Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

428 Thermodynamics of planetary volatiles
The effect of pressure on oxygen fugacity along the three buffering reactions at a constant
temperature of 1000
◦
C is shown in the bottom panel of Fig. 9.3. From 1 bar to 100 kbar
(which is within the range of pressures that Holland and Powell’s data base is expected
to be reliable) oxygen fugacity along the QFI buffer reaction stays several orders of mag-
nitude below the other two, but the QFM and HM buffers intersect at P ≈ 57 kbar. This
is an intersection between two pseudounivariant reactions (Section 6.3.1 ), that defines a
pseudoinvariant point. The HM reaction is degenerate, so it crosses the pseudoinvariant
point without becoming metastable, but the QFM reaction must become metastable at the
pseudoinvariant point. Two other reactions, magnetite-absent and oxygen-absent, must also
radiate from the pseudo-invariant point. These reactions, shown in Fig. 9.3 in their correct
calculated locations, are:
2Fe
2
SiO
4
+O
2
→
←
2Fe
2
O
3
+2SiO
2
(9.20)
2Fe
3
O
4
+SiO
2
→
←
2Fe
2
O
3
+Fe
2
SiO
4
. (9.21)
The first reaction is an analog to the QFM buffer which is not stable in the Earth’s crust
and shallow mantle, but that can be expected to substitute for QFM as the oxide–silicate
buffer at greater depths. The second reaction is called an iron disproportionation reaction.
It entails no change in oxidation state, but rather the segregation of Fe
2+
and Fe
3+
, that
occur together in magnetite, into a ferric phase (hematite) and a ferrous phase (fayalite).
Because it is oxygen-absent it plots parallel to the f (O
2
) axis.
9.2 Liquid–vapor equilibrium. Critical phase transitions redux
In Section 1.15, see in particular Fig. 1.16, we saw that non-ideal gases span a range of
densities between those of ideal gases and liquids. In order to develop equations of state
appropriate for non-ideal gases, then, we must first discuss the nature of the liquid–gas
phase transition in some detail. We recall that gases are non-condensed fluids because
they expand indefinitely as pressure decreases, whereas we call liquids condensed fluids
because they do not behave in this manner. A vapor is a gas in equilibrium with its liquid.
As temperature increases, the properties of liquid and vapor at equilibrium approach each
other, until the two phases become indistinguishable at the critical temperature. Above the
critical temperature a single fluid phase is stable, called a supercritical fluid. The density of
supercritical fluids may vary from values typical of liquids to values typical of ideal gases.
The defining property of a supercritical fluid is that in it there is no discontinuous phase
transition separating both states. This behavior is reminiscent of that of solid solutions and
order–disorder transitions in crystalline solids (Chapter 7).
9.2.1 The van der Waals equation of state
Ideal gases do not condense at any temperature or pressure, as by hypothesis they lack
intermolecular forces. Thus, in contrast to the ideal gas EOS, we require that any EOS for
a real gas be able to reproduce condensation and the existence of a critical phase transition.
The simplest equation of state that can achieve this was proposed by the Dutch physical
chemist J. D. van der Waals in 1873. The van der Waals EOS is not quantitatively accurate,

429 9.2 Liquid–vapor equilibrium
but thanks to its simplicity and its correct qualitative behavior it remains a powerful tool
with which to gain insight into the behavior of real fluids. Some of the more refined EOS
that we will discuss in later sections follow the van der Waals equation in spirit, if not in
algebraic detail.
We begin by defining the (poorly named) compressibility factor of a gas, Z, as follows:
Z ≡
PV
RT
. (9.22)
For an ideal gas, Z = 1. In a real gas we expect that there are both attractive and repulsive
forces between molecules, that are not present in an ideal gas. Intermolecular repulsion will
cause Z to increase, whereas attraction will cause it to decrease. We can then write Z for a
real gas as follows:
Z = 1 +Z
repulsion
−Z
attraction
. (9.23)
The idea behind van der Waals’equation, and in fact behind most EOS for real gases, is to find
explicit expressions for the Z terms that match the experimentally measured behavior of
fluids over as wide a range of conditions as possible. Many successful approaches, beginning
with that of van der Waals himself, entail some combination of intuition, educated guesses
and trial and error.
