Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

548 Dilute solutions
Since we required that all thermodynamic potentials of (B
β
A
α
)
bl
be given by an equation
of the form of (11.83), the standard state chemical potential of the bulk aqueous electrolyte
is given by:
µ
0,B
β
A
α
bl
≡βµ
0,B+
aq
+αµ
0,A−
aq
. (11.86)
This definition of standard state properties is the crucial difference with the treatment of
weak electrolytes. A consequence of equation (11.86) is that we can think of the dissoci-
ation constant of a strong electrolyte as being identically equal to 1 at all pressures and
temperatures for which the electrolyte undergoes complete dissociation. Equation (11.86)
is, however, never valid for a weak electrolyte in which, as we saw, the standard state is
defined in the usual way for a dilute solute.
From (11.85) and (11.86) we find that the activity of the bulk electrolyte is given by:
a
B
β
A
α
bl
=
a
B+
aq
β
a
A−
aq
α
. (11.87)
Note that this is different from the activity of an undissociated weak electrolyte, given
by (11.81). Substituting mean ionic molality and mean ionic activity coefficient, (11.87)
becomes:
a
B
β
A
α
bl
=
γ
±
aq
m
±
β+α
, (11.88)
which should be compared with equation (11.81) for a weak electrolyte. Exercise 11.3 asks
you to apply these relations to the equations that relate activity and osmotic coefficients
(Section 11.2.3), and may be helpful in clarifying the meaning of (11.87) and (11.88).
11.4.3 The reference for standard state properties of ionic species
A problem that arises in the treatment of ionic species is that their concentrations cannot
be varied independently of those of ions of opposite charge. It is possible to determine
the infinite dilution standard state properties of dissolved molecular species for a weak
electrolyte, or the standard state properties of (fictive) bulk strong electrolytes, for example
by measurements of enthalpies of dissolution. Once these data are available one knows
the sum of the standard state properties of the corresponding anion and cation, e.g. via
equations (11.78)or(11.86). In all cases we have one equation with two free parameters,
so that there is no unique solution unless one specifies the value of one of the parameters.
The convention is to make the standard state enthalpy, entropy, Gibbs free energy and heat
capacity of the H
+
cation in aqueous solution at infinite dilution equal to zero, i.e.:
f
H
0,H
+
aq
=
f
G
0,H
+
aq
=S
0,H
+
aq
=C
P
H
+
=0. (11.89)
Because the heat capacity is set equal to zero equation (11.89) is valid at all temperatures.
Of course, the entropy of protons at any temperature other than zero is not zero, but since all
we care about when performing thermodynamic calculations are entropy (or free energy)
differences at constant temperature and pressure the convention does not introduce any
difficulties. Also, H
+
ions in aqueous solutions are always hydrated, i.e. attached electro-
statically to one or more H
2
O molecules, and do not exist as free protons. The symbol H
+
in equation (11.89) and all subsequent discussions must be understood as thermodynamic
shorthand for the actual molecular entity that exists in solution.

549 11.4 Thermodynamic formulation of electrolyte solutions
11.4.4 Dissociation of water and pH
Water is a polar liquid, so it should not be surprising that H
2
O molecules dissociate in liquid
water to some extent (Section 11.3). We can write this dissociation reaction as follows:
(
H
2
O
)
liq
H
+
aq
+OH
−
aq
. (11.90)
Choosing the standard state of water as the pure liquid at the temperature and pressure of
interest, we get the following equation for the dissociation constant of water, K
w
(compare
equation (11.80) for the dissociation of a weak electrolyte):
K
W
=a
H
+
aq
·a
OH
−
aq
=
m
H
+
·m
OH
−
γ
H
+
aq
·γ
OH
−
aq
. (11.91)
At 298.15 K the constant K
W
, also known as the ionization product of water, has a value
of K
W
= 1.011 × 10
−14
, which is commonly rounded off to K
W
≈ 10
−14
. The ionic
concentration in pure water is very low. Therefore, as a first approximation that is consistent
with the infinite dilution standard state, we can assume that the activity coefficients of the
ionic species are unity. The charge balance constraint, equation (11.69), then requires:
m
H
+
=m
OH
−
≈10
−7
. (11.92)
This was the basis of the original definition of pH as the negative of the decimal logarithm
of the hydrogen ion concentration:
pH =−log
10
m
H
+
(11.93)
and the definition of a neutral solution, with pH = 7, as one in which the hydrogen ion
molality is 10
−7
. Rigorously, however, pH is defined on the basis of hydrogen ion activity:
pH ≡−log
10
a
H
+
aq
=−log
10
γ
H
+
aq
m
H
+
(11.94)
and a neutral solution is one in which the hydrogen ion activity is 10
−7
. In dilute electrolyte
solutions the difference between (11.93) and (11.94) is negligible, but in concentrated
solutions it may be necessary to calculate the activity coefficient of H
+
and use the rigorous
definitions (11.91) and (11.94).
