Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


488 Melting in planetary bodies
0 5 10 15 2
0
1800
1900
2000
2100
2200
M=m
r
/m
z
Melting temperature (K)
C
z,m
= 10 wt%
C
z,m
= 1 wt%
Melting point depression of forsterite
Fig. 10.3
Melting temperature of forsterite as a function of the parameter M =ratio of molecular weight forsterite, m
r
,to
molecular weight of “impurity” component in the melt, m
z
. The concentration of impurity in the melt is constant
along each curve, at 1 and 10 wt%.
Defining the ratio of molecular weight of major component to molecular weight of impurity
M =m
r
/m
z
, we get:
X
z,m
=
MC
z,m
(
M −1
)
C
z,m
+100
(10.19)
and:
T ≈ T
0
1 −
MC
z,m
(
M −1
)
C
z,m
+100
R
m
S
. (10.20)
We see that the molecular weight of the impurity relative to that of the major component
has a large effect on the magnitude of the melting point depression, simply because a given
mass of impurity represents a greater number of mols the lower its molecular weight is
relative to that of the host. This is shown by the curves of T vs. M in Fig. 10.3. The curves
are calculated with equation (10.20) for values of T
0
= 2174 K and
m
S = 56 J mol
−1
K
−1
, which correspond to melting of forsterite at 1 bar, and constant values of C
z,m
of 1
and 10 wt%.
10.4.2 Effect of partitioning of incompatible trace components on melt generation
In Section 10.3 I was somewhat careless about the thermodynamics of melting. It is now
necessary to clean up the act. Naively, one could think that the “progressive melting” that

489 10.4 The effect of “impurities”
I referred to in Section 10.3 corresponds to melting at constant temperature, as a result
of slow addition of heat (enthalpy of melting). This is not the case, however. Consider an
idealized system in which forsterite is pure Mg
2
SiO
4
. Melting in this system is univariant.At
constant pressure there is a unique temperature at which crystal and melt are at equilibrium,
i.e. the solidus and liquidus temperatures are the same. Suppose now that olivine contains
a trace component dissolved in its crystalline structure. This could be a cation substituting
for Mg, or it could be a molecular species, such as H
2
O or some other volatile component,
accommodated interstitially in its crystalline structure. In any case, we are now dealing
with a two-component system, and since the extra component does not cause a new phase
to form, the melting reaction becomes divariant: solidus and liquidus temperatures do not
coincide, nor will they in general be the same as the melting temperature of pure forsterite.
Whether this thermodynamically inevitable effect of incorporation of trace components is
petrologically significant is another question, to which we now seek answers. This topic
has been explored in detail by Hirschmann and collaborators in a number of recent and
clear contributions (Hirschmann, 2006; Hirschmann et al., 2009; Tenner et al., 2009). Here
I will derive a set of very general equations applicable to simple systems of what I shall
call “one component plus a trace”. By this I mean that the system is binary when the
trace component is included, and that we will compare its behavior to that of the unitary
trace-free system.
We seek an equation for the solidus temperature of a crystal that contains a trace
component dissolved in it. From (10.15) and (10.19) we write:
X
z,m
=
MC
z,i
(
M −1
)
C
z,i
+100D
s/m
(
1 −ϕ
)
+ϕ
. (10.21)
Substituting this expression in (10.17) and setting ϕ =0 yields the desired equation for the
solidus temperature, T
S
:
T
S
=T
0
1 −
MC
z,i
(
M −1
)
C
z,i
+100D
s/m
R
m
S
. (10.22)
This equation allows us to explore the effects of the three parameters, M, D
s/m
and C
z,i
on the melting point of a crystal. Figure 10.4 shows curves of T
S
vs. C
z,i
calculated for
melting of forsterite at 1 bar (T
0
= 2174 K,
m
S = 56 J mol
−1
K
−1
, same as Fig. 10.3).
The horizontal axis extends to a maximum value of 0.1 wt% (=1000 ppm), which makes
it clear that even trace amounts of “impurities” can have a dramatic effect on the solidus
temperature of a crystal (emphasis important, as we shall soon see). The values of the
partition coefficient and of the molecular weight of the trace component relative to that of
the host both have major effects on the magnitude of the melting point depression. The more
incompatible a component is, the more it partitions into the melt and therefore the greater
the melting point depression is. We discussed the effect of molecular weight in Section
10.4.1, but let us now consider specific examples.