As both the repulsive and attractive terms arise from intermolecular potentials, it is
reasonable to postulate that the two Z terms will vary inversely with some power of
volume: the closer the molecules are, the stronger they will interact. This is essentially the
same argument used to derive the Mie EOS, e.g. equation (8.47). Note that, just as in that
case, the qualitative statement about the relationship between volume and intermolecular
potentials says nothing about the actual value of the exponents. The van der Waals EOS
assumes that both the attractive and repulsive terms go as V
−1
, but the value of the exponent
could be different, and in fact this is one of the problems with this equation.
Real gas molecules have a finite volume, which in the van der Waals equation is defined
as the distance at which intermolecular repulsion becomes infinite: no matter how high
we make the pressure we cannot squeeze molecules any closer than this value. The total
molecular volume defined in this way for a mol of gas is called the excluded volume and
symbolized with b. We write the repulsive term as follows:
Z
repulsion
=
b
V −b
. (9.24)
This equation gives the desired behavior: repulsion becomes stronger with decreasing vol-
ume, and diverges as the excluded volume is approached (the term b must appear in the
numerator too because Z is a dimensionless number). We can immediately see two pitfalls
in this equation: first, molecules are assumed to be perfectly rigid, and second, the effect of
temperature, which is likely to affect the b parameter, is ignored. We shall return to this in
later sections.
In contrast to repulsion, the effect of attractive forces is assumed to decrease with
increasing thermal agitation, so we write:
Z
attraction
=
a
RT V
, (9.25)
where a is a constant with the units required to make Z non-dimensional. Again there are
pitfalls that we will address later: a is assumed to be independent of temperature, and the
430 Thermodynamics of planetary volatiles
exponents of T and V are arbitrarily assumed to be −1. Ignoring all of these problems we
substitute in (9.23) and obtain:
Z = 1 +
b
V −b
−
a
RT V
, (9.26)
which, by using (9.22), we recast as follows:
P =
RT
V −b
−
a
V
2
. (9.27)
Equation (9.27) is the van der Waals EOS. We shall now see what this equation predicts
about the behavior of fluids, following a path that parallels our prior discussions of critical
phase transitions in Chapter 7.
9.2.2 The critical point of a van der Waals fluid
For a system at equilibrium the derivative (∂P /∂V )
T
must be negative. This is easy to