Worked Example 11.3 Atmospheric CO
2
and the pH of rainwater
Dissolution of atmospheric gases in water is described by equation (11.18). In Worked
Example 11.1 we calculated the concentrations of the molecular species N
2
,O
2
, Ar and
CO
2
in water at equilibrium with the terrestrial atmosphere. When CO
2
dissolves in water,
however, it reacts with H
2
O and produces bicarbonate and carbonate ions, according to:
CO
2aq
+H
2
O HCO
−
3aq
+H
+
aq
(I )
HCO
−
3aq
CO
2−
3aq
+H
+
aq
(II). (11.95)
If the solution is dilute and we take the activity coefficients to be unity then we can write
the dissociation constants as:
K
ds,I
=
m
HCO
−
3
m
H
+
m
CO
2
(11.96)

550 Dilute solutions
and:
K
ds,II
=
m
CO
2−
3
m
H
+
m
HCO
−
3
. (11.97)
At equilibrium the chemical potential of gaseous CO
2
in the atmosphere is the same as
the chemical potential of molecular CO
2
dissolved in water. Therefore, by choosing the
standard state of CO
2
as the pure gas at the temperature of interest and 1 bar, rather than
infinitely dilute CO
2
in aqueous solution, we can re-write equation (11.96) as follows:
K
ds,I
=
m
HCO
−
3
m
H
+
f
CO
2
. (11.98)
Equations (11.96) and (11.98) are equivalent, differing only in the choice of standard state
for CO
2
and, therefore, in the numerical value of the dissociation constant. Although it is
customary to use an equation of the form of (11.96), I believe that (11.98) is preferable, as this
gets around the problem of calculating activity coefficients for dissolved molecular gases.
If one wishes to calculate the concentration of dissolved gases this is simply accomplished
by means of Henry’s law (equation (11.18)), i.e. in this case:
m
CO
2
=f
CO
2
·K
CO
2
H ,s
. (11.99)
There are two additional equations that must be satisfied by the molalities of dissolved
species at equilibrium. One is the water ionization equation (11.91) (taking the activity
coefficients to be unity), and the other is the charge balance equation (11.69) which in this
case is:
m
H
+
−m
HCO
−
3
−2m
CO
2−
3
−m
OH
−
=0. (11.100)
If we fix the fugacity of CO
2
in equilibrium with water then equations (11.91) (setting
the activity coefficients equal to 1), (11.97), (11.98) and (11.100) constitute a system of
four equations in which the four aqueous molalities are the unknowns. The equations are
non-linear, but a numerical solution with Maple is straightforward (Software Box 11.1).
The two ionic dissociation constants can be calculated from standard state properties for
aqueous species given, for example, by Wagman et al.(1982) or Robie and Hemingway
(1995). If one wishes to calculate equilibrium at 298.15 K then the dissociation constants
are simply obtained from the listed standard state Gibbs free energy of formation of the
species, as in equation (11.79). Equilibrium at other temperatures can be calculated from
listed standard state enthalpy and entropy values:
r
G
0
=
r
H
0
−T
r
S
0
, including heat
capacity integrals if the C
P
values are known. The constants at 298.15 K and 1 bar are:
K
ds,I
=1.465 ×10
−8
(with a standard state of pure CO
2
gas) and K
ds,II
=4.685 ×10
−11
.
