Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

518 Melting in planetary bodies
240 260 280 300 320 340
0
5
10
15
Temperature (K)
Pressure (kbar)
200 300 400 500 600
10
–5
10
–4
10
–3
10
–2
10
–1
1
10
1
Ice VI
Ice V
Ice Ih
Ice III
Ice II
Liquid
Liquid
Gas
Ices
adiabat
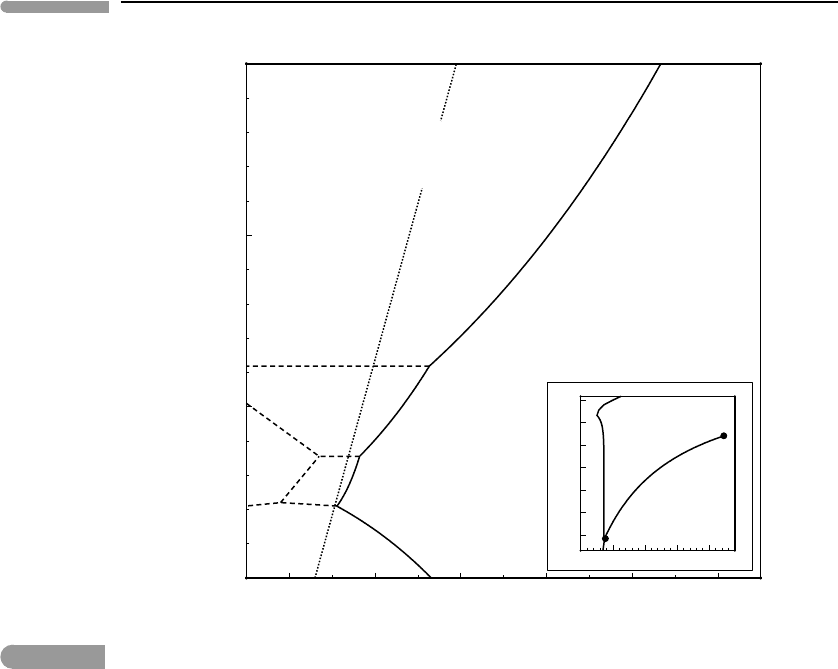
Fig. 10.16
Phase diagram of H
2
O, calculated with the thermodynamic model of Choukroun and Grasset (2007). The dotted line
shows the hottest ice adiabat along which no melting takes place. The inset shows the ice solidus together with the
liquid–vapor coexistence curve, terminating at the critical point (note logarithmic pressure scale, in kbar).
that low-pressure H
2
O ice contracts when it melts. The explanation for this resides in the fact
that hydrogen bonds have preferred orientations, and this produces a crystalline structure
with plenty of open spaces. These open spaces become occupied by H
2
O molecules in the
disordered melt, causing a decrease in volume upon melting. Higher-pressure polymorphs
of ice are, in contrast, “normal” in the sense that their density is greater than that of the
melt. Because of the peculiar freezing behavior of H
2
O the nature of magmatic activity in
H
2
O-rich icy planetary bodies is different from that in silicate bodies.
Let us begin by studying the phase diagram of H
2
O, shown in Fig. 10.16 (calculated
with thermodynamic data from Choukroun & Grasset, 2007). Over the pressure range 0–
15 kbar, shown in the figure, four ice polymorphs undergo stable melting reactions: ice Ih
(the familiar low-density ice) and three high-pressure ices, denser than liquid: ice III, V
and VI. Another polymorph, ice II, is not stable to the solidus. For comparison, the inset
in the figure shows the composite solidus curve from the main figure, together with the
sublimation and boiling curves of H
2
O, the latter terminating at the critical point.
A pressure of 15 kbar in large icy satellites such as Ganymede or Titan corresponds to
a depth of ∼1000 km, which is about as deep as the outermost H
2
O-dominated shell is
thought to extend in those worlds. Europa may have a much thinner H
2
O veneer, whereas
Callisto may be largely undifferentiated, and a mixture of ice and silicates may exist all the
519 10.8 The nature of solid–melt equilibrium
way to the satellite’s center, at a pressure of ∼50 kbar. Maximum pressures in small icy
satellites such as Enceladus or Mimas are of order 500 bar, so that high-pressure ices do
not form in them.