prove formally from the equilibrium conditions for Helmholtz free energy (Exercise 9.4),
but we can also accept it on the grounds of physical intuition: in a system at equilibrium
volume can only decrease in response to an increase in pressure. For the ideal gas EOS
this condition is always true, but the van der Waals EOS may have a positive (∂P /∂V )
T
derivative under certain circumstances. To see why, and what these circumstances may be,
we differentiate (9.27):
∂P
∂V
T
=−
RT
(
V −b
)
2
+
2a
V
3
. (9.28)
It is obvious that (9.28) may take positive or negative values, according to the relative
magnitudes of T and V. Inside any T –V region over which the derivative is positive a single
fluid phase cannot exist at equilibrium (note the emphasis on single). In order to identify
such regions we note that the derivative vanishes where it changes sign, so we make (9.28)
equal to 0 and solve for T:
T =
2a
R
(
V −b
)
2
V
3
. (9.29)
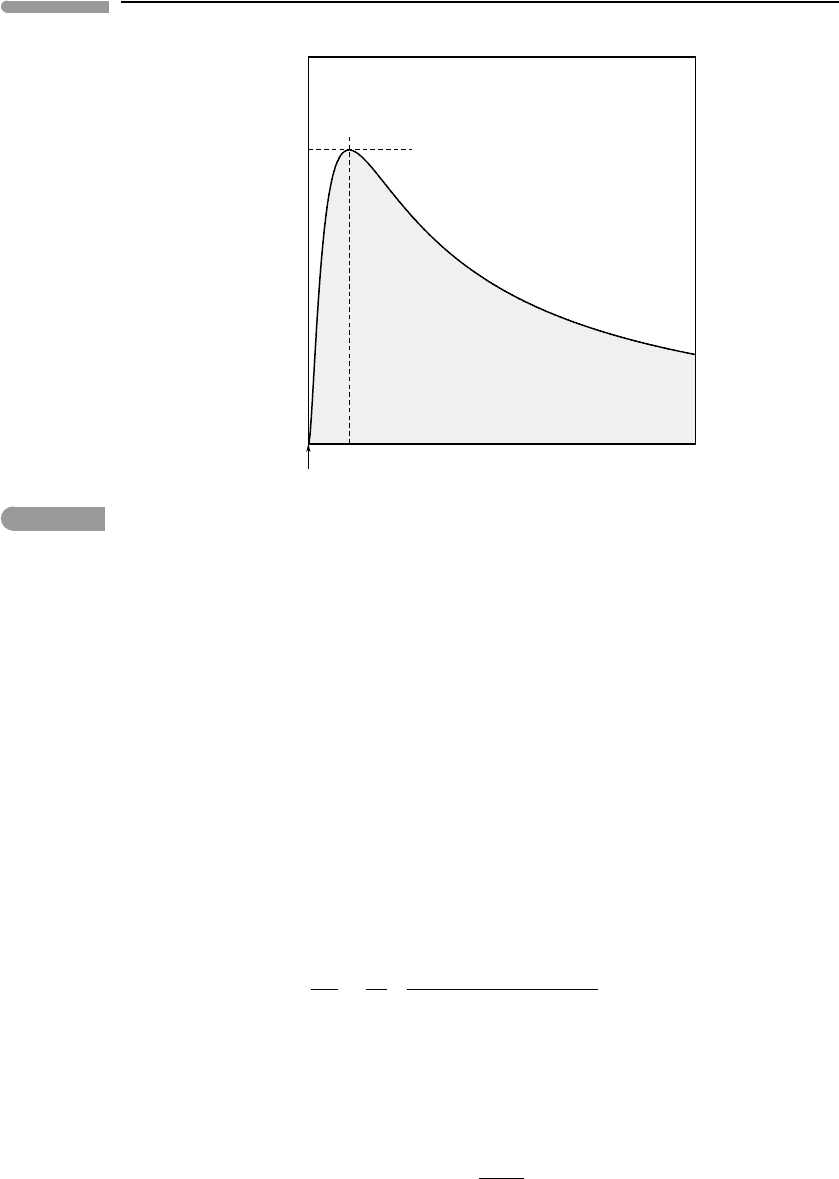
The shape of this function is shown in Fig. 9.4. The origin of the coordinate system is fixed
at T = 0 and V = b, as the region V<bis not physically meaningful. By construction,
(∂P /∂V )
T
vanishes along the curve, it is negative for temperatures above the curve and
positive for temperatures below it. Thus, the region under the curve in Fig. 9.4 is the
T –V region within which a single fluid cannot exist at equilibrium, which I will call the
“prohibited region”.
The peak of the curve is the maximum temperature at which (∂P /∂V )
T
may take non-
negative values. For temperatures higher than this value, let us call it T
c
, (∂P /∂V )
T
is
negative for all values of V. This means that for T>T
c
a single fluid phase that obeys van
der Waals’ EOS (equation (9.27)) is stable for all V and P. By “being stable for all V and P ”
I mean that we can vary the intensive variables continuously and, as long as we stay inside
the region T>T
c
there will be no discontinuity in the material properties of the phase. We
shall return to this, but for now we notice that the properties of equation (9.27) assure us
that this continuity is certainly true of density.
431 9.2 Liquid–vapor equilibrium
V
T
V=b
T
c
V
c
Single phase not stable within this region
Fig. 9.4
Conditions for reversal of the sign of the derivative (∂P/∂V)
T
for a van der Waals gas. In the shaded region below
the curve the derivative is positive, implying that a single fluid phase cannot be stable. The peak of the curve is the
critical temperature: above this temperature a single fluid phase of any density can be stable.
