Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


578 Non-equilibrium thermodynamics
We define the external contribution as the entropy change that arises from exchange of heat
with the environment, i.e.:
d
e
S ≡
dQ
T
=
dE +PdV
T
. (12.2)
This component can be positive, negative or, in the case of adiabatic or isolated systems,
zero. The internal contribution to entropy arises from processes that occur inside the sys-
tem. These could be, for example, heat transfer, chemical diffusion, chemical reactions,
viscous dissipation, or dissipation of electric currents. An important part of the study of
non-equilibrium thermodynamics consists of finding rigorous mathematical expressions
for these and other entropy production processes. At this point we note that d
i
S must obey
the following relationship:
d
i
S ≥ 0. (12.3)
The equality holds for a system at equilibrium, i.e. static. If a system is not static then d
i
S>0.
Of course, there is nothing new here: if we apply (12.1) to an isolated system (d
e
S = 0)
then (12.3) recovers the property of entropy given by equation (4.7). The importance of
separating external and internal entropy contributions as in (12.1) is that internal entropy
production (d
i
S)isalways positive in a non-static system, regardless of how the system
interacts with its environment. Moreover, it is positive not only for the system as a whole
but also for any (non-static) part of the system that we may wish to analyze independently.
If a system is not at internal equilibrium then gradients in intensive variables must exist
within it. If entropy gradients exist then the concept of molar entropy for the system as
a whole loses meaning. If the system is not far from equilibrium, however, it is possible
to define a lengthscale within which local equilibrium holds (i.e. such that gradients in
intensive variables can be neglected over distances of this magnitude). It is then convenient
to work with entropy per unit volume, so that the entropy of sufficiently small volume
elements is well-defined. More precisely, because we are interested in non-static conditions,
we consider the rate of entropy production per unit volume, σ , defined as:
σ ≡
1
V
d
i
S
dt
. (12.4)
We note that the units of σ are entropy per unit volume per unit time, for instance: J K
−1
m
−3
s
−1
.
Entropy production is the result of flows that occur as long as the system is not at
equilibrium, and that cease when the system reaches equilibrium. We shall call these ther-
modynamic flows. They could be, for instance, flow of heat, matter, electric charge or
momentum, or the change in the number of molecules of a given species during a chemical
reaction (a special case of mass flow). The thermodynamic flows are driven by potential
gradients, which are also called thermodynamic forces. For a general system in which
several different entropy production processes operate simultaneously we have:
σ = T
i
J
i
F
i
, (12.5)
where the J
i
are flows (vectors), the F
i
are potential gradients (one-forms) and (12.5)is
an inner product that produces the scalar quantity σ (Box 1.1). Consider heat flow as an
example. In this case J
q
is the heat flux vector (Section 3.1), which has units of J s
−1
m
−2
. On dimensional grounds we see that the units of the potential gradient that drives this
579 12.1 Non-equilibrium thermodynamics
flow must be K
−1
m
−1
. Restricting our discussion to flow in one spatial dimension, z,we
conclude that the thermodynamic gradient that drives heat flow is:
F
q
=
∂
∂z
1
T
. (12.6)
We shall now make the assumption that, if the potential gradients are small, then the flows
are linear functions of the potential gradients. This linear relationship is the formal definition
of a system close to equilibrium. If the linear relationship between flows and forces does
not hold then the system is far from equilibrium and the discussion in this and subsequent
sections does not hold. We write the linear relationship as follows:
J
i
=T
k
L
i
k
F
k
, (12.7)
where the L
i
k
are constants called phenomenological coefficients. Note that this equation
says that a flow J
i
may be driven not only by the gradient F
i
but also by all other poten-
tial gradients that may exist in the system. For instance, if gradients in temperature, F
q
(equation (12.6)) and chemical potential, F
d
, exist in a system, then according to (12.7),
both heat flow, J
q
, and mass flow, J
d
, each includes two separate contributions, one driven
by F
q
and the other by F
d
. Expanding (12.7) for this case we would have:
J
q
=L
q
q
F
q
+L
q
d
F
d
J
d
=L
d
q
F
q
+L
d
d
F
d
.