We now seek answers to a number of questions about the interiors of icy satellites.
First, do icy satellites convect? To answer this question, we solve the Rayleigh number
(equation 3.62) for the thickness of the convective layer, D:
D =
Ra µκ
gαρ
0
T
1/3
. (10.60)
Characteristic values for H
2
O ices are: µ = 10
12
Pa s, κ = 1.5 × 10
−6
m
2
s
−1
, α =
2 ×10
−4
K
−1
and ρ
0
= 1000 kg m
−3
. Recall that the temperature difference T is the
temperature drop across the thermal boundary layer. For an icy satellite a conservative
value is T ∼10 K. Assuming that the onset of convection occurs at Ra ∼ 1000, we get
D ∼1000 g
−1/3
m. For a large icy satellite g ∼ 1ms
−2
,soD ∼ 1 km, whereas for a small
icy satellite g ∼ 0.1ms
−2
and D ∼2 km. As these values are orders of magnitude smaller
than likely ice thicknesses (100–1000 km), we conclude that icy satellites convect, and in
fact do so vigorously (i.e. with a high Nusselt number, equation (3.65)). This conclusion is
valid for pure H
2
O ice, and probably for mixtures of H
2
O–NH
3
–CH
4
ices as well.
Because they convect, temperature distribution inside icy satellites must be adiabatic.
Using values for high pressure ices, α = 2 × 10
−4
K
−1
, V = 1.9 J bar
−1
mol
−1
, C
p
=
47JK
−1
mol
−1
and taking T = 300 K we get from equation (3.32) an adiabatic gradient
of ∼2.5 K kbar
−1
. This leads to the second question: what is the minimum potential tem-
perature in a convective icy satellite that allows the existence of liquid? For a convecting
silicate mantle, the answer is approximately 1400 K (≈1100
◦
C, see Fig. 10.7). An adiabat
with this potential temperature would intersect the peridotite solidus at zero pressure. For a
satellite composed of pure H
2
O we see from Fig. 10.16 that the answer is approximately 247
K. Adiabats with a higher potential temperature will intersect the solidus, but in contrast to
silicate systems they will do so at P>0. Moreover, if the potential temperature is between
∼247 K and ∼273 K then the liquid is only stable at pressures higher than the pressure at
the satellites’ surface. The conclusion that follows is that, in contrast to a silicate planet,
extrusion of cryolavas in a pure H
2
O world is impossible.
The surface of our pure H
2
O world could be covered by a convective ocean with a
potential temperature higher than 273 K. If the ocean was deep enough (equivalent to at
least a few kbar) then its bottom would freeze to high-pressure ice. There is no satellite
like this in the present-day Solar System. If the potential temperature of the convecting
satellite was less than 247 K then it would be solid throughout. This is perhaps the case for
most of Saturn’s small icy moons, with the exception of Enceladus. Large H
2
O satellites
with potential temperatures between 247 K and 273 K must contain a layer of liquid H
2
O
sandwiched between ice Ih at the surface and high pressure ices at depth. In this case there is
a maximum possible thickness for the ice Ih shell, which is given by the pressure of the ice
Ih–ice III–liquid invariant point, about 2 kbar (see Fig. 10.16). For the Solar System’s large
icy satellites this corresponds to a depth of ∼170 km. This is the greatest possible depth
to the top of the liquid layer, if such layer exists. In small icy satellites such as Enceladus
liquid H
2
O can exist under an ice Ih layer, but the deep high pressure ice layer cannot.
Present day Titan, and quite possibly Ganymede as well, are thought to follow the sand-
wich model, with a layer of liquid H
2
O between low-pressure and high-pressure ice layers.
The case of Europa appears to be different, as the H
2
O layer is not thick enough to allow

520 Melting in planetary bodies
crystallization of high-pressure ices, so that liquid H
2
O would be sandwiched between the
ice Ih shell and the planet’s silicate mantle (or crust?). Focusing on Titan and Ganymede,
then, we ask our next question: is cryomagmatism possible in these worlds? If the com-
position of the icy envelope is pure H
2
O then, as we saw, the answer is no. Liquid H
2
O
would freeze when attempting to ascend through the ice Ih shell (this is a convoluted way
of saying that ice floats on water). And yet, there is geologic evidence for cryolavas in both
of these worlds. The most likely explanation for this is that the material that encases these
satellites is a mixture of H
2
O with other volatile compounds, chiefly NH
3
and perhaps CH
4
as well. These additional components have two effects that may facilitate cryomagmatism:
they lower the melting point of ice, and they lower the density of the liquid.
