is the inverse of the viscosity µ (equation (3.7)).
Because viscosity, rather than its inverse, is the parameter that is used to measure the rate of
momentum transfer the negative sign does not appear in the exponential factor of equation
(3.3.1). Otherwise that equation is identical to (12.135).
Equation (12.135) is known as Arrhenius law, after the Swedish physical chemist Svante
Arrhenius, who discovered it experimentally. If we think of Arrhenius law as an empirical
macroscopic relationship then it is not necessary to attach any physical meaning to the
constants A and E
a
. We can nonetheless note that E
a
has units of energy, and it is a
quantity known as the activation energy for the process in question. The units of A, known
as the pre-exponential factor, vary depending on what the parameter Ψ represents, but
they always include the factor [T ]
−1
. From a purely macroscopic point of view we can
think of A as the limiting value of Ψ at very high temperature. We can, however, attach
more specific physical meanings to the two constants, which relate to the microscopic
nature of the processes in question. In this view A is a quantity that is proportional to
the rate at which microscopic events take place. The exponential factor exp(−E
a
/RT )
is the probability that a given event will produce a specific outcome. For example, if Ψ
corresponds to the rate constant of a chemical reaction then the events would be molecular
collisions, and theexponential factor woulddescribe the probability that a given collision has
enough energy to break atomic bonds and result in chemical recombination. If Ψ describes
diffusion of matter or momentum then the events would be excursions of atoms away from
their equilibrium positions and the exponential factor would be the probability that a given
excursion is energetic enough to cause an atom to jump from its initial equilibrium position
to a neighboring one. In every case the probability tends to zero as temperature goes to zero.
Then no events have enough energy to accomplish the desired result, and the macroscopic
process stops. The probability tends to one at high temperature, so that all events accomplish
610 Non-equilibrium thermodynamics
the desired result and the rate of the macroscopic process approaches some maximum, but
finite, value.
Arrhenius law is a reasonably good first approximation to the behavior of thermally acti-
vated processes. Chemical diffusivities and reaction rate constants are commonly tabulated
in the form of values of the A and E
a
parameters in equation (12.135). An example is the
large database of reaction rate constants, from which the values used in Worked Exam-
ple 12.3 were obtained, maintained on-line by the U.S. National Institute of Science and
Technology (NIST Chemical Kinetics Database). A compilation of chemical diffusivity
parameters of geological interest (used in Worked Example 12.1) can be found in Zhang
(2008).
Equation (12.135) is not a complete physical picture, however. For example, it is found
that the pre-exponential factor is generally a function of temperature, and pressure depen-
dencies of the pre-exponential factor and the activation energy also exist. These macroscopic
effects point to a microscopic mechanism that is more complex than what I describe here.
Rigorous discussions of these topics can be found, for example, in the books by Logan
(1996); Houston (2006); Borg and Dienes (1988), and with specific focus on geological
processes, Poirier (1985) and Zhang (2008).
12.4.2 Photochemical processes
Thermal energy is incapable of initiating a chemical reaction if the resulting kinetic energy
of the molecules is not sufficient to break interatomic bonds. Even at low temperature, how-
ever, individual molecules may acquire excess energy and become reactive by absorbing
photons of specific wavelengths (see also Chapter 13). An example of this is the initiation
step of the Chapman cycle. At the temperature of the stratosphere, and even at temperatures
considerably higher than this, O
2
molecules do not have sufficient kinetic energy to disso-
ciate as a result of collisions, but a molecule of O
2
, regardless of its temperature, dissociates
when it absorbs an ultraviolet photon. This is not a thermal process, which means that it
does not rely on a statistical distribution of molecular speeds and on the probability that the
speeds of some molecules will exceed some characteristic threshold. The rate constants for
photochemical reactions are therefore not described by equation (12.135). Partly for this
reason it is customary to use a different symbol for the rate constants (j instead of k), even
if the rate laws are the same regardless of the nature of the activation process.