(12.8)
Each phenomenological coefficient is a scalar, and the matrix composed of all phenomeno-
logical coefficients arranged as in equations (12.8) is a geometric object called a tensor.
The two diagonal coefficients are easily interpreted: L
q
q
relates temperature gradient to heat
flow, so it must be somehow related to the heat conductivity, k (Chapter 3), whereas L
d
d
links the gradient in chemical potential to mass flow, so it must be related to chemical
diffusivity (Section 12.2.2).
The other two coefficients are more obscure and perhaps unexpected. L
q
d
=0 implies that
a gradient in chemical potential drives heat flow, a phenomenon know as the Dufour effect,
whereas L
d
q
= 0 means that a gradient in temperature causes chemical diffusion, which
is known as the Soret effect. These and other “cross-flow” phenomena have been known
since the nineteenth century. For example, a temperature engenders an electric current (the
Seebeck effect) and a gradient in electrical potential gives rise to heat flow at constant
temperature (the Peltier effect). Cross chemical diffusion terms arise in systems in which
there are gradients in the chemical potentials of more than one component (Section 12.2.3).
Lars Onsager (1931a, and b) demonstrated that these effects are not random, but rather
a fundamental property of non-equilibrium systems. His work is one of the cornerstones
of non-equilibrium thermodynamics. We shall not discuss it here, but we will mention a
fundamental result that is due to Onsager.This is the fact that the matrixof phenomenological
coefficients is symmetric. In other words, all cross coefficients obey the identity:
L
i
k
=L
k
i
. (12.9)
This is known as Onsager’s reciprocal relation.

580 Non-equilibrium thermodynamics
12.1.2 Heat diffusion revisited and the principle of minimum
entropy production rate
Consider a one-component system, in which compositional gradients are by definition
impossible. If we impose a thermal gradient on this system then because F
d
= J
d
= 0it
must be L
q
d
=L
d
q
=0, and (12.8) collapses to:
J
q
=L
q
q
F
q
. (12.10)
The thermodynamic flow is in this case the heat flux, given by equation (3.5):
J
q
=q =−k
dT
dz
. (12.11)
The potential gradient F
q
is given by equation (12.6), which we can also write as:
F
q
=
∂
∂z
1
T
=−
1
T
2
dT
dz
. (12.12)
We then obtain a relationship between the phenomenological coefficient L
q
q
and the thermal
conductivity, k:
k =
L
q
q
T
2
(12.13)
and, using (12.5), the rate of entropy production per unit volume is:
σ =
k
T
2
dT
dz
2
. (12.14)
As expected, σ is a non-negative quantity, and vanishes only if temperature is uniform and
hence there is no heat flow.
We recall from Chapter 3 that the steady state for heat diffusion, i.e. ∂T /∂t = 0, is
attained, in a system with no heat generation, when the thermal gradient is uniform, i.e.
∂T /∂z =constant (e.g. equation (3.15)). It can be proved beginning from equation (12.14)
that, for any non-zero temperature gradient, σ is minimized if T(z)is a linear function, so
that ∂T /∂z = constant. The formal demonstration of this will not be presented here (see,
for example, Kondepudi & Prigogine, 1998, p. 399). The result is, however, general, and is
known as the theorem of minimum entropy production rate, originally due to Prigogine. In
words, it states that any non-equilibrium system in which at least some of the thermodynamic
forces do not vanish, and in which the linear phenomenological law (12.70) and Onsager
reciprocal relations are valid, evolves to a non-equilibrium steady state in which the rate of
entropy production is minimum. One way to think of this, suggested by Onsager, is that the
rate of entropy production behaves as a potential, that is minimized when a non-equilibrium
system reaches a dynamic state that remains stationary with time.Although the reality of this
result is not in question, different opinions exist on whether minimum entropy production
is a principle (i.e. non-demonstrable from simple statements) or a theorem, as envisioned
by Prigogine (see, for example, Jaynes, 1980).