Melting phase relations for the binary system H
2
O–NH
3
at low pressure were summa-
rized by Kargel (1992). Three intermediate crystalline compounds form between Ice Ih and
solid ammonia: ammonium dihydrate (NH
3
.2H
2
O), ammonium hydrate (NH
3
.H
2
O) and
diammonium hydrate (2NH
3
.H
2
O). These give rise to three eutectics, and a peritectic at
which ammonium dihydrate melts incongruently to ice Ih plus a liquid richer in ammonia
that NH
3
.2H
2
O. Everywhere between the composition of the peritectic (∼33 wt% NH
3
) and
the H
2
O end of the binary join the H
2
O–NH
3
melt is at equilibrium with ice Ih. Therefore,
a subsurface liquid layer in a water–ammonia icy satellite containing between 0 wt% and
33 wt% NH
3
can exist at equilibrium with an ice Ih shell.
What is the buoyancy of these H
2
O–NH
3
melts relative to the Ice Ih country rock? An
equation of state for H
2
O–NH
3
liquids has been calibrated by Croft et al. (1988). Their
equation consists of an isothermal Murnaghan EOS (Section 8.2.1) and a polynomial zero-
pressure thermal expansion term. It is somewhat unwieldy because the calibration changes
with composition along the H
2
O–NH
3
join. We cannot discuss it here owing to space
constraints. We can state some of the key results of the Croft et al. EOS, though. First, it
predicts that the density of H
2
O–NH
3
liquids is always lower than that of pure H
2
O liquid at
the same pressure and temperature, and that density decreases with increasing NH
3
content.
Second, it shows that H
2
O–NH
3
melts may be more or less dense that ice Ih, depending on
pressure and melt composition. Third, it suggests that the melts that form by incongruent
melting of ammonium dihydrate are less dense than ice Ih, which is the solid phase that
these melts are at equilibrium with. The melt becomes denser than ice Ih with decreasing
NH
3
content, but a compositional interval exists within which the Clapeyron slope of the
ice Ih melting reaction changes from negative (i.e. as in the pure H
2
O system, Fig. 10.16)
to positive. Under those circumstances the melt can ascend through the ice Ih crust and
eventually be extruded on the surface of the satellite.
And herein lies a significant problem: if a satellite-wide liquid layer of low-density H
2
O–
NH
3
melt forms, it is gravitationally unstable relative to the ice Ih shell. This would produce
a short-lived surge of magmatic activity, at the end of which refractory ice Ih would underlie
a less-dense mixture of H
2
O–NH
3
ices, making further igneous activity difficult. This is
not altogether different from the formation of anorthositic crust from a primordial lunar
magma ocean. The problem is that, in contrast to the volcanically dead Moon, there appears
to be geologically recent cryovolcanism on Titan, and perhaps on Ganymede as well. One
possible way out is that the liquid layer underlying the ice Ih shell is not positively buoyant
after all, but merely close to being neutrally buoyant, and that cryomagmas are “squeezed”
to the surface by tectonic processes driven by convection of the ice Ih shell (see Mitri
& Showman, 2008). Another possibility is that a satellite-girdling liquid layer does not
exist at all, because the average internal temperature is lower than the solidus, and that
convection causes only local decompression melting of ices, as in silicate planets. If the

521 Exercises for Chapter 10
NH
3
concentration is high enough to preempt the negative Clapeyron slope of the ice Ih
melting reaction then the resulting liquids could be extruded on the satellite’s surface.
Exercises for Chapter 10
10.1 The two melting point depression equations, (6.49) and (10.8), are approximations.
Derive an equation for the difference between both approximations and discuss the
conditions under which one or the other, or neither, is a better approximation.
10.2 Show that a peritectic cannot be a minimum melting point because it would violate
Schreinemakers’ rule.