Software Box 11.1 Calculation of carbonate speciation in aqueous solution, assuming ideal
behavior
Procedure zeco2 in Maple worksheet aq_spec_ideal.mw calculates molalities
of dissolved carbonate, bicarbonate and carbon dioxide, in equilibrium witha1bar
atmosphere at 298.15 K. The aqueous solution is assumed to be ideal (see Worked
Example 11.3). A range of CO
2
atmospheric concentrations (in ppm) is specified in the
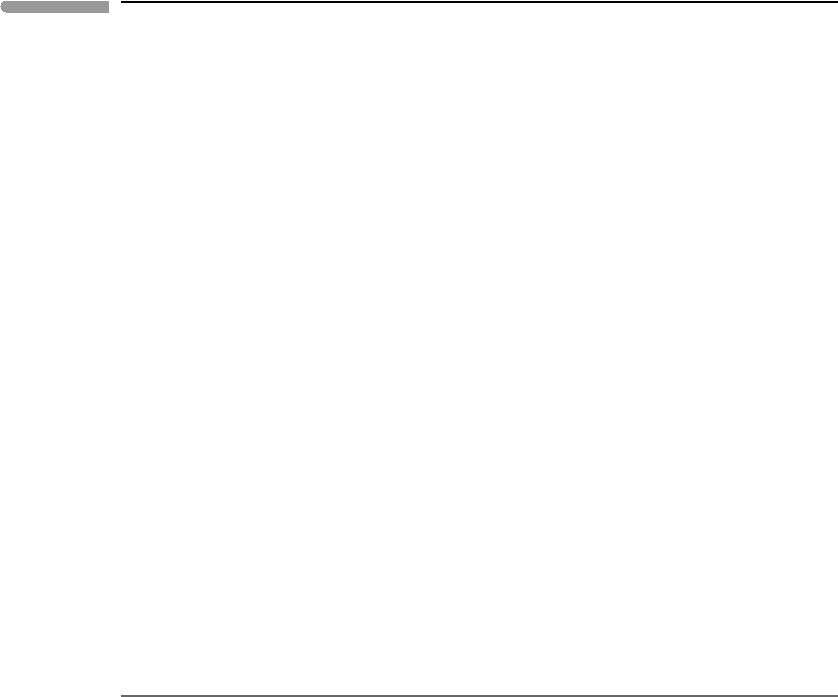
551 11.4 Thermodynamic formulation of electrolyte solutions
procedure call, as well as the number of intermediate values to calculate between these
boundaries, and a file name to send the output to. Output fields are: CO
2
atmospheric
concentration (ppm), pH , m
CO
2
, m
HCO
−
3
, m
CO
2−
3
, total dissolved CO
2
molality.
A second procedure, zecalcite, adds calcite saturation (equation (11.106), see
Worked Example 11.4) but is otherwise identical to zeco2. An additional field at the
end of each line of output contains m
Ca
2+
.
The results of the calculations as a function of f
CO
2
for values ranging from 0 to 5 ×
10
−4
bar (≈ 500 ppm CO
2
by volume in a 1 bar atmosphere) are shown in Fig. 11.5. The
top panel shows molalities of dissolved molecular CO
2
(calculated from f
CO
2
and Henry’s
law constant given by Sander, 1999, K
CO
2
H
=0.034) and of carbonate and bicarbonate ions,
as well as the sum of all three species, which is the total amount of dissolved CO
2
, called
the carbonate analytical concentration. At very low CO
2
fugacity the dominant species is
the bicarbonate anion, but as CO
2
concentration increases and the solution becomes more
acidic (bottom panel) the increased H
+
concentration displaces equilibrium (I) to the left and
limits dissociation, so that molecular CO
2
is the most abundant species. The bottom diagram
0
10
–4
2*10
–4
3*10
–4
4*10
–4
5*10
–4
10
–11
10
–10
10
–9
10
–8
10
–7
10
–6
10
–5
f (CO
2
) – bar
0
10
–4
2*10
–4
3*10
–4
4*10
–4
5*10
–
4
f (CO
2
) – bar
molality
5.5
6.0
6.5
7.0
pH
CO
3
2–
HCO
3
–
CO
2
total
Carbonate speciation in rain water at 25º C
pH of rain water at 25º C
Fig. 11.5 Carbonate species distribution (top) and pH (bottom) in “pure” water (∼rainwater) in equilibrium with the
terrestrial atmosphere at 25
◦
C. The analytical CO
2
concentration equals the total concentration of CO
2
-bearing
species =m
CO
2
+m
HCO
(−)
3
+m
CO
(2−)
3
.