581 12.2 Chemical diffusion
12.2 Chemical diffusion
12.2.1 Fundamental relationships
Transport of chemical species down a chemical potential gradient is a non-equilibrium
process. It therefore generates entropy. Mass transfer can take different forms. One of them
is chemical diffusion without chemical reaction. We seek an equation that relates this type
of matter flow to entropy production. We begin with the fundamental equation (4.101),
which we re-write as follows:
dS =
dE +PdV
T
−
1
T
i
µ
i
dn
i
. (12.15)
Comparing to (12.1) and (12.2) we find that internal entropy production is given by:
d
i
S =−
1
T
i
µ
i
dn
i
. (12.16)
Consider diffusive mass transfer of a single component, which requires that there be a
gradient in the chemical potential of only that component. Physically this could be possible,
for example, in a one-component system that is not in equilibrium in a gravitational field
(Chapter 13), or in a two-component system in which the solute is dilute enough that the
concentration of the solvent can be considered to be constant even if the solute concentration
varies (Chapter 11). The latter case is known as tracer diffusion. For simplicity we will
consider diffusion in a single spatial dimension, z, but the equations are easily extended
to diffusion in three dimensions (see Kondepudi & Prigogine, 1998; Zhang, 2008; Borg &
Dienes, 1988).
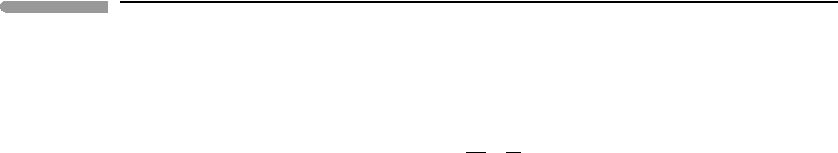
Consider two parallel surfaces of cross-sectional area a, separated by a small distance
δz, and such that the chemical potentials of the diffusing component at each surface are µ
1
and µ
2
, with µ
1
>µ
2
(Fig. 12.1). Define δµ = µ
2
−µ
1
. Matter flows from 1 to 2, so that
if we call dn = dn
2
=−dn
1
> 0, we have:
i
µ
i
dn
i
=µ
1
dn
1
+µ
2
dn
2
=δµdn. (12.17)
Substituting in (12.16):
d
i
S =−
1
T
δµdn, (12.18)
which is always positive, as δµ and dn always have opposite signs. Passing to the limit
and noting that this amount of entropy is produced inside a volume of size aδz, we get, by
using (12.4):
σ =
1
a
dn
dt
−
1
T
dµ
dz
. (12.19)
Comparing (12.19)to(12.5), and allowing for the possibility that temperature gradients may
also exist (Section 12.2.4), we identify the thermodynamic flow and the thermodynamic
582 Non-equilibrium thermodynamics
a
J
d
δ
z
>
a
µ
1
µ
2
Fig. 12.1
Matter flow, J
d
, between two parallel surfaces of area a on which the chemical potentials of the diffusing species are
µ
1
>µ
2
.
potential gradient as follows:
J
d
=
1
a
dn
dt
F
d
=−
d
dz
µ
T
.