10.3 Use the Maple worksheet described in Software Box 10.1 to study how each of the
following variables affects melt production by decompression melting: (i) Clapeyron
slope of the solidus; (ii) temperature difference between solidus and liquidus, (iii) ratio
of heat capacity to enthalpy of melting.
10.4 Use the Maple worksheet described in Software Box 10.1 to study decompression
melting in Venus, assuming that the composition of the Venusian mantle is the same
as that of the Earth’s, and using the thermal structure of the Venusian mantle discussed
in Section 3.9.
10.5 Discuss how the “envelope” between batch melting and fractional melting shown
in Fig. 10.9 would be affected by the non-isentropic processes discussed in Section
10.6.5. Show this (at least) semi-quantitatively.
10.6 Use the Maple worksheet described in Software Box 10.2 to study the effect of fluid
temperature on melt production by fluid-fluxed melting. How likely is it that the
temperature of the fluid will differ significantly from the temperature of the rock?
Use the appropriate equations from Chapter 3 to justify your answer.
10.7 Show that there is no contradiction between the facts that, during fractional volatile-
fluxed melting, individual melt increments have higher fluid mol fractions than batch
melts, whereas the total melt produced by fractional melting has lower fluid mol
fractions than batch melts (see Fig. 10.15).
11
Dilute solutions
The focus of this chapter is on liquid solutions in which one component is present in much
greater abundance, say at least one order of magnitude greater, than all others. Examples that
underscore the importance of this type of solutions include seawater, and natural terrestrial
waters in general, but one can imagine more exotic possibilities, such as hydrocarbon-based
solutions on Titan’s surface and ammonia-based solutions in its interior. What all of these
examples share is the fact that it is convenient to make a distinction between dilute solutes
that may not be liquid in their standard states, and a liquid solvent that is generally close
to being in its standard state. Depending on the nature of the solvent and of the solutes
the latter may exist as electrically neutral chemical species, as ions, or as a combination
of both. Solutions in which solutes dissociate into ions are known as electrolyte solutions.
Among these, those in which water is the solvent are by far the most important ones, at
least in terrestrial environments. The chapter emphasizes aqueous electrolyte solutions, but
virtually all of the thermodynamic framework is applicable to any type of dilute solution.
We begin with a discussion of dilute solutions in general, and shift the focus to electrolyte
solutions in Section 11.3.
11.1 Some properties of dilute solutions
In our discussions so far on the thermodynamic properties of solutions we have made no
distinction between the treatment of the different solution components. All our equations
have been symmetric, in the sense that we could interchange the components and arrive
at the same final result. This was true whether the solution was above or below its critical
mixing point (Section 7.1). In the latter case we considered two distinct subcritical phases
(for binary systems), and we allowed for the possibility that one or the other, or both, could
exist within some region of interest. This is not always the case, however. Consider aqueous
solutions as an example. One of the components in such solutions is liquid H
2
O. Within the
P–T region in which liquid H
2
O is stable the solubility of many chemical species of interest
in the planetary sciences is small (although there are important exceptions, such as NH
3
).
One way to look at this is that most aqueous solutions correspond to conditions that are so
far below the critical mixing point that their compositional range is restricted to a narrow
interval between the solvus and the H
2
O end of the composition axis. The other branch
of the solvus is generally inaccessible, either because the necessary bulk compositions are
not realized in nature, or because the solvus is suppressed by a first-order phase transition
and the corresponding liquid component is not stable at the conditions at which liquid
H
2
O is stable (consider, for example, a solution of NaCl in liquid H
2
O). In such cases it
is convenient to distinguish between a solvent and one or several solutes, such that the
total solute concentration is much lower than the solvent concentration. “Much lower” is of
522

523 11.1 Some properties of dilute solutions
course not a proper quantitative definition, but a good rule of thumb is “at least one order
of magnitude lower”. Solvent and solutes are best described by different sets of equations,
and the symmetry that characterizes the equations for other types of solutions is lost.
Two points must be clearly understood. First, although the liquid solvent that we most
commonly encounter in planetary environments is water this is not necessarily always the
case. Second, many aqueous solutions are electrolyte solutions, meaning that the solutes
dissociate into ions upon dissolving. Although thermodynamic description of electrolyte
solutions requires some specific techniques, that we will study beginning in Section 10.3,
there are fundamental aspects of the way in which dilute solutions are handled that are the
same regardless of whether or not they are electrolytes.