552 Dilute solutions
shows that the pH of water in equilibrium with the present-day terrestrial atmosphere
(380 ppm CO
2
) should be about 5.6. This is approximately the pH of rainwater in regions
far removed from sources of pollution (particularly burning of high-sulfur coal and diesel
fuel). It is not the pH of ocean water, nor of surface waters in general, as these waters contain
other solutes in addition to CO
2
and may also be in equilibrium with solid phases.
11.4.5 Equilibrium between electrolyte solutions and solid phases
Aquestion that repeatedly comes up when studying aqueous solutions is whether the solution
becomes saturated in one or more crystalline phases. This is the same problem that is at
the core of igneous petrology, but aqueous solutions are generally better understood from a
theoretical point of view than silicate melts. We can represent equilibrium between an ionic
crystalline solid, (B
β
A
α
)
xs
, and its dissociation products by:
B
β
A
α
xs
β
(
B+
)
aq
+α
(
A−
)
aq
. (11.101)
This equilibrium is equally valid whether the substance is a strong or weak electrolyte.
In the latter case we could also, if we wished, write an equilibrium equation with the
undissociated aqueous species. As usual, the standard state for the crystalline phase is the
pure solid at the temperature and pressure of interest. Note that this is not equal to the
standard state chemical potential of the molecular species (B
β
A
α
)
aq
for a weak electrolyte.
The equilibrium condition for (11.101) is:
µ
0,B
β
A
α
xs
=βµ
B+
aq
+αµ
A−
aq
=βµ
0,B+
aq
+αµ
0,A−
aq
+RT ln
a
B+
aq
β
a
A−
aq
α
. (11.102)
Let us call the equilibrium constant for this reaction K
sp
, so that:
K
sp
=exp
−
r
G
0
RT
=exp
−
βµ
0,B+
aq
+αµ
0,A−
aq
−µ
0,B
β
A
α
xs
RT
(11.103)
and:
K
sp
=
a
B+
aq
β
a
A−
aq
α
=
(
m
B+
)
β
(
m
A−
)
α
γ
±
aq
β+α
. (11.104)
These equations are valid in general, but it is convenient to distinguish between relatively
soluble and relatively insoluble substances. For the former the molalities at saturation are
large enough that the activity coefficients cannot be ignored. For relatively insoluble elec-
trolytes the molalities are small enough that it may be acceptable to ignore the activity
coefficients. In such cases K
sp
is known as the solubility product and it provides a conve-
nient way of checking for saturation of specific phases. At a given temperature and pressure
the solubility product is a constant for each phase in equilibrium with an aqueous solution.
It is independent of the composition of the solution, as it is a combination of standard state
properties only (see equation (11.103), but recall that if the solvent is a liquid other than
water then the standard states for the solutes will be different, and so will the solubility prod-
uct). At saturation the product of the activities (or molalities if the solution is sufficiently

553 11.4 Thermodynamic formulation of electrolyte solutions
dilute) of the ionic species, with the stoichiometric coefficients as exponents, is equal to
the solubility product. If the activity (or molality) product is less than the solubility product
then the solution is not saturated in that particular phase. If it is greater then the solution is
supersaturated with respect to the phase, which is a non-equilibrium condition.