Photochemical reactions are activated by radiation within a specific range of wave-
lengths. For example, photodissociation of O
2
(the first reaction in the Chapman cycle)
requires ultraviolet photons with wavelengths shorter than 240 nm. There are reactions,
called radiolytic reactions, that require higher energy photons, in the X-ray and gamma
part of the spectrum. We can expect that the rate constant will vary directly with the inten-
sity of the radiation of the required wavelength (number of photons per unit of area per
unit of time, i.e., photon flux) and with the absorption cross section, which we can think
of as the effective target area offered by the molecules to the photon flux (more on this in
Chapter 13). We write this relation as follows:
j =
I (λ)σ (λ)dλ, (12.136)
where I (λ) is the photon flux at wavelength λ, σ (λ) is the absorption cross section, which
also varies with wavelength, and the integral is over the range of wavelengths within which
photoactivation takes place.

611 12.5 An introduction to kinetics
This equation is appropriate, for example, to photoactivation of a chemical reaction
in a laboratory environment. Photochemical reactions in planetary atmospheres are more
complicated, because the photon flux varies with elevation. It is maximum, and equal to the
incident solar flux, at the top of the atmosphere, and then decreases as photons are absorbed
by atmospheric gases. If, for the sake of simplicity, we consider only a hypothetical situation
in which solar radiation is always perpendicular to the planet’s surface (e.g. noon at the
equator on the day of the equinox), then we have, by Beer–Lambert’s law of radiation
(Section 13.2.4):
I (λ) =I
0
(λ)e
−τ
λ
, (12.137)
where I
0
(λ) is the photon flux at the top of the atmosphere and τ
λ
is a non-dimensional
absorption length, known as the optical thickness (Section 13.2.4), and given by (see also
equation (13.55)):
τ
λ
=
i
σ
i
(λ)
N
i
(z)dz, (12.138)
where σ
i
is the absorption cross section of chemical species i, N
i
is the number density
of species i (molecules per unit volume), the integral is from the top of the atmosphere to
the elevation of interest, and the summation is over all atmospheric species that absorb at
wavelength λ. If the incident solar radiation is not perpendicular to the planet’s surface then
a geometric correction must be applied to Beer–Lambert’s law.
Substitution of (12.138)in(12.137), and then in (12.136), yields the value of the rate
constant as a function of elevation:
j
a
(z) =
I
0
(λ)e
−
i
σ
i
(λ)
N
i
(z)dz
σ
a
(λ)dλ, (12.139)
where σ
a
is the absorption cross section of the photoactivated molecule of interest. As we
should expect, this equation states that the rate constant decreases with increasing optical
thickness, i.e. as the planet’s surface is approached. Photochemistry is therefore a more
important process in the stratosphere than in the troposphere, for the simple reason that
there is a higher photon flux. The rate constants will obviously also vary with time of
day and season, as the angle of the incident solar radiation and hence the photon flux will
vary. Rate constants for atmospheric photochemistry vary over many orders of magnitude
across a planetary atmosphere. The values of j
1
and j
3
used in Worked Example 12.3
are approximate average values for the terrestrial stratosphere at an elevation of ∼30 km.
Since the rate of production and destruction of ozone is critically dependent on these rate
constants, it follows that the numerical results obtained there change with elevation.
12.5 An introduction to kinetics of heterogeneous processes
The kinetic behavior of homogeneous processes, such as chemical reaction in a homoge-
neous phase or diffusion in a single continuous phase, can be set-up mathematically in
a relatively straightforward and unambiguous fashion – which of course does not mean
that the resulting equations are necessarily easy to solve. The mathematical description
of the kinetics of heterogeneous processes is less straightforward. It commonly involves

612 Non-equilibrium thermodynamics
sets of coupled differential equations that describe rates of chemical reaction, heat transfer
and mass transfer. Simplifying assumptions are sometimes possible, though. Consider the
following three simple examples.