For T<T
c
there is a “prohibited” volume (or density) interval within which a single
phase is not stable. Any volume inside this region can, however, be obtained algebraically as
a linear combination of two arbitrary volumes outside and on opposite sides of the curve – in
particular, by two volumes that satisfy equation (9.27) at the same temperature and pressure
(the equation is cubic in V, so it must have either one or three real roots). Note that these
volumes are not the two intersections of a temperature coordinate with the curve in Fig. 9.4,
as these intersections simply define the condition (∂P /∂V )
T
= 0. The two volumes that
we seek correspond to two stable phases of different densities, so they must lie outside of
the curve, and they must be such that they minimize the free energy of the system. We shall
return to this crucial point shortly.
At the temperature T
c
the system changes from one in which two different fluids are
possible (for T<T
c
) to one in which only one fluid is possible (for T>T
c
). This looks a
lot like a critical temperature, and in fact it is. In order to find its value we need to locate
the maximum of the curve in Fig. 9.4, so we differentiate (9.29) and equate to 0:
dT
dV
=
2a
R
2V
(
V −b
)
−3
(
V −b
)
2
V
4
=0. (9.30)
The solution to (9.30) is the critical volume V
c
, i.e. the volume at the critical temperature:
V
c
=3b. (9.31)
Substituting in (9.29) we get the critical temperature, T
c
:
T
c
=
8a
27Rb
(9.32)

432 Thermodynamics of planetary volatiles
and using (9.31) and (9.32)in(9.27) we get the critical pressure, P
c
:
P
c
=
a
27b
2
. (9.33)
The set {T
c
,P
c
,V
c
} gives the coordinates of the critical point of a van der Waals gas with
constants a and b. In the next section we will show rigorously that a single supercritical fluid
phase is stable at T>T
c
, whereas two subcritical phases, a liquid and its vapor, can exist at
equilibrium for T<T
c
.At the temperature T
c
the properties of the subcritical phases become
identical, marking the critical phase transition. Before we demonstrate these conclusions
formally it is important to focus on some of the similarities between the critical point of a
fluid and those that we discussed in Chapter 7, for solutions and for order–disorder phase
transitions in crystals.
We recall that the Helmholtz free energy, F, is a function of the natural variables T and
V, such that (see Section 4.8.6 ):
∂F
∂V
T
=−P . (9.34)
Thus:
∂
2
F
∂V
2
T
=−
∂P
∂V
T
=
RT
(
V −b
)
2
−
2a
V
3
(9.35)
and:
∂
3
F
∂V
3
T
=−
2RT
(
V −b
)
3
+
6a
V
4
. (9.36)
Substituting the values of T
c
and V
c
we find that both the second and third derivatives of
free energy vanish at the critical point. This is the same behavior that we found at the critical
point of a solution, and at order–disorder critical phase transitions in general (Chapter 7). We
recall that the order parameter is a quantity that vanishes at the critical point and takes non-
zero values only for T<T
c
. For example, the difference in the compositions of coexisting
phases or the difference in the dimensions of the crystallographic axes. For a fluid we
may take the difference in density, or molar volume, between the two subcritical phases,
liquid and vapor, as the order parameter (see Fig. 7.8). Note also that, because the second
derivative of free energy (=−∂P /∂V ) vanishes at the critical point, so does the isothermal
bulk modulus. Equivalently, the compressibility becomes infinite. Divergence of quantities
such as compressibility and heat capacity (Fig. 7.9) is a characteristic of critical phenomena.