10.4.3 Volatiles and melting
Volatiles, and in particular H
2
O, have a strong effect on the melting point of silicate systems.
The key to this behavior is, to a considerable extent, the low molecular weight of volatile
species. What determines the maximum magnitude of the melting point depression is the
solubility of the volatile in the silicate melt at the conditions of interest, i.e. how large C
z,m

490 Melting in planetary bodies
0 0.02 0.04 0.06 0.08 0.1
1800
1900
2000
2100
C
z,i
(wt%)
Melting temperature (K)
Melting point depression of forsterite
M=10
D
s/m
= 0.005
M=10
D
s/m
= 0.01
M=1
D
s/m
= 0.005
M=1
D
s/m
= 0.01
Fig. 10.4
Melting temperature of forsterite as a function of bulk initial concentration of impurity, for different values of the
parameter M, and different solid–melt partition coefficients, D
s/m
.
can get. But for a given mass solubility, the lower the molecular weight of the volatile,
the stronger its effect on the melting point will be. Volatile solubility in silicate melts is a
complicated function of volatile and melt compositions, pressure and temperature. A vast
literature on this topic exists, of which a reasonably recent summary can be found in Volume
30 of Reviews in Mineralogy. Volatile solubilities in silicate melts increase with pressure at
constant temperature. If any generalizations beyond this one are possible, one could say that:
(i) H
2
O solubility in silicate melts is significant (in the wt% level) beginning at pressures
of a few hundred bars, (ii) CO
2
solubility reaches wt% levels only at pressures of order
10 kbar, (iii) fluorine solubility may be comparable to that of H
2
O, whereas chlorine may
be at least one order of magnitude less soluble, (iv) sulfur solubility is strongly dependent
on oxygen fugacity and the oxidation state of iron, and (v) nitrogen solubility may become
quite large only at lower mantle pressures.
Another important consideration is the nature of the sub-solidus host for the volatile
species. One possibility (see Worked Example 6.3) is that a volatile phase exists at equilib-
rium with the rock. If the volatile phase is pure (say, pure H
2
O), then the melt is saturated in
the volatile species. In this case X
z,m
takes its maximum value at the given P and T , and the
solidus temperature takes its minimum value (equation (10.17)). A variation on this theme,
that we discuss in Section 10.7, is a situation in which the volatile phase percolates through
the rock but the volatile mass flux is low enough that the melt does not become saturated
in volatiles, at least initially. Another possibility is that the volatile species is an essential
structural component of one or more of the mineral phases in the rock, for example, micas,

491 10.4 The effect of “impurities”
amphiboles, apatite or carbonates. In this case it does not become available until the host
crystalline phase breaks down. This is dehydration melting (discussed in Worked Exam-
ple 6.3). Chemography requires that in this case the concentration of the volatile in the melt
be lower than the saturation concentration. The dehydration-melting solidus temperature
must therefore be higher than the vapor-saturated solidus (equation (10.17), Fig. 6.9). Yet
another possibility, that may be important in planetary mantles, is that the volatile species
is present as a trace component in a nominally anhydrous mineral.
10.4.4 Melting point depression by trace volatiles
Olivine at upper mantle pressures can dissolve a few hundred ppm of H
2
O, and measured
values of D
olivine/melt
for H
2
O are in the range 0.006 to 0.009; see, for example, Hirschmann
(2006); Hirschmann et al. (2005); Tenner et al. (2009). As pointed out by these authors, a
reliable estimate of the melting point depression is complicated by the fact that the speciation
of H
2
O in silicate melts is far from being completely understood. We can postulate, however,
that the molecular weight of H
2
O species is likely to be much lower than that of silicate
species, so perhaps the curves for M = 10 in Fig. 10.4 yield a reasonable estimate of the
effect of H
2
O on the melting point of forsterite. If this is the case then a few hundred ppm
of H
2
O dissolved in forsterite will lower its solidus temperature by as much as 150–200 K,
which may be significant when considering magma generation in planetary mantles (see
Section 10.6). Suppose, on the other hand, that forsterite contains the same mass of a trace
incompatible cation in solution, for example potassium. We can reasonably assume that
when K enters the melt it does so as a silicate species, so in this case M may be closer to 1.