(12.20)
For isothermal diffusion of a single component we write the phenomenological relationship
(12.7) as follows:
J
d
=−
L
T
dµ
dz
. (12.21)
12.2.2 Fick’s laws of chemical diffusion
Equation (12.21) describes isothermal chemical diffusion of a single chemical species, but
it is not convenient because chemical potential is not a directly measurable quantity. We
seek to recast this equation in terms of concentration of the diffusing species. If c
i
is the
molar concentration of the diffusing species per unit volume and V is the molar volume
of the system, then the mol fraction X
i
is X
i
= Vc
i
. Taking the standard state as the pure
substance at the temperature and pressure of interest:
µ
i
=µ
0,i
+RT ln
(
γ
i
c
i
V
)
. (12.22)

583 12.2 Chemical diffusion
By the chain rule, and assuming that the specis concentration is low enough that it follows
Henry’s law (γ
i
approximately constant):
dµ
i
dz
=
dµ
i
dc
i
dc
i
dz
=
RT
c
i
dc
i
dz
. (12.23)
Substituting in (12.21):
J
d
=−
LR
c
i
dc
i
dz
(12.24)
and defining:
D ≡
LR
c
i
(12.25)
we arrive at:
J
d
=−D
dc
i
dz
, (12.26)
which is known as Fick’s first law of diffusion. Equation (12.26) is identical to Fourier’s
law of heat conduction (equation (3.5)) and it is no more of a “law” than the latter. Rather,
it is another constitutive equation that is a special case of the general transport relation
expressed by equation (3.4).
We now recall that c
i
has units of mols per unit volume, and that the matter flux J
d
has
units of mols per unit area per unit time. It follows that the dimension of D is area per unit
time, e.g. m
2
s
−1
, which are units of diffusivity (Section 3.2.3). The parameter D is called the
chemical diffusivity or diffusion coefficient. In particular, for the dilute one-component case
that we are considering here it is called the tracer diffusion coefficient. Diffusion coefficients
are a strong function of temperature (Section 12.4.1) and equation (12.25) shows that they
are also a function of composition. This latter point can cause considerable complications
in the mathematics of diffusion, but the compositional dependency can usually be ignored