(i) Crystallization of enstatite from a basaltic melt, which we can schematize as follows:
MgO
melt
+SiO
2 melt
MgSiO
3 crystal
.
(ii) Devolatilization of the assemblage magnesite + quartz:
MgCO
3 crystal
+SiO
2 crystal
MgSiO
3 crystal
+CO
2 vapor
.
(iii) Weathering of enstatite at the Earth’s suface:
MgSiO
3 crystal
+3H
2
O Mg
2+
aq
+H
4
SiO
4aq
+2OH
−
.
One way of analyzing the kinetic behavior of heterogeneous reactions such as these is to
assume that the locus of the chemical reaction is a homogeneous region of infinitesimal
extent, which we may call the reaction volume or reaction interface (Zhang, 2008). The
rate of the chemical reaction is then a function of its rate constant and of the affinity of the
reaction inside the infinitesimal reaction volume (equation (12.74)). The rate constants of all
three of these reactions are functions of temperature (equation (12.135)), and the affinity,
which is a linear combination of chemical potentials, varies with temperature, pressure
and composition. As the reactions proceed enthalpy is liberated or absorbed, and chemical
species are consumed and produced. The temperature and affinity in the reaction volume are
therefore determined by the relative rates of chemical reaction and heat and mass exchanges
between the reaction volume and its environment.
Crystallization of enstatite from a melt (reaction i) liberates enthalpy (
r
H<0) and
consumes MgO and SiO
2
melt components. If the reaction volume were a closed system
then its temperature would increase and the concentrations of the reactants would decrease.
From the definition of affinity, equation (12.70), we can see that:
∂E
∂T
=
r
S =
r
H
T
(12.140)
and:
∂E
∂X
i
=
ν
i
RT
X
i
> 0, i : reactant. (12.141)
In a closed reaction volume the affinity would decrease and hence the reaction would slow
down, and eventually stop. There would also be an effect on the rate constant that would act
in the opposite direction, but this is likely to be minor at magmatic temperatures, at which
chemical reaction rates are always fast. In reality, however, crystallization sets up gradients
in temperature and chemical potential between the reaction volume and the surrounding
melt, so that heat is carried away from the reaction volume and chemical components are
transported towards it. If we assume that diffusion is the transport mechanism for both
heat and chemical components then we can conclude that mass transfer is the rate-limiting
process, as heat diffusivity at magmatic temperatures is ∼6 orders of magnitude greater
than chemical diffusivity (see Worked Examples 12.1 and 12.2). As a first approximation,
then, the kinetics of reaction (i) could be treated as a chemical diffusion problem.

613 12.5 An introduction to kinetics
Consider now reaction (ii). This reaction is endothermic (
r
H>0) so that, if it took
place in a closed system, the temperature would decrease and the fugacity of CO
2
would
increase. If the gas phase is a product then differentiation of (12.70) yields:
∂E
∂f
j
=
ν
j
RT
f
j
< 0, j : product (12.142)
so that it follows from (12.140) and (12.142) that the affinity in a closed reaction volume
would go down. Let us assume for the sake of argument that the decarbonation reaction (ii)
occurs in a permeable setting, in which,for example, a network of cracks allows the gas phase
to escape as soon as it forms. If, as in the previous case, the temperature is high enough that
the reaction rate is much faster than heat diffusion, then we can consider two end-member
situations. If grain size is “infinitesimally fine” and the mixture of minute magnesite and
quartz crystals is perfectly random then we might be able to ignore mass transfer rates and
assume that the rate-limiting process is heat diffusion. For any “geologically reasonable”
grain size, however, it is likely that mass transfer will determine the rate of reaction (ii), but
there is an important qualitative difference with the crystallization reaction (i). In that case
diffusion of Mg and Si cations in the melt phase does not present a conceptual problem, but
in the case of the solid-state reaction (ii) this is not so. How do SiO
2
and CaCO
3
components
migrate to the reaction volume where enstatite and CO
2
are produced? Does this happen
by solid-state diffusion? If so, which are the chemical species that actually diffuse, and
what is the underlying atomic lattice through which they diffuse? Or does mass transfer
take place by dissolution of the reactants in a fluid phase, for example the CO
2
produced
by the reaction itself, and migration of the fluid phase? The rate-determining process may
be difficult to pin down, and even more difficult to quantify.