9.2.3 The phase diagram of a van der Waals fluid
To continue our study of real fluids it is best to recast van der Waals’ EOS into non-
dimensional form. We define the non-dimensional pressure, π, volume, φ, and temperature,
τ, as the ratios P /P
c
, V/V
c
and T/T
c
, respectively. The variables π, φ and τ are also known
as reduced variables (do not confuse this φ with the fugacity coefficient that we defined in
Section 9.1). With these coordinate transformations the van der Waals EOS becomes:
π =
8τ
3φ −1
−
3
φ
2
. (9.37)
433 9.2 Liquid–vapor equilibrium
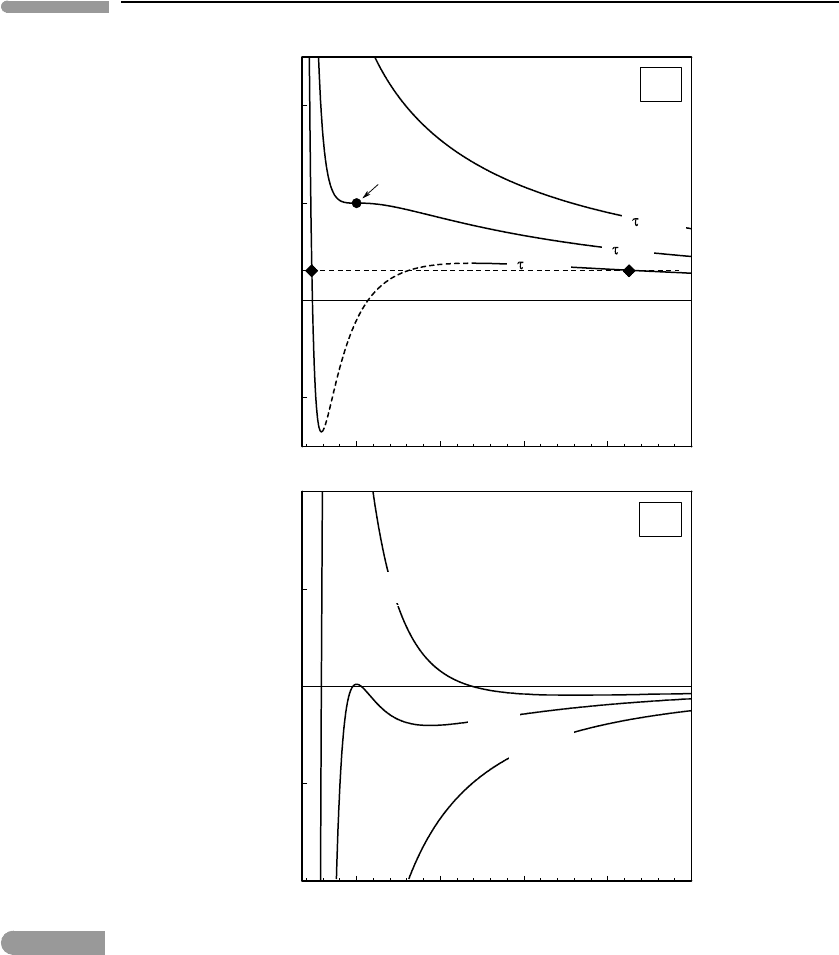
1234
5
–1
0
1
2
1234
5
–1
0
1
=1.5
=1
=0.7
τ = 0.7
∂π /∂φ
critical point
(a)
(b)
τ =1
τ = 1.5
π
φ
φ
Fig. 9.5
Reduced pressure of a van der Waals gas (a) and its volume derivative (b), plotted as a function of reduced volume.
For a given temperature below the critical temperature (e.g. τ = 0.7) the width of the “prohibited” region in Fig. 9.4
corresponds to the dashed segment of the curve in (a). For any pressure between 0 and the local maximum of the
isothermtherearetwo phases atequilibrium.Anexample is shownbythetwo diamonds joinedbythethin dashed line.
Because the parameters a and b disappear in this equation, this would be a universal EOS if
all fluids followed van der Waals behavior (i.e. equation (9.26)) exactly, but unfortunately
this is not the case. We will need to develop more complex EOS to deal with real fluids in a
quantitative fashion. In the meantime (9.37) is an excellent tool with which to gain physical
insight.

434 Thermodynamics of planetary volatiles
Figure 9.5a shows π =π(φ) calculated with (9.37) along three isotherms, and Fig. 9.5b
shows (∂π/∂φ)
τ
along the same three isotherms. The supercritical isotherm, τ = 1.5, is
reminiscent of the hyperbola P ∼ 1/V that describes an ideal gas. Its slope is always
negative, implying that a single phase of continuously variable density is stable everywhere
along the isotherm. The critical isotherm, τ =1, tends to become parallel to the supercritical
isotherm at low densities (large φ) but is generally steeper than the latter at high densities.