Worked Example 11.4 Limestone saturation and the pH of ocean water
Shallow ocean water is saturated in calcium carbonate, or at least nearly so. We can be
reasonably certain of this because, for instance, the shells of marine invertebrates do not
dissolve when the animals die. We will study how calcium carbonate saturation affects our
calculation of carbonate speciation and pH (Worked Example 11.3). Three of the equations
that we used in those calculations, (11.97), (11.98) and the water ionization equation (11.91),
remain unchanged. Because now we must also consider aqueous Ca
2+
ions, the electrical
neutrality equation (11.100) is modified as follows:
m
H
+
+2m
Ca
2+
−m
HCO
−
3
−2m
CO
2−
3
−m
OH
−
=0 (11.105)
and we have an additional equation, which is the solubility product of calcite:
K
sp,cc
=m
Ca
2+
·m
CO
2−
3
·
γ
±,CaCO
3
aq
2
. (11.106)
There are now five equations in five unknowns: the five ionic molalities listed in equation
(11.105).Thesolubilityproductofcalciteat298.15KcalculatedwithdatafromWagman
et al.(1982)isK
sp,cc
= 4.965 ×10
−9
(using aragonite instead of calcite makes a small
difference). For now we will assume that we can ignore the activity coefficient in equation
(11.106), and will return to this in Section 11.6. Modifying the Maple procedure discussed in
Software Box 11.1 to include the additional equation (11.106) is straightforward (Exercise
11.4).
For an atmospheric CO
2
concentration of 380 ppm we calculate an oceanic pH of
∼8.3, which is similar to typical values measured in the Earth’s oceans. The agreement
is remarkably good, considering that we have ignored all excess mixing properties. Table
11.1 compares the calculated solution compositions with and without calcite saturation
(Worked Example 11.3), for a fixed atmospheric CO
2
concentration of 380 ppm. The con-
centration of dissolved molecular CO
2
is of course the same in both cases, as this is fixed by
equilibrium with the gas phase. Total dissolved carbonate, however, is almost two orders of
magnitude higher in water saturated with calcite. The calculated Ca
2+
molality is ∼5.4 ×
10
−4
, which is more than one order of magnitude lower than measured molality in seawater
(∼10
−2
). The discrepancy arises to a large extent from ignoring the activity coefficient in
equation (11.106).
Why is the pH of seawater controlled by CaCO
3
saturation? The answer is that among
the most abundant species in seawater carbonate is the only weak electrolyte, for which it is
possible to write a partial dissociation reaction such as (11.95)(II). Table 11.2 (taken from
Millero, 2004) shows the concentrations of the most abundant ions in seawater. The four
most abundant cations are strong bases, and the halide and sulfate anions are strong acids.
None of these ions react with H
2
O to consume or produce H
+
ions. The pH of seawater
is therefore fixed by the solid carbonate for which the solubility product is first exceeded,
and for present day seawater this is calcite.

554 Dilute solutions
Table 11.1 Calculated ionic speciation in simplified natural waters
Rainwater Calcite-saturated water
ideal Debye–Hückel ideal Debye–Hückel
CO
2
1.29 ×10
−5
1.29 ×10
−5
1.29 × 10
−5
1.29 × 10
−5
HCO
−
3
2.36 ×10
−6
2.36 ×10
−6
1.05 × 10
−3
1.15 × 10
−3
CO
2−
3
4.68 ×10
−11
4.71 ×10
−11
9.28 × 10
−6
1.22 × 10
−5
Total CO
2
1.53 ×10
−5
1.53 ×10
−5
1.07 × 10
−3
1.18 × 10
−3
Ca
2+
−−5.35 × 10
−4
5.89 × 10
−4
Ionic strength 2.36 ×10
−6
2.37 ×10
−6
1.61 × 10
−3
1.78 × 10
−3
pH 5.63 5.63 8.28 8.30
Calculations are based on 380 ppm atmospheric CO
2
at 1 bar and 25
◦
C, assuming either ideal
aqueous solutions or Debye–Hückel activity coefficients. Concentrations given in molality.
Table 11.2 Most abundant ionic species in seawater (after Millero, 2004)
Cation Molality Anion Molality
Na
+
0.4691 Cl
−
0.5459
Mg
2+
0.0528 SO
2−
4
0.0282
Ca
2+
0.0103 HCO
−
3
0.0018
K
+
0.0102 Br
−
0.0008
F
−
0.0007
CO
2−
3
0.0003
11.5 Speciation in ionic solutions. Iron solubility in ocean water
as an example
Iron cations occur in two oxidation states, Fe
2+
and Fe
3+
, that have vastly different solu-
bilities in water. Both ferrous and ferric ions react with water to form several ionic species
whose relative abundances are a function of pH . These properties make iron aqueous solu-
tions an excellent test subject on which to apply the concepts that we have discussed so
far. We will estimate the solubility of iron in ocean water, and study how this may have
varied with changes in atmospheric composition. The problem was introduced in Worked
Example 6.7, where we discussed banded iron formations and the information that they
preserve about conditions in the early Earth.