In the case of the weathering reaction (iii) we may assume that circulation of meteoric
water, i.e. advection, is the mechanism that accounts for both heat and mass transfer. Given
that advection is much faster than diffusion, and that fromArrhenius law (equation (12.135))
we can expect a small value of the rate constant for the chemical reaction at room temper-
ature, we may infer that weathering rates are likely to be controlled by chemical reaction
rates. The chief difficulty in quantifying the process in this case would be in the experimental
determination of the required rate constants.
Worked Example 12.4 Relationships between affinity and progress variable
The attentive reader should have noticed that the statement that I made regarding reactions
(i) and (ii), to the effect that in a closed system the affinity of a reaction decreases as the
reaction proceeds, is always true. In fact, it is an alternative way of stating the Second Law
of Thermodynamics, which we can summarize as follows:
∂E
∂ξ
≤0. (12.143)
The inequality holds true in a closed system – it simply states the fact that in a spontaneous
chemical reaction the Gibbs free energy of the products is lower than that of the reactants.
In order for the equality to be true the rates of heat and mass transfer between the reaction
volume and its environment must exactly match the rates of change of enthalpy and chemical
species concentrations caused by the reaction.Assume for simplicity that a chemical reaction

614 Non-equilibrium thermodynamics
occurs in the linear regime defined by equation (12.80) (i.e. close to equilibrium, see also
Exercise 12.2). We can re-write this equation as follows:
∂ξ
∂t
=k
1
E , (12.144)
where k
1
is a positive constant. To begin with, we note that (12.144) says that a chemical
reaction can only take place beginning from a non-equilibrium condition, as the reaction
rate vanishes for E =0. This means that some degree of overstepping is always required in
order for reactions to occur in nature. For example, melting can only begin at a temperature
higher than the solidus, and crystallization cannot begin until temperature drops below the
liquidus. According to equation (12.144), the amount by which the reaction is overstepped
(i.e. the value of E ) will determine the rate at which melting or crystallization proceeds. We
can now look at different ways in which E may vary with the progress variable ξ .
Assume first that E is a constant. This could happen, for instance, during crystallization
of a well-stirred one-component system (e.g. ice from pure H
2
O), or during crystallization
from a very large reservoir of low-viscosity liquid (e.g. ocean water). Defining the value of
the progress variable at t =0asξ
0
=0 we have:
ξ =k
1
E t, (12.145)
which states that the reaction will proceed indefinitely. The behavior becomes more inter-
esting if we consider the inequality in (12.143). This means that either heat or mass transfer
are not able to keep up with the chemical reaction rate. The exact functional form of the
derivative ∂E /∂ξ may be difficult to determine, but we can choose the simplest possible
function as an example. This is the linear law:
∂E
∂ξ
=−k
2
, (12.146)
where k
2
is another positive constant. This constant must contain information about the
efficiency of the rate controlling process. For instance, if the rate is controlled by chemical
diffusion then k
2
must vary inversely with diffusivity: the lower the diffusivity, the more
the affinity will decrease with reaction progress, and thus the larger the magnitude of k
2
must be. Let the affinity at ξ
0
=0beE
0
. Then:
E = E
0
−k
2
ξ (12.147)
so that, substituting in (12.144) and integrating:
ξ =
E
0
k
2
1 −e
−k
1
k
2
t
. (12.148)
We now note that the reaction goes to completion as t →∞and that, because k
1
and k
2
are
positive constants:
lim
t→∞
ξ =
E
0
k
2
. (12.149)
Say that the process that we are modeling is crystallization from a silicate melt and that
we assume, rather simplistically, that the magnitude of the progress variable ξ represents
615 Exercises for Chapter 12
crystal size. Chemical diffusivity in melts varies inversely with viscosity, so that k
2
varies
directly with viscosity. Equation (12.149) then states that crystals that grow from a viscous
melt (e.g. a water-poor rhyolite) should be smaller than those that grow from a fluid melt
(e.g., a water-saturated pegmatite).Although during magmatic crystallization there are addi-
tional complications that we have ignored, most notably the generation and availability of
nucleation sites, equation (12.149) provides at least a qualitative kinetic explanation for an
observation that we became familiar with during our introductory physical geology course.