11.1.1 Concentration scale in dilute solutions
The concentration of the solvent in dilute solutions is expressed in mol fraction, as we have
done so far. For the solutes this is inconvenient for two reasons. First, solute mol fractions
are small numbers ( 1). Second and most importantly, many dilute solutions of interest,
and in particular aqueous solutions, may contain a large number of solutes. This makes it
advisable to describe the concentration of each solute with a function that remains invariant
when the amounts of other solutes are varied. Mol fraction does not behave in this way,
because if the amount of one component is varied all mol fractions vary. For the solvent in
a dilute solution this variation is generally trivial, but the same is not true for the solutes.
Solute concentration in dilute solutions is expressed in molality, defined as mols of solute
per kg of solvent. We will use the fixed subscript s to refer to the solvent, and the variable
subscript i to refer to solutes, where i can take as many different values as there are solutes
in the solution. Let the number of mols of solvent be n
s
, and the number of mols of the ith
solute be n
i
. The mol fraction of the solvent is then given by:
X
s
=
n
s
n
s
+
i
n
i
. (11.1)
The number of mols of the ith solute per kg of solvent is given by:
n
i
n
s
M
s
, (11.2)
where M
s
is the molecular weight of the solvent, in units of kg mol
−1
. The units of (11.2) are
mol kg
−1
, which agrees with our definition of molality but presents some formal problems.
In particular, units of concentration should always be dimensionless (e.g. equation (11.1))
because they end up as arguments of logarithmic functions. The problem is in this case easily
solved by defining the dimensionless molecular weight of the solvent, M
∗
s
, as follows:
M
∗
s
=
M
s
1kg mol
−1
, (11.3)
which allows us to define the non-dimensional molality of i, m
i
, as follows:
m
i
≡
n
i
n
s
M
∗
s
. (11.4)
Note that, in contrast to fugacity and partial pressure, which retain their pressure dimension
but are divided by the 1 bar standard state pressure when they are the argument of a logarithm

524 Dilute solutions
(even if we omit the denominators for simplicity), equation (11.4) defines a non-dimensional
molality. This function has the desired property, that the molality of a solute in a dilute
solution depends only on the amount of the solute itself. The amounts of all other solutes
j = i can be changed and this does not affect m
i
.
11.1.2 Standard states in dilute solutions
The standard state for the solvent in a dilute solution is taken as usual, as pure solvent at the
temperature and pressure of interest. Commonly the solvent is a liquid at the temperature
and pressure of interest, but it could also be a solid or a supercritical fluid. Using the same
standard state convention for dilute solutes is not convenient, however. One reason for this
is that the pure liquid solute at the temperature and pressure of interest is in many cases
not thermodynamically stable. This is the case, for instance, when we consider aqueous
solutions of substances that are solids or gases at room temperature. Another reason is that
the chemical potential of a very dilute solute may be orders of magnitude lower than that
of the pure substance at the same P and T, even if the latter was stable. It is thus more
convenient to define the standard state of dilute solutes at the infinite dilution limit, rather
than at the pure solute state. This requires some arbitrary definitions, and the first one is to
postulate that an infinitely dilute solution of a single solute is ideal, and that its molality
equals its activity. We write this as follows:
lim
m
i
→0
a
i
m
i
≡1, m
j=i
=0, (11.5)
where a
i
is the activity of solute i. Equation (11.5) carries the assumption that the activity
coefficient γ
i
of every solute tends to 1 as the total solute molality
m
i
goes to zero.
We now recall the usual relation between µ and µ
0
:
µ
i
=µ
0,i
+RT ln a
i
(11.6)
and define the standard state chemical potential of solute i at infinite dilution as follows:
µ
0,i
≡ lim
m
i
→0
µ
i
−RT ln m
i
, m
j=i
=0. (11.7)
There are two important aspects of this definition. The first one is explicitly stated: the
standard state of solute i is defined in an infinitely dilute solution in which i is the only
solute. The second one is also contained in (11.7) but it may be less obvious. It is the fact
that the standard state of a solute depends on the identities of both the solute and the solvent.