This hints at the existence of two distinct fluids: a highly incompressible high density
fluid for which (∂π/∂φ)
τ
→−∞and a fairly compressible low density fluid for which
(∂π/∂φ)
τ
approaches a small negative value (see Fig. 9.5b). One could identify the former
with a condensed phase (liquid) and the latter with a non-condensed phase (gas), but along
the critical isotherm there is continuity between both states, because there is no first-order
phase transition between them. To see this, note that the critical point, τ = π = φ = 1, is
the only point on this isotherm for which (∂π/∂φ)
τ
= 0 and, since this point corresponds
to a maximum of the first derivative function (Fig. 9.5b), the second derivative vanishes
too, as we expected from (9.36). At the critical point the bulk modulus vanishes, but since
it never becomes negative there is no region of the critical isotherm inside which a single
fluid phase is prohibited from existing.
Along the subcritical isotherm, τ = 0.7, there is an interval, shown by the broken seg-
ment in Fig. 9.5a, inside which (∂π/∂φ)
τ
is positive, as seen in the corresponding curve
in Fig. 9.5b. This “prohibited” interval extends from a minimum to a maximum on the
isotherm, as it must given that (∂π/∂φ)
τ
vanishes at both ends of the interval. Of course, it
is possible to have an equilibrium system with {τ,π,φ} coordinates inside this region, but
the thermodynamic relations summarized in Fig. 9.5, which we will explore in more detail
in a moment, mandate that this system cannot consist of a single phase. It must be made up of
two phases that lie on the same isotherm but on opposite sides of the prohibited region, and
at the same pressure. In this case we can identify the low-volume phase as a liquid and the
high-volume phase as its vapor. Note, however, that the diagrams in this figure by themselves
cannot tell us what the volumes of the phases that coexist at equilibrium are. In particular,
these are not the volumes at the ends of the prohibited interval, first because they do not lie
at the same pressure and second because, in this case, one of the volumes corresponds to
the unphysical condition π < 0. The latter is not a general constrain, as isotherms closer to
τ =1 never take negative π values, but the former is (as an aside, negative pressures are not
altogether impossible, but their magnitude is limited by the cohesiveness of the material).
There is a π interval, between 0 and the maximum on the isotherm located at the right end
of the broken segment, within which (9.37) has two solutions in φ for each value of π,on
opposite sides of the prohibited interval. The thin dashed line is an example, with the two
solutions shown by the diamonds. The phase rule assures us that one and only one of these
solution pairs along each isotherm represents thermodynamic equilibrium, as we have a
system of one component and two phases, and therefore one degree of freedom, which we
have chosen to be the temperature. The phase rule does not tell us, of course, which is
the equilibrium pair. What we can be certain about, however, and what characterizes the
subcritical region, is that the two phases must be separated by a first-order phase transition,
because there is a discontinuity in volume and hence in enthalpy and entropy.
In order to find the volumes of coexisting liquid and vapor at equilibrium we begin by
calculating the Helmholtz free energy of the fluid. This we do by integrating (9.34) and
substituting the van der Waals EOS (equation (9.27)). The result is (check with Maple):

435 9.2 Liquid–vapor equilibrium
F =−
V
V
0
PdV =−RT ln
(
V −b
)
−
a
V
+
F
0
+RT ln
(
V
0
−b
)
+
a
V
0
, (9.38)
where F
0
, V
0
are the Helmholtz free energy and volume at some arbitrary reference state.
Let us now define a non-dimensional Helmholtz free energy, W = F/RT
c
. Substituting
(9.31) and (9.32)in(9.38) we find:
W =−τ ln
(
3φ −1
)
−
9
8φ
+
W
0
+τ ln
(
3φ
0
−1
)
+
9
8φ
0
. (9.39)
We can, without loss of generality, set the constant term in square brackets equal to zero –
this will move the curve of W(φ) up or down, but will not change its geometric properties.
We also find, using the chain rule and required substitutions:
∂W
∂φ
=
∂W
∂F
∂F
∂V
∂V
∂φ
=−
3
8
π, (9.40)
which is the non-dimensional equivalent of (9.34).