Exercises for Chapter 12
12.1 Derive the full set of interdiffusion equations, including the Onsager reciprocal terms,
for a ternary system consisting of two dilute solutes and a solvent (Section 12.2.3).
12.2 Find a linear approximation to (12.74) by writing r
f
= r
f ,eq
+δr
f
, where r
f ,eq
is
the rate of the forward reaction at equilibrium, by lineariting the exponential function
for
E
RT
<< 1. Use your linearized expression to find a relationship between the phe-
nomenological coefficient L and r
f
. Establish a criterion by which a reaction can be
considered to be close to equilibrium.
12.3 Write differential and integral rate laws for the elementary reactions:
(i) 2A →products
(ii) 3A →products
(iii) 2A +B →products
(iv) A +B +C → products.
(Hint: use Maple wherever possible.)
12.4 Find expressions for the half lifes and time scales of the elementary reactions:
(i) 2A →products
(ii) A +B →products
(iii) 2A +B →products.
For (ii) and (iii) consider three cases: [A
0
]=[B
0
], [A
0
]>> [B
0
] and [A
0
]<< [B
0
].
12.5 Compare the exact solution for the concentration of a short-lived species [B]
(equation (12.104)) with the steady state solution (12.105). Establish a criterion for
how much time must be allowed to elapse for the steady state solution to become
an acceptable approximation. Also, describe some conditions under which the steady
state solution is never an acceptable approximation.
12.6 Estimate what would be the half life of ozone in the terrestrial atmosphere if ultraviolet
radiation were to stop suddenly. Comment on likely night vs. day variations in ozone
concentration.
13
Topics in atmospheric thermodynamics and
radiative energy transfer
This chapter seeks to answer three basic questions about the physical nature of planetary
atmospheres. First, why do some planets have atmospheres and others do not? Second, what
controls the mass and the thickness of a planetary atmosphere? Third, how is a planet’s
surface temperature affected by the existence of an atmosphere? Some of the answers that
we find here will be the starting point for our discussion, in the last chapter, of the chemical
evolution of atmospheres in the terrestrial planets and the possible relationships between
atmospheric composition and the origin of life.
13.1 Gravitational binding of planetary atmospheres
Whether or not a planet has an atmosphere depends on the relationship between the gravita-
tional potential at the planet’s surface and the translational kinetic energy of gas molecules.
The latter is of order k
B
T (Section 1.14), whereas the gravitational potential at the planet’s
surface can be written as Φ =4/3πGr
2
ρ (from equation (1.8)). Recalling that Φ is gravita-
tional potential energy per unit mass, we take the product Φu, where u =1.66054×10
−27
kg
is the atomic mass unit, and form the non-dimensional ratio:
^ =
3k
B
T
4πGr
2
ρu
. (13.1)
The parameter ^ is an estimate of the tendency of a planetary atmosphere to escape. For
reasons that we will see later the absolute value of ^ does not have a rigorous interpretation,
but the relative value among different planets is a good indicator of whether or not a planet
is likely to have an atmosphere, and, to some extent, of what type of volatile species may
be most abundant. The smaller the value of ^, the more strongly bound an atmosphere is,
and the more likely it is to contain a large proportion of low molecular weight species.