For a partition coefficient similar to that of H
2
O the melting point depression would be only
∼20 K. These estimates ignore the effect of any excess chemical potential of the major melt
component (i.e. γ
r, m
in equation (10.17)). A value of this factor greater than 1 would raise
the solidus temperatures relative to those in Fig. 10.4, but the behavior depicted in Figure
10.4 is nonetheless qualitatively correct.
These results correspond to generation of an infinitesimal amount of melt at the solidus.
A different question is that of how much melt forms as a function of temperature above the
solidus. For a strongly incompatible component we expect that, as melt fraction increases
above the solidus,the concentration of the trace component in the melt will initially drop very
rapidly (Fig. 10.2), giving rise to a negative feedback effect that will limit melt generation.
We can examine this by substituting (10.21)in(10.17) and solving for the melt fraction, ϕ.
After some uninspiring algebra:
ϕ =
1
1 −D
s/m
C
z,i
100
M
1 −
T
T
0
m
S
R
−M +1
−D
s/m
. (10.23)
Note that this equation blows up for T = T
0
, as it must, for the liquidus (ϕ = 1) must
also be attained at a temperature lower than the melting point of the pure system (T
0
).
The liquidus temperature can be calculated by substituting (10.21)in(10.17) and setting
ϕ =1. It is (obviously) independent of D and for all likely values of C
z,i
and M it is only a
fraction of a degree to a couple of degrees lower than the melting point of the pure system.
Equation (10.23) is plotted in Fig. 10.5, for a constant value of C
z,i
= 0.05 (= 500 ppm).
The plot confirms our suspicion that, even if a strongly incompatible trace component with
a low molecular weight can cause a strong depression of the solidus, the increase in melt

492 Melting in planetary bodies
1950 2000 2050 2100 2150
0
0.02
0.04
0.06
0.08
0.1
Temperature (K)
Melt fraction
Melting point depression of forsterite
C
z,i
= 500 ppm
M=10
D
s,m
= 0.005
M=10
D
s,m
= 0.01
M=1
D
s,m
= 0.005
M=1
D
s,m
= 0.01
Fig. 10.5
Forsterite melt fraction as a function of temperature, for different values of the parameter M and different solid–melt
partition coefficients, D
s/m
. The bulk initial concentration of impurity is 500 ppm in all cases.
fraction above the solidus is painfully slow, requiring perhaps 100 K to generate 1% melt.
The rate of melting for a system in which the impurity is closer in molecular weight to the
major component is greater, but the melting point depression is relatively minor to begin
with. There is no free lunch.
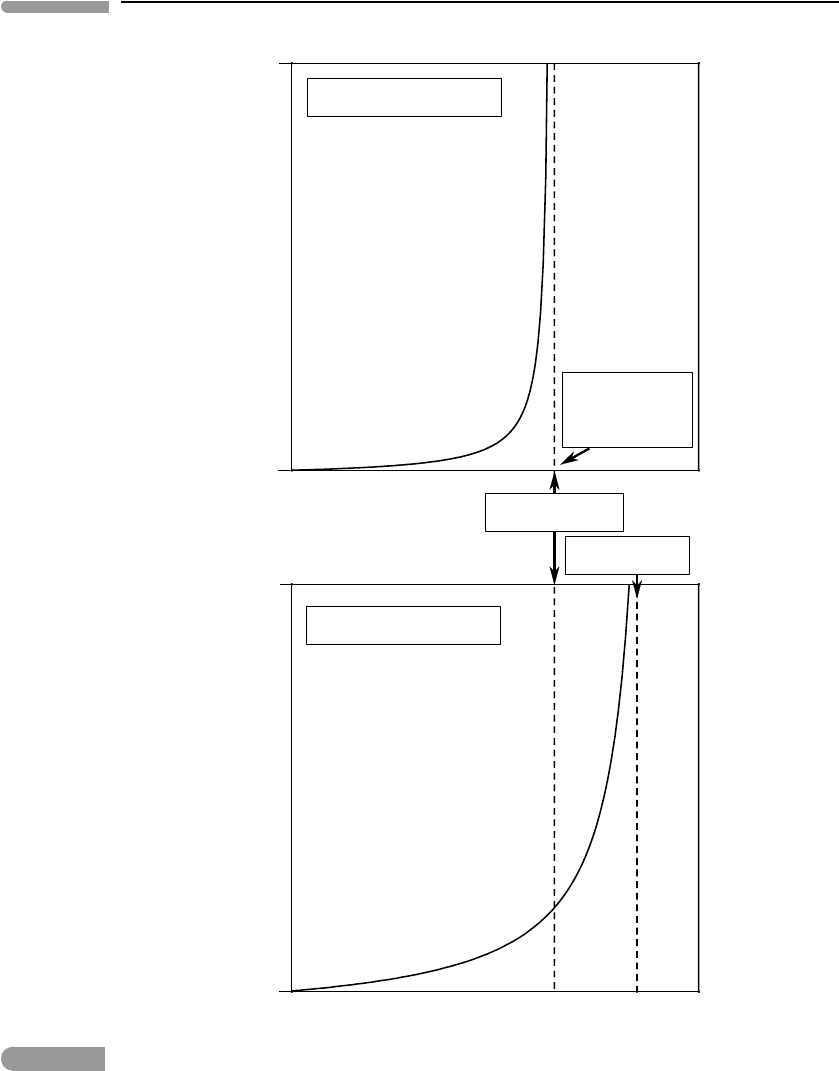
The principles summarized in Figs. 10.4 and 10.5 apply to multicomponent systems, such