in tracer diffusion problems.
Equation (12.26) still has the disadvantage that it includes a matter flux term that is
generally not easily measured, especially if D is small. A better alternative would be
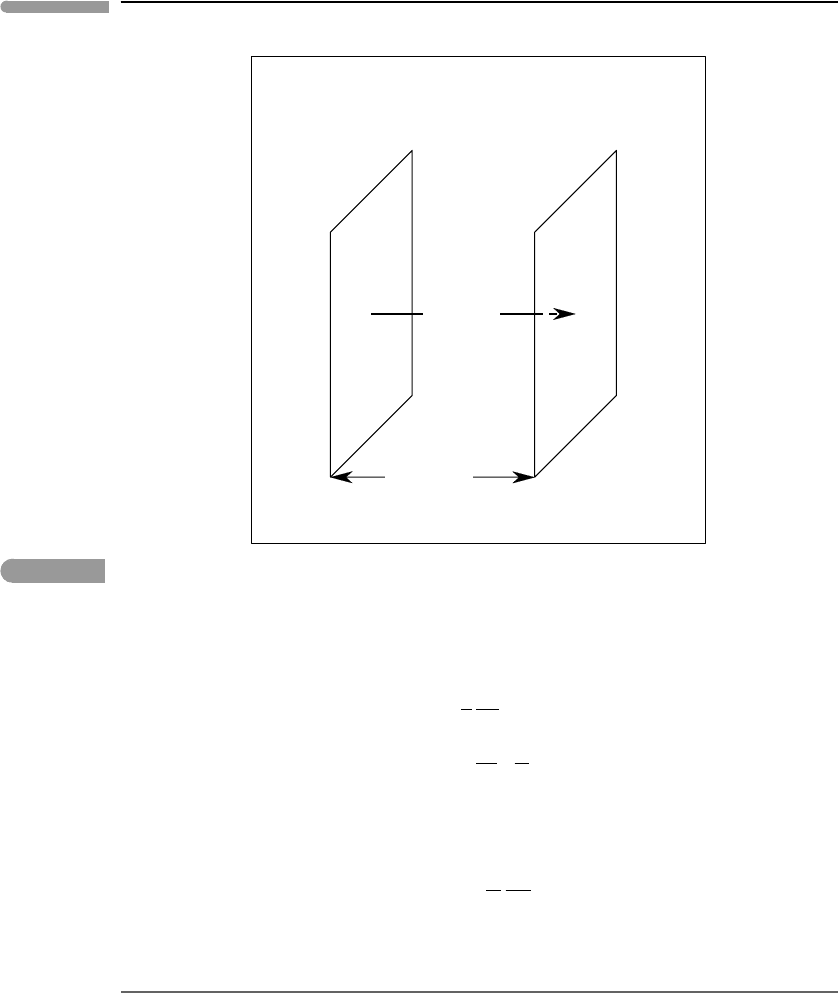
to measure the change in concentration with time. Consider a volume element of unit
cross-sectional area and width δz (Fig. 12.2), and let the matter fluxes across its two faces
be J
z
and J
z+δz
. The change per unit time in the number of mols of solute contained
in the volume is J
z
−J
z+δz
, so that the rate of change of concentration (mols per unit
volume) is:
dc
i
dt
=
1
δz
(
J
z
−J
z+δz
)
. (12.27)
Using (12.26):
∂c
i
∂t
=
D
δz
∂c
i
∂z
z+δz
−
∂c
i
∂z
z
=
D
δz
∂
2
c
i
∂z
2
δz (12.28)
584 Non-equilibrium thermodynamics
a=1
J
z
J
z + δz
δ
z
a=1
Fig. 12.2
Geometry of the chemical diffusion equation in one dimension. The volume element has unit cross-sectional area
perpendicular to the matter flow direction and thickness δz along this direction.
or:
∂c
i
∂t
=D
∂
2
c
i
∂z
2
. (12.29)
Equation (12.29), which is identical to the heat diffusion equation (3.15) without a source
term, is known as Fick’s second law of diffusion. You may also recognize in equations
(12.27) and (12.28) a compact version of the derivation of equation (3.15) in Section 3.2.2.
As I mentioned there, equation (12.29) is a differential equation that shows up in many
branches of physics and is simply known as the diffusion equation. The mathematics of
diffusion are the same regardless of what is the physical entity that is transported.
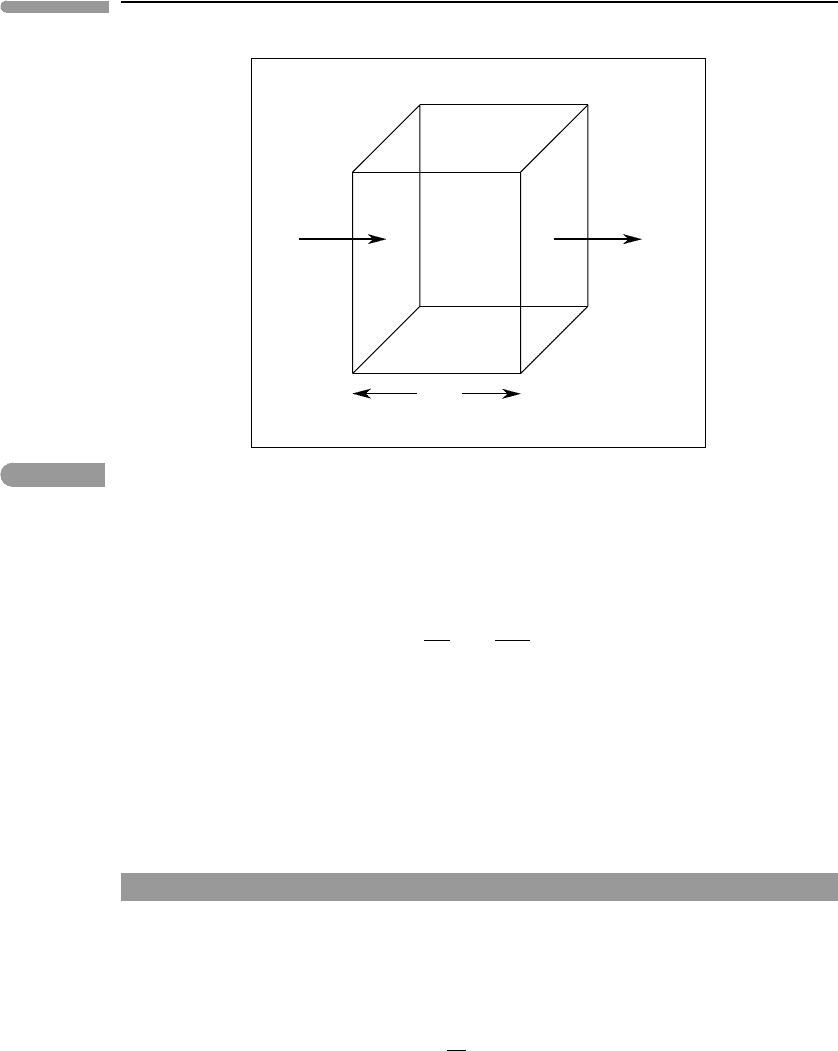
Worked Example 12.1 Chemical diffusion on planetary time and lengthscales
We can get a feeling for the relevance of chemical diffusion in planetary processes by
focusing on a few examples. In particular, we will look at diffusion in the atmosphere, the
oceans, magmatic systems and minerals at metamorphic conditions. As for heat diffusion,
we have the relationship:
λ
2
τ
∼D, (12.30)
where λ is the characteristic diffusive lengthscale, τ the characteristic time scale, and D the