The solubility of iron in the present day shallow oceans, i.e. in ocean water that is in
equilibrium with the atmosphere, is limited by precipitation of Fe
3+
species. This fol-
lows from the fact that the present day atmospheric oxygen fugacity is about 70 orders of

555 11.5 Speciation in ionic solutions
magnitude higher than the oxygen fugacity along the hematite–magnetite buffer at room
temperature (Fig. 9.3). We can expect the solubility-limiting phase to be either ferric oxide
or ferric hydroxide. The Gibbs free energy change for the reaction 2 Fe(OH)
3
→ Fe
2
O
3
+
3H
2
Oat298Kis∼−30 kJ mol
−1
, which means that hematite is the stable phase.
The phase that usually precipitates from seawater is not hematite, however, but rather
an amorphous solid or a hydrated crystalline ferric oxide with a composition intermedi-
ate between those of hematite and ferric hydroxide. Over time the precipitate dehydrates
and becomes hematite, and this may have been the mechanism by which Precambrian
hematite iron formations formed. This is a kinetic problem, that we will not address here
(see Chapter 12). We will use hematite in our calculations, in order to be consistent with
Worked Example 6.7. Because the metastable phase that actually forms has higher Gibbs
free energy than hematite, the results of our calculations are an absolute minimum of
solubility.
In these calculations we will only consider dissolved iron species that exist in an aqueous
solution with no other solutes, except carbonate anions. The effect of this simplification is
to underestimate the solubility of iron relative to the actual solubility in seawater, in which
formation of other Fe-bearing ionic species is possible. We will also assume that the activity
of dissolved iron species is ideal (i.e. a = m). Our estimated total iron contents in solution
will be about two orders of magnitude lower than the analytical concentrations of iron in
present day seawater (∼10
−10
molal in oxygen-rich near-surface water). The qualitative
behavior with changes in oxygen fugacity and pH that we will uncover are, however, robust,
and will illuminate the significance of banded iron formations (see also Worked Example
6.7). A rigorous discussion of iron solubility in seawater is given, for example, by Millero
et al.(1995) and Liu and Millero (2002).
We can think of dissolution of Fe ionic species in water as occurring in one of two ways.
The one that is perhaps more intuitively appealing is to think of iron hydroxides as weak
bases. Just as dissolved CO
2
, which is a weak acid, undergoes successive ionization reactions
(Worked Example 11.3), so a molecular aqueous species such Fe(OH)
3aq
can be thought
to dissociate in steps to Fe(OH)
2
+
, FeOH
2+
and finally Fe
3+
. All of these stoichiometric
species are known to exist in ferric iron solutions. Alternatively, we may imagine that Fe
3+
exists in solution as free ions that associate electrostatically with OH
−
groups to form
complex ions with the same stoichiometries as the molecular species that would form by
dissociation. The difference is that whereas in the former case the intermediate ionic species,
as well as neutral Fe(OH)
3
, are taken to be covalently bonded molecules, in the latter case the
ionic species are complexes in which Fe
3+
and OH
−
ions are attached electrostatically. The
more accurate physical picture is the latter one, but from a macroscopic thermodynamic
point of view the two approaches are equivalent. I will therefore use the “weak base”
model, because of its intuitive appeal. According to this model dissolution of hematite in
water proceeds as follows:
1
2
Fe
2
O
3
+
3
2
H
2
O Fe
(
OH
)
3aq
(III,hm)
Fe
(
OH
)
3 aq
Fe
(
OH
)
+
2 aq
+OH
−
aq
(III,1)
Fe
(
OH
)
+
2 aq
FeOH
2+
aq
+OH
−
aq
(III,2)
FeOH
2+
aq
Fe
3+
aq
+OH
−
aq
(III,3)
(11.107)
556 Dilute solutions
for which the equilibrium constants are (assuming an ideal electrolyte solution):
K
III,hm
=m
Fe(OH)
3
K
III,1
=
m
Fe(OH)
+
2
·m
OH
−
m
Fe(OH)
3
K
III,2
=
m
FeOH
2+
·m
OH
−
m
Fe(OH)
+
2
K
III,3
=
m
Fe
3+
·m
OH
−
m
FeOH
2+
.