as rocks, but there is a potentially important difference with the one-component system,
which is shown schematically in Fig. 10.6. In this figure I use the labels “dry” solidus
and “dry” liquidus to refer to the solidus and liquidus of the system in the absence of a
trace component. As we saw, the latter could be a volatile species, but it could also be
an incompatible trace element, such as an alkali in a magnesium silicate. Regardless of
the number of components in the system, addition of a trace component lowers both the
solidus and the liquidus temperatures. Depression of the solidus can be drastic, depending
on the nature of the impurity, its abundance, and its partition coefficient. Depression of the
liquidus, in contrast, is always vanishingly small, as for ϕ =1 the concentration of the trace
in the melt is always small. The initial rate of increase of melt fraction may be very slow
(see also Fig. 10.5) but, if the solidus depression is large enough, the melt fraction at the
temperature of the dry solidus of a multicomponent system may be substantial. One way of
looking at this is that the importance of trace amounts of volatiles in nominally anhydrous
mantle phases is not so much that they lower the solidus temperature as the fact that they
raise the amount of melt produced at temperatures near that of the dry solidus. Quantifying
these effects, however, requires a detailed thermodynamic model of multicomponent silicate
melts, which is beyond the scope of this book (see, for example, Hirschmann et al., 1998,
1999a,b).
493 10.4 The effect of “impurities”
"Dry" solidus
"Dry" liquidus
T
T
One component system
Multicomponent system
0
1
0
1
ϕ
ϕ
"Dry" liquidus
=
"Dry" solidus
Fig. 10.6
Schematic diagrams comparing variation in melt fraction with temperature in one-component and multicomponent
systems. Bulk initial concentration of impurity and all other parameters are the same in both diagrams. Liquidus
depression is negligible in both cases.

494 Melting in planetary bodies
10.5 Melting in planetary interiors
In Chapter 2 we discussed the sources of thermal energy in planetary interiors. With the
exception of catastrophic events, such as planetary-sized impacts, core formation, tidal
de-spinning and perhaps decay of abundant short-lived isotopes, all of which may have
been common in the early Solar System, the rate of heating of planetary interiors is not
sufficient to cause wholesale melting. Formation of magmas is always localized and entails
less than complete melting of the parent solid assemblage. We seek to understand what
causes localized partial melting in planetary interiors.