Recall that in equation (11.5)wespecified that the infinitely dilute solution is ideal, and
that its activity equals its molality. This implies that the molecules of solute and solvent do
not interact with one another, which is never the case. The energetic contributions of these
interactions, which depend on the nature of the molecules of both solute and solvent, are
lumped into the standard state chemical potential by equation (11.7). Consider for example
N
2
as a dilute solute in two different solvents, liquid H
2
O and methanol (CH
3
OH), at
the same temperature and pressure. The interaction energies of N
2
molecules with H
2
O
molecules and CH
3
OH molecules are different, and therefore the standard state chemical
potential of N
2
in a water solution is different from that in a methanol solution. This is
different from the way in which we have dealt with other solutions, where the standard state
properties depended only on the identity of the substance itself. Now we need to consider

525 11.1 Some properties of dilute solutions
the solute and the solvent but, by requiring that m
j=i
= 0, the standard state properties are
independent of any other solutes.
You may object that equation (11.7) does not necessarily tell us how to measure the
standard state chemical potential at infinite dilution. This is true, but if you think about it
it is also true of all the definitions of standard state properties that we have used so far. In
this book I take the position that we will not worry about this. It is possible to determine
values of standard state chemical potentials, enthalpies, entropies and heat capacities, and
extrapolate them to infinite dilution (see, for example, Robinson & Stokes, 1959; Pitzer,
1995; Anderson, 2005). Standard state values for many species in aqueous solution are
tabulated at the usual reference conditions: 298.15 K and 1 bar (see Wagman et al., 1982;
Robie & Hemingway, 1995). Standard state properties in other solvents are less well known.
What matters to us is that equation (11.7) allows us to operate on the chemical potential of
dilute solutes, and hence perform chemical equilibrium calculations.
We also note that we can expand (11.6) as follows:
µ
i
=µ
0,i
+RT ln
γ
i
m
i
. (11.8)
For a hypothetical ideal 1 molal solution we would have γ
i
=m
i
=1, and therefore µ
i
=
µ
0,i
. Because of this relationship the infinite dilution standard state defined by equation
(11.7) is also referred to as the “1 molal” standard state. This means that the standard state
chemical potential at infinite dilution would also be the chemical potential of the solute in
a hypothetical 1 molal solution in the same solvent that behaved ideally. The actual 1 molal
solution is never ideal, however, even if no other solutes are present. The chemical potential
of a solute in a real 1 molal solution is never equal to its standard state chemical potential.
This may be rather confusing, but it is nothing more than an algebraic manipulation.
11.1.3 Activity coefficient of a dilute solute and excess Gibbs free energy of mixing
From equation (5.129), the excess chemical potential of solute i is given by:
µ
i,ex
=RT ln γ
i
. (11.9)
The relationship between excess chemical potential (a partial molar property) and excess
Gibbs free energy of mixing, G
ex
(Section 5.9.1), is given by equation (5.28):
µ
i,ex
=
∂G
ex
∂n
i
P ,T ,n
j≡i
,n
s
(11.10)
where n
s
is the number of mols of solvent, and n
j
are the number of mols of solutes other
than i. From (11.9) and (11.10) we find:
RT ln γ
i
=
∂G
ex
∂n
i
=
∂G
ex
∂m
i
∂m
i
∂n
i
(11.11)
and from (11.4):
∂m
i
∂n
i
=
1
n
s
M
∗
s
(11.12)

526 Dilute solutions
from which we get:
ln γ
i
=
1
RT n
s
M
∗
s
∂G
ex
∂m
i
P ,T ,m
j≡i
. (11.13)
Therefore, if one has an explicit formula for the excess Gibbs free energy of the solution
(Section 11.6), the activity coefficient of each solute can be calculated with (11.13).