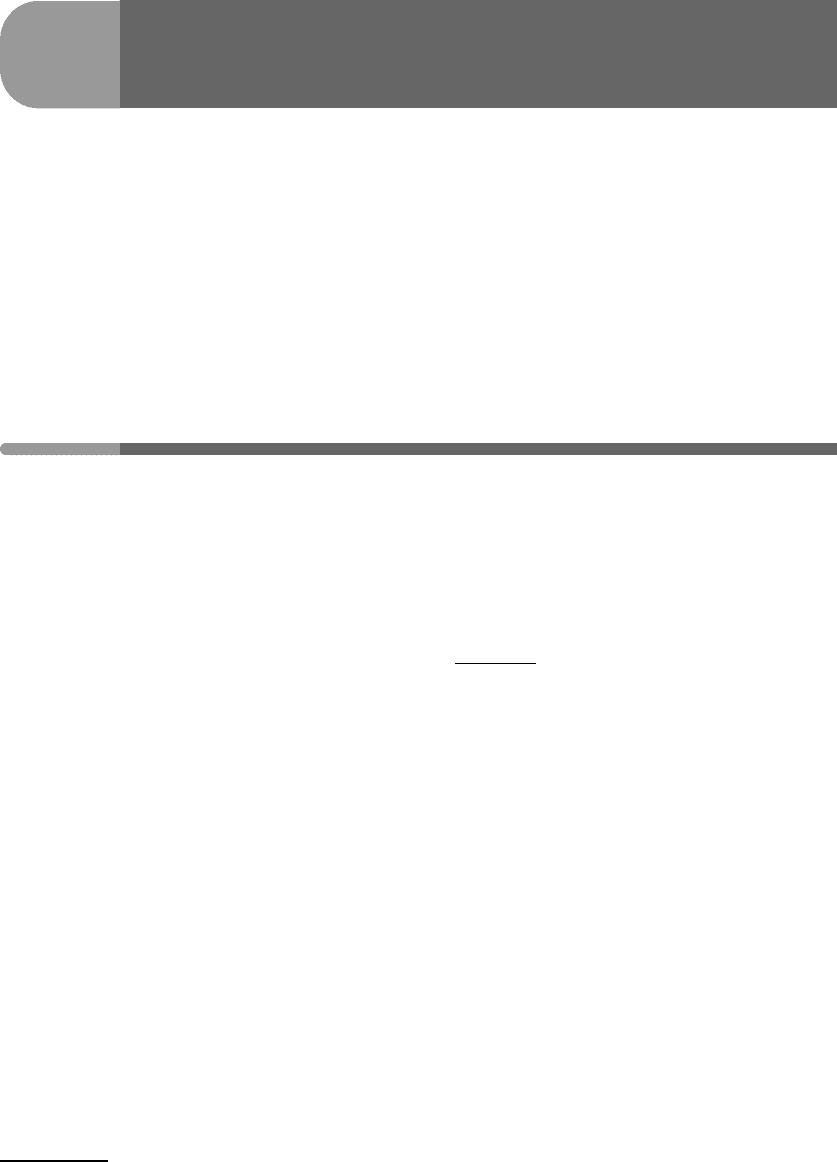
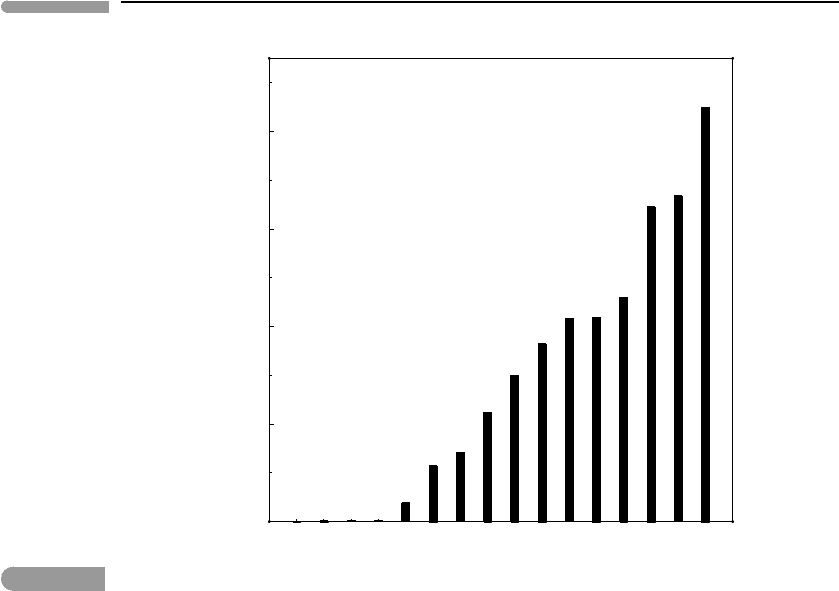
Calculated values of ^ for planets and major moons are shown in Fig. 13.1, arranged
in order of increasing value of ^. The value of T used in this graph is the temperature at
the planet’s surface (or its equilibrium temperature, Worked Example 13.1). As we shall
see this is not the best choice, but it is the simplest one. Regardless, we note that, with
one exception, the parameter ^ discriminates correctly between bodies with “substantial”
atmospheres (to the left of Triton) and those without one (to the right of Triton). Triton’s
faint atmosphere marks the boundary. The exception is Pluto, which is thought to have an
atmosphere comparable to that of Triton, but given the non-trivial uncertainties in Pluto’s
physical parameters the significance of this is not clear – it will likely remain unresolved
until the New Horizons spacecraft arrives in the Pluto–Charon system in 2015. Ignoring this
exception, equation (13.1) shows, for instance, that the reason why Titan has an atmosphere
but none of the Galilean satellites of Jupiter does is the fact that they lie closer to the
616
617 13.1 Gravitational binding of planetary atmospheres
0
0.2
0.4
0.6
0.8
Planetary body
Ξ
Jupiter
Saturn
Neptune
Uranus
Earth
Venus
Mars
Titan
Triton
Ganymede
Pluto
Io
Callisto
Mercury
Europa
Moon
Fig. 13.1
Value of the non-dimensional parameter ^ (equation (13.1)) for the planets and large moons of the Solar System.
Triton marks the boundary between bodies with atmospheres (to the left) and airless bodies (to the right), the one
outlier being Pluto.
Sun. Interestingly, the plot suggests that Europa is as unlikely to be able to hold on to an
atmosphere as Mercury, and that the Moon is even more hopeless in this respect.