(11.108)
The values of the equilibrium constants at 298.15 K and 1 bar, calculated with data from
Wagman et al.(1982) are: K
III,hm
=1.499 ×10
−12
, K
III,1
=5.997 ×10
−12
, K
III,2
=1.011×
10
−9
, K
III,3
=1.515 ×10
−12
. From equations (11.108) it follows that the solubility product
for hematite is K
sp,hm
=K
III,hm,
·K
III,1
·K
III,2
·K
III,3
= 1.38 ×10
−44
. If we were to calculate
the solubility of hematite from this figure we would get a molality of Fe
3+
of ∼10
−23
at
pH =7. This is the correct concentration of Fe
3+
cations in equilibrium with hematite, but
the analytical concentration of dissolved ferric iron is many orders of magnitude higher than
this, as the solubility product fails to account for the abundances of the other ionic species.
This is a point that one must always be careful with when calculating solubilities: failure to
account for speciation leads to grossly erroneous results. In fact, at pH =7 the most abundant
species is neutral Fe(OH)
3
, and the second most abundant one, with a concentration over
four orders of magnitude lower, is Fe(OH)
2
+
.Fe
3+
becomes the dominant aqueous species
only in very acidic solutions (Exercise 11.5).
The total concentration (= analytical concentration) of dissolved ferric iron is obtained
by solving each equation in (11.108) for one of the species molalities, and adding up the
resulting equations. Using the ionization product of water (equation (11.91)) to convert
m
OH
−
to m
H
+
we get the following equation for total dissolved ferric iron, m
Fe
3+
,total
,in
equilibrium with hematite, as a function of pH:
m
Fe
3+
,total
hm sat
=
=K
III,hm
1 +
K
III,1
K
W
10
−pH
+
K
III,1
·K
III,2
K
2
W
10
−2pH
+
K
III,1
·K
III,2
·K
III,3
K
3
W
10
−3pH
.
(11.109)
Although in the present day terrestrial oceans the concentration of Fe
2+
is vanishingly
small (at least in those parts of the ocean that are close to equilibrium with the atmosphere),
the same may not have been true during theArchaean. To account for this we write equations
for equilibrium between hematite and dissolved ferrous iron, which will necessarily involve
oxygen. In contrast to Fe(OH)
3