The left-hand-side panels in Fig. 9.6show W(φ) calculated for three different values
of τ. It is now important to recall that, whereas Gibbs free energy defines the equilib-
rium condition at constant temperature and pressure, Helmholtz free energy does the same
thing at constant temperature and volume (Sections 4.8.5 and 4.8.6 ). This means that the
equilibrium state of a system at constant temperature and volume is the one that mini-
mizes Helmholtz free energy, F, or its non-dimensional avatar, W. We see in Fig. 9.6 that,
for τ > 1, i.e. above the critical temperature, the Helmholtz free energy has continuously
negative curvature, meaning that at any given volume there is only one possible stable
phase. The situation is different below the critical temperature, τ < 1. There are now two
inflection points, and two points on the curve that have a common tangent. Because the
tangent to the Helmholtz free energy curve is the negative of the pressure (equation (9.40))
the φ coordinates of these two points are the volumes of two different fluids at the same
pressure. Moreover, for all volumes in between these two the Helmholtz free energy of a
combination of these two fluids (i.e. a point on the common tangent) is lower than that
of a single fluid with the same volume, implying that the single fluid is unstable relative
to formation of liquid + vapor. The interval between φ
liq
and φ
vap
in the bottom left
panel of Fig. 9.6is thus the “prohibited” density interval. Outside of this interval Helmholtz
free energy has negative curvature and a single phase is stable, a high-density liquid or a
low-density gas.
The transition between the two different behaviors occurs at the critical temperature,
τ = 1. As the critical temperature is approached from below the common tangent points
approach one another, and merge at a single point at τ = 1. At this temperature the free
energy curve becomes “flat” in the neighborhood of the coordinate φ = 1 (the critical
volume), reflecting the fact that its second and third derivatives vanish (equations (9.35)
and (9.36)). A comparison of Fig. 9.6with Fig. 7.1should bring out the similarities between
the critical point of a fluid and the critical mixing point of a solution, but there are also
differences, most notably the fact that symmetry between the subcritical phases is never
possible in a fluid.
The diagram on the right of Fig. 9.6shows the phase relations of the fluid in terms of the
reduced variables π, φ and τ, and completes the analogy with critical mixing (Fig. 7.1). The
thick curve, called the liquid–vapor loop, bounds the “prohibited region”. Calculation of the
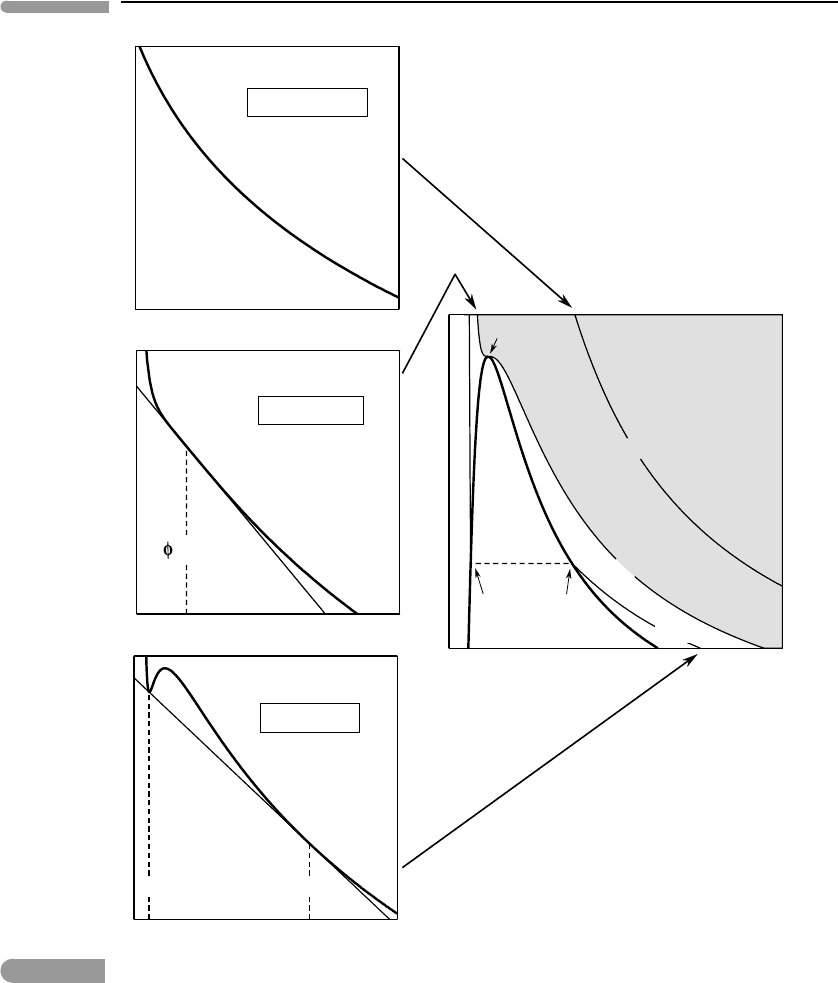
436 Thermodynamics of planetary volatiles
c
=1
supercritical fluid
ψ
φ
π
φ
φ
φ
φ
liq
φ
vap
φ
vap
ψψ
liquid + vapor
"critical" fluid
liquid
+
vapor
critical point
supercritical fluid
τ = 1
τ = 1
τ = φ = π = 1
τ < 1
τ > 1
τ > 1
τ < 1
φ