11.1.4 Gases as low-concentration solutes
Consider a molecular species, A, that is present in a gas phase and that dissolves in a
coexisting condensed phase. The usual case is that the condensed phase is a liquid, but the
following treatment is equally valid for solid–gas equilibrium. At equilibrium we have:
µ
A(g)
=µ
A(s)
, (11.14)
where µ
A(g)
is the chemical potential of A in the gas phase, and µ
A(s)
its chemical poten-
tial in the liquid solution. Because at low pressures, say those corresponding to planetary
atmospheres and near-surface environments (including planetary oceans), solubilities of
most common gas species in water and other possible liquid solvents are rather low, it is
convenient to refer the chemical potential of species A dissolved in the liquid phase to the
infinite dilution standard state. We therefore expand (11.14) as follows:
µ
0,A(g)
+RT ln f
A
=µ
0,A(s)
+RT ln a
A(s)
, (11.15)
where µ
0,A(g)
is the standard state chemical potential of pure gaseous A at the temperature
of interest and 1 bar, µ
0,A(s)
is the standard state chemical potential of infinitely dilute A in
the solvent of interest and at the temperature of interest (equation (11.7)), f
A
is the fugacity
of A in the gas phase and a
A(s)
is the activity of solute A in the liquid solution. Writing
activity as the product of molality, m
A
, times activity coefficient, γ
A
, (equation (11.8)) we
rearrange (11.15) as follows:
m
A
f
A
=
exp
−
s
G
0
RT
γ
A
, (11.16)
where
s
G
0
is the standard state Gibbs free energy of dissolution at infinite dilution, defined
as:
s
G
0
=µ
0,A(s)
−µ
0,A(g)
. (11.17)
It is an empirical observation that, at constant temperature and for very dilute solutions
(i.e. as m
A
→ 0), the ratio m
A
/f
A
is approximately constant. This is Henry’s law (Section
5.9.2), and the resulting constant, K
A
H ,s
, is one way of defining Henry’s law constant. We
then have:
m
A
f
A
=K
A
H ,s
, m
A
1, (11.18)
where the constant K
A
H ,s
is equal to the right-hand side of equation (11.16) and therefore
includes contributions both from the standard state Gibbs free energy of dissolution and from

527 11.1 Some properties of dilute solutions
the excess chemical potential of A in the condensed solution (i.e. the activity coefficient).
It follows that the value of Henry’s law constant is specific to each solute–solvent pair.
Henry’s law (equation (11.18)) describes the equilibrium between a molecular species in
a gas and the same molecular species in a dilute condensed solution. The quantity m
A
refers
only to the molality of the molecular species A in the solution. If the species dissociates,
or reacts with the solvent in some other way, then m
A
will be less than the total amount of
dissolved A, which is a quantity known as the analytical concentration of A. This is a key
point, that will become clear in several numerical examples.
Henry’s law constant as defined by equation (11.18) describes the solubility (hence the
subscript s) of the gas in the condensed phase. It is also common to use the inverse of
equation (11.18) and define Henry’s law constant as the ratio f
A
/m
A
as m
A
goes to zero. The
resulting constant, K
A
H ,
v
, describes the volatility of the gas and is simply equal to 1/K
A
H ,s
.
This alternate formulation is used in many thermodynamics textbooks (e.g. Pitzer, 1995;
Anderson, 2005). I prefer the solubility constant defined by equation (11.18) because it is
the convention used in comprehensive data bases for gases in aqueous solutions (see below).
The solubility of most gases in liquids decreases with increasing temperature. Within
the temperature range in which aqueous solutions are stable the effect is not negligible. We
account for the temperature dependency of Henry’s law constant by writing, from equations
(11.16) and (11.18):
ln K
A
H ,s
=−
s
G
0
RT
−lnγ
A
. (11.19)
Differentiating with respect to T, and assuming that γ is constant:
∂ ln K
A
H ,s
∂T
=
1
R
s
S
0
T
+
s
G
0
T
2
(11.20)
or:
∂ ln K
A
H ,s
∂T
=
s
H
0
RT
2
, (11.21)
where
s
H
0
is the molar enthalpy of dissolution at infinite dilution. Using the chain rule
we simplify this further to:
∂ ln K
A
H ,s
∂
(
1/T
)
=
∂ ln K
A
H ,s
∂T
∂T
∂
(
1/T
)
=−
s
H
0
R
. (11.22)
Assuming that the enthalpy of dissolution remains constant we integrate from some
reference temperature T
0
(universally taken as 298.15 K) to the temperature of interest:
K
A
H ,s
=K
A,0
H ,s
exp
−
s
H
0
R
1
T
−
1
T
0
. (11.23)