Focusing on the left side of the plot, we note that the four giant planets have very small
^ values, ranging from 0.0007 to 0.003. This means that their atmospheres are bound very
tightly, but it also means something else. In equation (13.1) I used a constant mass, u,to
obtain the non-dimensional parameter ^, but the masses of different gas species, and hence
their gravitational binding energies, differ. For instance, the gravitational potential energy
of an H
2
molecule (mass =2) is more than twenty times smaller than that of a CO
2
molecule
(mass =44).At a given temperature, however, their kinetic energies are the same, so that the
hydrogen molecule is much more likely to escape than the carbon dioxide molecule. We can
also think of this in terms of velocities: the gravitational potential determines the planet’s
escape velocity (Section 2.4.1), whereas molecular speeds are a function of temperature
and molecular mass (Section 1.14). Molecular speeds follow a statistical distribution with
long “high speed” tails (Fig. 1.12), so that it is generally the case that only some fraction
of the total number of gas molecules in a planetary atmosphere have speeds greater than
the planet’s escape velocity. The rate at which gases can escape a body’s gravitational
attraction varies directly with the fraction of molecules that have speeds that exceed the
escape velocity, and at a given temperature this fraction is greater for light species (e.g. H
2
)
than for heavy ones (e.g. CO
2
). We can then (qualitatively) interpret a very small value of
the parameter ^ as signifying that even light molecules will generally be able to remain
gravitationally bound to the planet, because only a very small portion of the high velocity