The ratio H
fusion
/C
P
for silicate minerals is of order 10
2
–10
3
K. Melting a given
amount of rock requires the same amount of energy as raising its temperature by hundreds
of degrees. This is a key part of the explanation for why magmas typically do not carry
significant superheat, or in other words, that eruption temperatures are not normally above
liquidus temperatures. It also tells us that melting must have a considerable effect on a
planet’s thermal gradient. Let us define our thermodynamic system as a region of the inte-
rior of a planetary body in which partial melting takes place. Focusing on its fundamental
thermodynamic aspects we can consider three simple end-member situations. First, melt-
ing may take place in an open system, in which there is mass and heat transfer across its
boundaries. This typically involves influx of a volatile phase that lowers the melting point
of the solid assemblage (Section 10.4.4). Melting above terrestrial subduction zones may
largely occur in this way. Second, melting may occur in a closed but non-adiabatic system,
i.e. one that can exchange heat but not matter with its environment. This may be a magma
generation process at major planetary-scale compositional discontinuities, at which jumps
in density, melting point and rheological properties may allow the juxtaposition of advec-
tive and diffusive heat-transfer regimes. Partial melting of the Earth’s continental crust
by ponding of mafic magmas near the Moho may be an example. The problem with this
mechanism is that it relies on diffusive heat transfer into the region that undergoes melting,
and the very long time scales for heat diffusion may render it inoperable (Chapter 12). One
way of getting around this difficulty is to decrease the diffusive lengthscale which would
be the case, for example, if mafic magmas intrude the crust as a complex of closely spaced
dikes. Finally, melting may occur under adiabatic conditions if the solidus of the mineral
assemblage intersects the adiabatic thermal gradient. This process, called decompression
melting, is likely to be the most widespread magma generation process in convective plan-
etary bodies. It is responsible for magmatism at Earth’s mid-ocean ridges, hot spots and
large igneous provinces, such as continental flood basalts and oceanic plateaus. It is almost
certainly responsible for magmatism in Venus and Mars, and perhaps for Io’s volcanoes as
well. Because of its importance we will discuss this process first.
10.6 Decompression melting
10.6.1 Fundamental relations
In Chapter 3 we derived expressions for the adiabatic temperature gradient, as a function
of either depth (equation (3.35)) or pressure (equation (3.32)). In Chapter 4 we saw that
these equations correspond to a specific type of adiabatic process, during which entropy

495 10.6 Decompression melting
is constant (Worked Example 4.6), and that adiabatic but not-isentropic transformations
are also possible (Section 4.4). We have calculated and used adiabatic thermal gradients in
several discussions without paying much attention to this distinction, which is generally
safe to do in systems in which neither inelastic deformation nor phase separation take place.
We can ask, however, is decompression melting truly adiabatic (see, for example, Asimow,
2002; Stolper & Asimow, 2007)? Of course, if a transformation is not adiabatic it cannot
be isentropic, as heat exchange entails entropy generation, but even if melt generation is
an adiabatic process, is it isentropic? We shall address these questions in a later section,
but the isentropic approximation is an excellent starting point. This is so, first and fore-
most, because the mathematics are simple, allowing us to focus on the physics of melt
generation during mantle upwelling. Second, in many instances decompression melting is
approximately isentropic, at least locally. Third, it is relatively straightforward to start with
the isentropic approximation and add to it the effects of entropy gain or loss arising from
exchange of heat and matter with the environment, or from energy dissipation. Through-
out this discussion, and unless otherwise stated, I will continue to use the terms adiabat,
and adiabatic decompression melting, to mean adiabatic and isentropic, as this is common
throughout the literature. When necessary I will explicitly state whether departures from
the constant entropy assumption need to be taken into consideration.
Using equation (3.35) and the thermodynamic properties of forsterite yields a characteris-
tic adiabatic gradient for the Earth’s upper mantle of ∼0.4 K km
−1
, or, using equation 3.32,
∼1.5 K kbar
−1
. The measured volume and entropy of melting of upper mantle minerals
(forsterite, diopside, enstatite and spinel) yield Clapeyron slopes for their melting reactions
(equation (5.6)) of 50–100 bar K
−1
, or equivalently 10–20 K kbar
−1
. Clearly, the adia-
bat and the melting curve for the mantle can intersect. Whether and where they intersect,
however, and what happens next, depend not only on their relative slopes but also on their
absolute locations.
We will consider melting under Earth’s mid-ocean ridges as our reference model – after
all, this is where most of Earth’s volcanic activity takes place. Let us assume that the
oceanic lithosphere extends to a depth of 150 km, and that the temperature at the base of the
lithosphere is 1650 K (= 1377
◦
C, see Chapter 3). The pressure at that depth is ∼50 kbar
(Chapter 8). From these values and a slope of 1.3 K kbar
−1
we derive the mantle adiabat
shown in Fig. 10.7. The intersection of the mantle adiabat with the Earth’s surface (P =0)
defines the mantle’s potential temperature, which in our example is T
p
=1312
◦
C. This is
the temperature that the mantle would have if it were allowed to decompress adiabatically
(and, remember, isentropically) to the planet’s surface, i.e. if the lithosphere did not exist
and phase changes did not take place. This may not happen, but knowing the potential
temperature is important because, given that an adiabat is fully determined if we specify a
single {P , T }point on it (Section 3.5), T
p
allows us to compare the thermal state of different
regions of a convective mantle, as well as the mantles of different planetary bodies. In other
words, comparing how much hotter or colder different parts of a convective region are
requires that we specify the pressure at which we make the comparison, and we choose zero
as the reference pressure.