diffusion coefficient. Diffusivities of trace gas components in air at 298 K and 1 bar are of
the order of 10
−5
m
2
s
−1
, whereas for molecular and ionic species in aqueous solution at
298 K typical values are ∼10
−9
m
2
s
−1
(see Kondepudi & Prigogine, 1998; Zhang, 2008).
Diffusivities in silicate melts are somewhat more variable; we will use a typical value for
585 12.2 Chemical diffusion
10
3
10
4
10
5
10
6
10
7
10
8
10
–10
10
–8
10
–6
10
–4
10
–2
1
10
2
10
4
Diffusion time (years)
Diffusion length (meters)
10
-5
10
-9
10
-12
10
–22
10
-32
atmosphere
ocean
magma chamber
metamorphism
Sr in feldspar
Pb in monazite
magma
atmosphere
ocean
Fig. 12.3 Some examples of chemical diffusion in planetary environments. The lines labeled with diffusion coefficients (in
m
2
s
−1
) are plots of equation (12.30). For the atmosphere and ocean we start from their characteristic lengthscales
and infer unrealistically long chemical diffusion time scales, implying that other homogenization mechanisms
operate, such as eddy diffusion. For igneous and metamorphic processes we start with estimates of characteristic time
scales, e.g. 10
6
years for crystallization of a magma chamber and 20 ×10
6
years for a metamorphic event, and infer
lengthscales for various processes (see text).
H
2
O in rhyolite melts at 900
◦
C, which is ∼10
−12
m
2
s
−1
(Zhang et al., 2007). Tracer
diffusion coefficients in minerals are much more variable. For example, Pb in monazite
at 700
◦
C has a diffusivity of ∼10
−32
m
2
s
−1
(Cherniak et al., 2004), whereas the value
for Sr in feldspars at the same temperature is ∼10
−22
m
2
s
−1
(Cherniak & Watson, 1994).
Figure 12.3 shows plots of equation (12.30) with each of these diffusivity values.
The terrestrial atmosphere and oceans have lengthscales of ∼10 km and 2 km, respec-
tively. We see from Fig. 12.3 that diffusive homogenization of the atmosphere would take
about 300 000 years. The corresponding value for the ocean would be about 130 million
years. Clearly, some other mass transfer mechanism must operate in both of these systems.