liq
Fig. 9.6
Non-dimensional Helmholtz free energy as a function of non-dimensional volume (left panels, for the critical
temperature and temperatures above and below the critical temperature), and the phase diagram of a van der Waals
gas (right panel). Compare with Gibbs free energy diagram for a non-ideal solution in Fig. 7.1.
loop is explainedin Box 9.1.Any π–φ combination inside the loop consists of liquid +vapor,
with the equilibrium volumes given by the two intersections of the pressure coordinate,
indicated by the dashed line, with the loop. The liquid–vapor loop corresponds to the solvus
in Fig. 7.1. The supercritical fluid phase unmixes into two subcritical phases as temperature

437 9.2 Liquid–vapor equilibrium
drops below T
c
. The width of the loop, φ
vap
−φ
liq
, is the order parameter. The thin curves
are isotherms, with the critical isotherm (τ =1) separating the supercritical region (shaded)
from the subcritical region. The critical isotherm is tangent to the liquid–vapor loop at the
critical point.
Box 9.1
Calculation of the liquid–vapor loop
The problem of calculating the liquid–vapor loop, e.g. in Fig. 9.7and 9.8, consists of finding the volumes of
the two subcritical phases at equilibrium. We seek to solve for three variables, the non-dimensional volumes
of the liquid and its vapor, φ
liq
and φ
vap
, respectively, and the non-dimensional pressure, π. Hence, we
need three independent equations, which in this case are non-linear. The first one is the equality of pressures
between the two phases, π
liq
=π
vap
, which from (9.37) yields:
8τ
3φ
liq
−1
−
3
φ
2
liq
=
8τ
3φ
vap
−1
−
3
φ
2
vap
. (9.1.1)
The second equation is the equality of Gibbs free energy between the two phases. Note that the Helmholtz
free energy of the two fluids at equilibrium is not the same – Fig. 9.6makes this clear. The two fluids have
different volumes, so Helmholtz free energy does not provide the correct equilibrium criterion. The two fluids
coexist at equilibrium at constant temperature and pressure, however (Fig. 9.6, right panel), so it must be
G
liq
=G
vap
. Now, applying the Legendre transform we find that:
G = F +PV (9.1.2)
so the equilibrium condition at constant P and T is:
G = F +PV =0 (9.1.3)
which in non-dimensional form becomes:
ψ +
3
8
πφ = 0 (9.1.4)
and substituting (9.39):
−τ ln
3φ
liq
−1
3φ
vap
−1
−
9
8
1
φ
liq
−
1
φ
vap
+
3
8
π
φ
liq
−φ
vap
=0. (9.1.5)
The third equation is the equation of state, which we can apply to either the vapor or the liquid, e.g.:
π =
8τ
3φ
vap
−1
−
3
φ
2
vap
. (9.1.6)
At a given temperature equations (9.1.1 ), (9.1.5 ) and (9.1.6 ) are a system of three non-linear equations in
the three unknowns φ
liq
, φ
vap
, and π . They are easily solved with a Maple procedure that you are asked to
write in Exercise 9.5. Solving for the vapor–liquid loop with the dimensional van der Waals EOS, or any other
EOS, is in principle no more complicated.