In Section 3.7.2 we defined the lithosphere as the thermal boundary layer for mantle
convection, meaning that heat transfer across the lithosphere is by diffusion. If we ignore
radioactive heat production (which, if not quite right, is not altogether unacceptable for the
oceanic lithosphere) then the equilibrium lithospheric geotherm is a straight line. In Fig. 10.7
I show this conductive geotherm with a straight line joining the 1650 K temperature at the
base of the lithosphere to a surface temperature of 300 K. This translates to a heat flux of

496 Melting in planetary bodies
1100 1200 1300 1400
0
10
20
30
40
50
T(°C)
P (kbar)
asthenosphere
lithosphere
garnet lherzolite
spinel lherzolite
conductive geotherm
adiabat
model peridotite
solidus
50 bar K
–1
100
bar
K
–1
T
p
basalts
Fig. 10.7
Simplified thermal conditions in the Earth’s oceanic upper mantle. The thick “dog-leg” line shows the geotherm far
away from a mid-ocean ridge. Under a mid-ocean ridge adiabatic upwelling of the mantle intersects the peridotite
solidus. If the adiabat were able to reach the surface unperturbed its temperature would be the potential
temperature, T
p
. The difference between T
p
and basalt eruption temperatures (range shown with an arrow) reflects
the enthalpy of melting.
∼50 mW m
−2
, which is a bit on the low side for old ocean floor. Including radioactive
heat production in the lithosphere would probably raise the value to the right ballpark, but
this is not important for the present discussion. What is important is that the geotherm
in an old sector of ocean floor, effectively infinitely removed from any region of active
magma generation, would look like the thick “dog-leg” line in Fig. 10.7, composed of an
adiabatic (advective) segment capped by a diffusive segment. There are places, however,
where advective flow is able to penetrate to depths that are significantly less than that of the
“typical” lithospheric base. This process may be driven either from “above”: the lithosphere
thins in response to stretching, or from “below”: the rate of heat advection is high enough
to thermally “erode” the lithosphere. For our purposes it does not matter which is the case.
What matters is that under those circumstances the adiabatic thermal gradient will extend
to shallower depths, as shown by the line labeled “adiabat” in Fig. 10.7.
The figure also shows the dry model peridotite solidus, after McKenzie and Bickle (1988).
The adiabat and the solidus intersect at a pressure of ∼17 kbar, indicating where decom-
pression melting begins during mantle upwelling. The intersection occurs in the spinel
lherzolite field. This is consistent with the phase equilibrium and geochemistry of MORBs,
suggesting that the processes and thermal conditions summarized in Fig. 10.7 are a feasible
model for melting under Earth’s mid-ocean ridges. The zero-pressure solidus temperature
is 1100
◦
C. Hypothetical solidi going through this temperature and with Clapeyron slopes
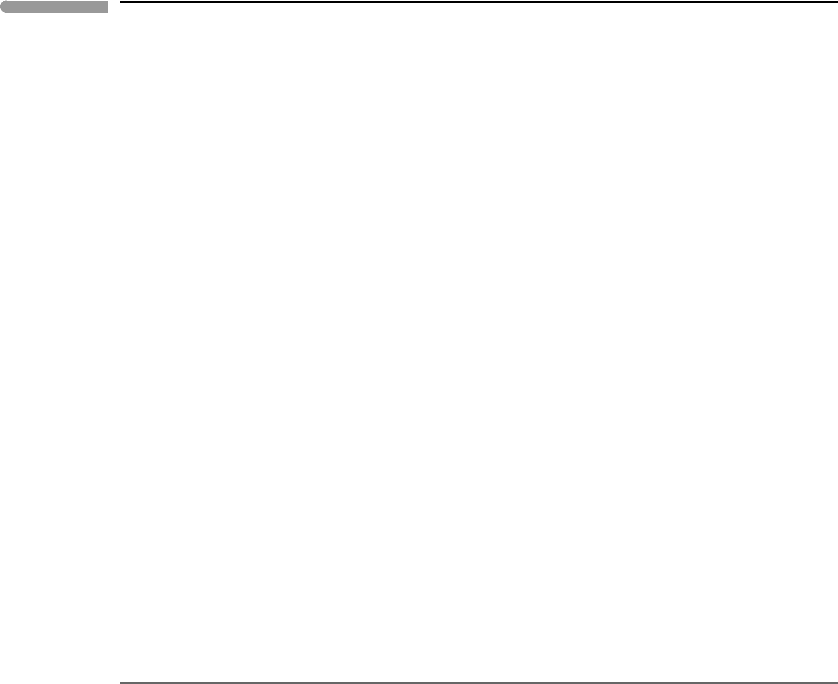
497 10.6 Decompression melting
of 50 and 100 bar K
−1
, which bracket the slope range for typical mantle minerals at low
pressure, are also shown. McKenzie and Bickle’s model solidus is almost exactly in the
middle, which may be reassuring at some (subjective) level.