For instance, changes in CO
2
concentration with a periodicity of less than one year are seen
in the atmosphere. A diffusive time for the ocean of 130 million years would imply that
it should not be possible to detect differences between Cretaceous and present-day ocean
chemistry, yet marine sediments record changes on much shorter time scales. If we assume
that the atmosphere mixes over times of order 1 year, and the ocean over times of ∼100 years
(rates are probably faster than these), then we can calculate with equation (12.30) that the

586 Non-equilibrium thermodynamics
effective diffusivities are ∼3 and 10
−3
m
2
s
−1
, respectively. These values are 5 to 6 orders
of magnitude greater than the corresponding chemical diffusivities. The explanation for the
discrepancy is that mass transfer in the atmosphere and ocean is chiefly controlled by a
process known as eddy diffusivity. The idea is that, owing to their relatively low viscosities,
neither air nor water are ever perfectly still. Rather, turbulent motions, i.e. eddies, occur
at all lengthscales, driven by causes such as temperature gradients and motion of animate
or inanimate bodies. Eddies cause local stirring and homogenization, and coupling among
eddies effectively diffuses matter at a rate that renders chemical diffusion in oceans and
atmospheres inconsequential.
A reasonable time scale for crystallization of a large igneous system may be 1 million
years. Over this time diffusive homogenization of compositional gradients would extend
over a distance of about 6 m.At least some magmatic systems are known to be homogeneous
over greater distances, as suggested for instance by the composition of km-size plutons and
of large volcanic eruptions. Again, some other mechanism for chemical mixing appears to
be required, but eddy diffusivity is unlikely to be the answer, given the very high viscosity
of silicate melts. Convective stirring in magma chambers is a possible explanation.
Let us now assume that a typical high-grade metamorphic recrystallization event lasts
20 million years. Over this time Sr in feldspar would diffuse a distance of perhaps 0.5 mm,
whereas Pb in monazite would homogenize over 10
−9
–10
−8
m. Radiogenic Pb formed
by decay of U and Th is essentially immobile relative to the size of a monazite crystal
(say, 10
−5
to 10
−4
m). Monazite is a refractory mineral that commonly survives high-
grade metamorphism. Therefore, monazite crystal cores can be used to date events that
preceded metamorphic recrystallization, even if rims of neoformed monazite grow during
metamorphism. In contrast, feldspar may break down and regrow in response to changes in
metamorphic conditions. Chemical reactions such as these are mass transfer mechanisms,
which may be much faster than simple chemical diffusion. However, even if feldspar growth
did not take place, Sr would homogenize over a length not too different from the size of
feldspar crystals. Rb–Sr dating may then yield the age of metamorphism.
An important generalization that follows from these examples is that, whereas chemical
diffusion is not an important mass transfer mechanism at planetary lengthscales, it pro-
vides the physical underpinnings and constraints for many powerful techniques used to
study planetary processes, such as radiometric dating and estimation of rates of geological
processes. A comprehensive and up to date treatment of these topics is given by Zhang
(2008).
12.2.3 Interdiffusion
Consider now the case of a binary system in which two components are present in com-
parable concentrations. If there is a compositional gradient then the two components will
diffuse in opposite directions. This is known as interdiffusion or, also, binary diffusion.It
is the process by which crystals that grow during metamorphism or igneous crystallization
tend to homogenize.
Let the matter fluxes be J
1
and J
2
. As always we consider diffusion in one dimension.