, there is no experimental evidence for the existence of the
neutral species Fe(OH)
2
in aqueous solution. We therefore write the reactions for hematite
saturation from Fe
2+
as follows:
1
2
Fe
2
O
3
+H
2
O FeOH
+
aq
+OH
−
aq
+
1
4
O
2
(II, hm)
FeOH
+
aq
Fe
2+
aq
+OH
−
aq
(II, 2). (11.110)
557 11.5 Speciation in ionic solutions
Taking the standard state of oxygen as the pure gas at the pressure and temperature of
interest the equilibrium constants are:
K
II,hm
=
f
O
2
1/4
·m
FeOH
+
·m
OH
−
K
II,2
=
m
Fe
2+
·m
OH
−
m
FeOH
+
(11.111)
and the values of the equilibrium constants (calculated with data from Wagman et al., 1982)
are K
II,hm
= 7.431 × 10
−32
and K
II,2
= 5.920 × 10
−8
. We can now derive an equation for
total dissolved ferrous iron in equilibrium with hematite, analogous to (11.109), which is:
m
Fe
2+
, total
hm sat
=
K
II,hm
K
W
10
−pH
f
O
2
1/4
1 +
K
II,2
K
W
10
−pH
. (11.112)
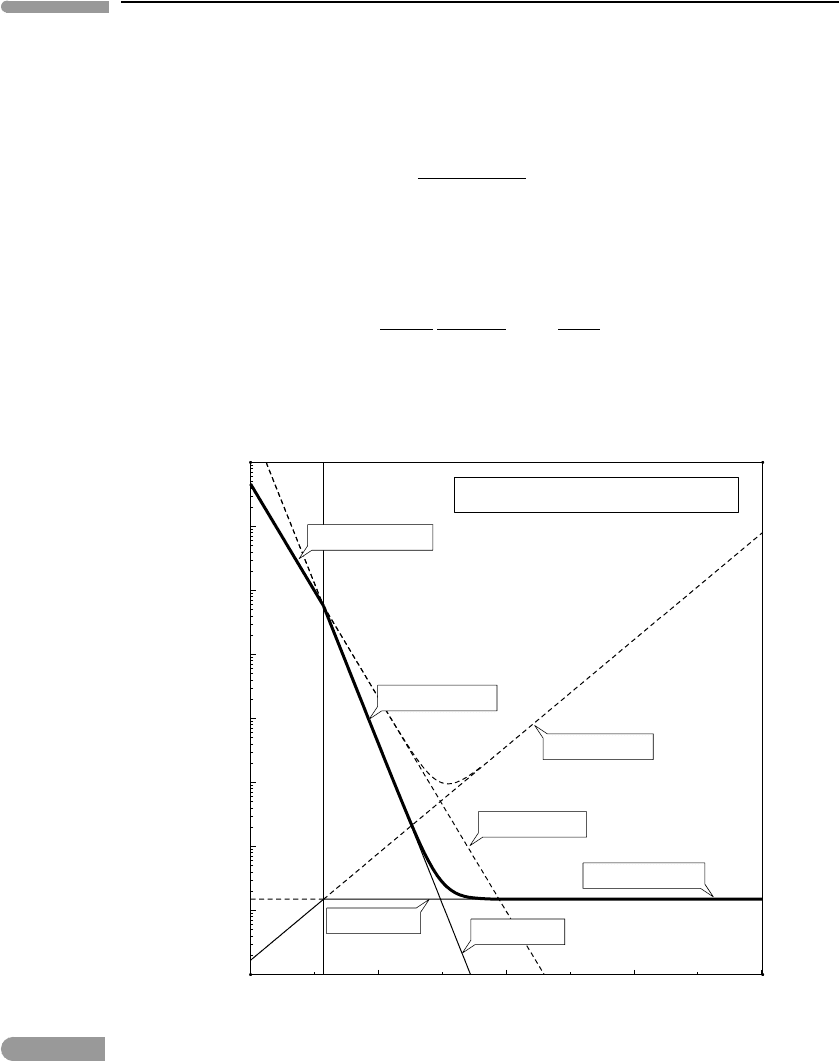
Equations (11.109) and (11.112) are plotted in Fig. 11.6 as a function of oxygen fugacity,
at a constant pH =8, which is approximately that of present day ocean water. Also shown
–80 –60 –40 –20
0
log f(O
2
) – bar
molality
Fe
2+
, hematite
Fe
3+
, magnetite
Fe
3+
, hematite
Fe
2+
, magnetite
Fe total, hematite
Fe total, magnetite
magnetite
hematite
Dissolved iron at pH = 8 and 25º C
Fe total, hematite
10
–11
10
–12
10
–13
10
–10
10
–9
10
–8
10
–7
10
–6
10
–5
Fig. 11.6
Concentration of dissolved iron species in water at 25
◦
C as a function of oxygen fugacity at pH =8(∼present day
seawater). The diagram shows total Fe
3+
species (Fe
3+
analytical concentration), total Fe
2+
species (Fe
2+
analytical
concentration) and total dissolved iron (=Fe
3+
+Fe
2+
species analytical concentrations) in equilibrium with either
hematite or magnetite. The stable phase is the one which attains saturation at lower concentration, shown with the
solid curves. The dashed curves show calculated concentrations in equilibrium with the metastable phase. The thick
solid curve shows total dissolved iron in equilibrium with the stable phase, which is hematite at log f (O
2
) > −68.59
and magnetite at log f (O
2
) < −68.59.