If the enthalpy of fusion of rocks were zero and the melts formed by decompression
melting rose isentropically then they would reach the surface at the mantle’s potential
temperature. This is not the case. Basalt eruption temperatures are typically ∼1200
◦
C. The
difference between this temperature and the mantle’s potential temperature must reflect at
least in part the conversion of sensible heat to latent heat during melting, as first-order phase
transitions are always accompanied by a non-zero enthalpy change. We will discuss this
issue in detail in the next section, but before doing so there are a few more things to learn
from Fig. 10.7.
First, the eruption temperature of a magma formed by adiabatic decompression melting
must be lower than the potential temperature of its mantle source region. Thus, whereas
Fig. 10.7 may be able to account for melting under mid-ocean ridges, it cannot explain
high-Mg basaltic lavas with eruption temperatures in excess of 1300
◦
C, let alone ultramafic
komatiite lava flows. These require mantle potential temperatures significantly higher than
1312
◦
C, which may have been the norm in the Archaean mantle (Worked Example 3.4).
Second, mafic lavas exist that have major and trace element characteristics indicative of
having formed from garnet-bearing peridotites. Figure 10.7 shows that melting in the garnet
lherzolite field can take place through a combination of higher potential temperature, steeper
Clapeyron slope and lower solidus temperature at a given pressure. In view of our discussion
in Section 10.4, the fact that some of these deep magmas tend to be rich in incompatible
elements (e.g. K) and dissolved volatiles may be significant.
10.6.2 Batch decompression melting of a one-component system
We initially consider decompression melting under isentropic conditions. This requires that
the melt remain in the system and in equilibrium with the solid, a process that as we saw
in Section 10.3 is known as batch melting. We will later relax this constraint and allow the
melt to leave the system at the same rate as that at which it is produced. This process, known
as fractional melting, is obviously not adiabatic, and hence not isentropic.
Imagine an idealized planetary mantle composed of a single component (Fig. 10.8). At
pressures greater than P
i
the temperature is below the solidus and the mantle consists of a
single solid phase, so that it is a divariant thermodynamic system. Upwelling of solid mantle
occurs along an adiabat with potential temperature T
p
, from A to B in the figure. At point
B the adiabat intersects the solidus at temperature T
i
and, as a second phase appears, the
system becomes univariant. Adiabatic decompression beyond the intersection point cannot
follow the adiabat calculated with equation (3.32). Rather, it must be constrained to the
solidus (which in this case is also the liquidus), from B to C in Fig. 10.8. It is important
to realize that the two-phase segment of the decompression path, BC, is also adiabatic.
However, it is not described by equation (3.32) because this equation does not take into
consideration the enthalpy of fusion.At point C, when the upwelling mantle reaches pressure
P
f
, melting is complete and the system becomes divariant once more. Further ascent, from
C to D, occurs along another adiabat calculated with equation (3.32), but with a different
potential temperature, T
p,m
, and perhaps a different slope arising from differences in material
properties between solid and liquid. The drop in potential temperature from the AB adiabat
to the CD adiabat reflects the enthalpy absorbed by the melting phase transition.