In some systems the following relationship is valid:
J
1
+J
2
=0. (12.31)
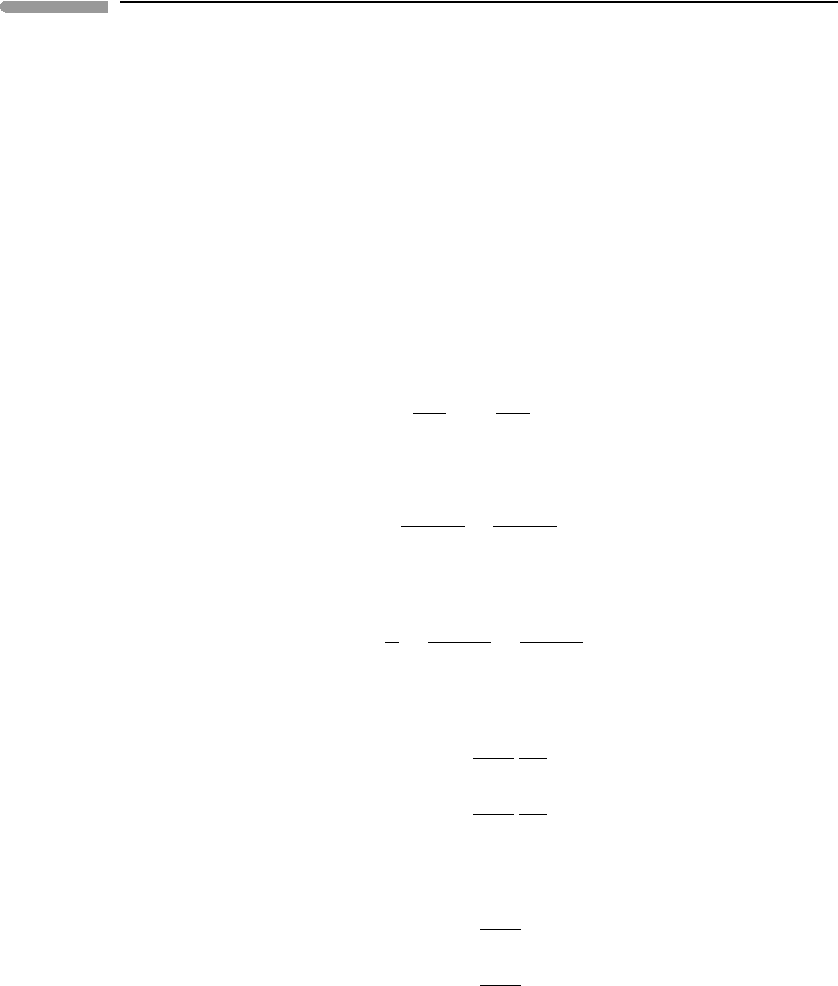
587 12.2 Chemical diffusion
Recall that we consider flux in units of mols, or particles, per unit of surface per unit of time.
Interdiffusion in a mixture of ideal gases at constant temperature and pressure obeys (12.31).
The same can be expected to be at least approximately true in a crystal, in which particles
exchange places among fixed sites in a crystalline lattice. But there may be instances in
which (12.31) is not valid, for example if the partial molar volumes of the two components
are significantly different. This requires only a slight modification to the equations, as we
shall see.
Calling the mol fractions of the two components X
1
and X
2
we have, from Gibbs–
Duhem’s equation (6.7) at constant temperature and pressure:
X
1
dµ
1
+X
2
dµ
2
=0 (12.32)
or:
X
1
dµ
1
dz
+X
2
dµ
2
dz
=0. (12.33)
We can combine (12.31) and (12.33) as follows:
J
1
X
2
dµ
1
/dz
=
J
2
X
1
dµ
2
/dz
(12.34)
and define a phenomenological coefficient, L, as follows:
−
L
T
≡
J
1
X
2
dµ
1
/dz
=
J
2
X
1
dµ
2
/dz
. (12.35)
Calling the molar concentrations per unit volume c
1
and c
2
, and using (12.23), we find:
J
1
=−
LR
X
2
c
1
dc
1
dz
J
2
=−
LR
X
1
c
2
dc
2
dz
(12.36)
so that the diffusion coefficients are (see equation (12.26)):
D
1
=
LR
X
2
c
1
D
2
=
LR
X
1
c
2
.
(12.37)
For X
2
→ 1 the coefficient D
1
becomes the tracer diffusion coefficient given by
equation (12.25), and as long as X
1
= 0, D
2
remains bound and the flux J
2
vanishes
as dc
2
/dz → 0. In other words, we recover the tracer diffusion case. We also note that, if
the molar volume of the mixture is V , then for all values of X
1
and X
2
we have X
1
=Vc
1
and X
2
=Vc
2
. Substituting these identities in (12.37) we find:
D
1
=D
2
=D
i
, (12.38)
