Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


408 Equations of state for solids
because solids only have vibrational degrees of freedom, the vibrational energy density
equals the internal energy density, so that we can differentiate (8.57) as follows:
∂P
th
∂T
V
=
γ
V
∂E
∂T
V
=
γC
V
V
. (8.60)
Comparing with (8.59) yields a definition of the Grüneisen ratio in terms of experimentally
accessible macroscopic quantities:
γ =
αK
T
V
C
V
. (8.61)
We now return to the calculation of thermal pressure. As thermal pressure is calculated
along a constant-volume path (see Fig. 8.1) we can find it by integrating (8.59):
P
th
=
T
0
(
αK
T
)
dT . (8.62)
The lower limit of integration is 0 because thermal pressure, which arises from atomic
vibrations, vanishes only at 0 K. Thus, and as I mentioned previously, in order for the
calculation to be rigorously correct isothermal compression must be calculated at 0 K.
In order to evaluate the integral (8.62) we not only need to know how the product αK
T
varies with temperature but also, and more subtly, how it changes with volume. The latter
information is needed even if the integration path is at constant volume because the value
of this volume will be different depending on how much the material is compressed at zero
temperature (see Fig. 8.1). From our discussion in Sections 8.2.2 and 8.2.3, see in particular
Figs. 8.2 and 8.3, we see that K
T
decreases linearly with temperature and, above the Debye
temperature, α increases approximately linearly with temperature. We can thus expect that
their product will not be very sensitive to temperature above θ
D
, and this expectation is
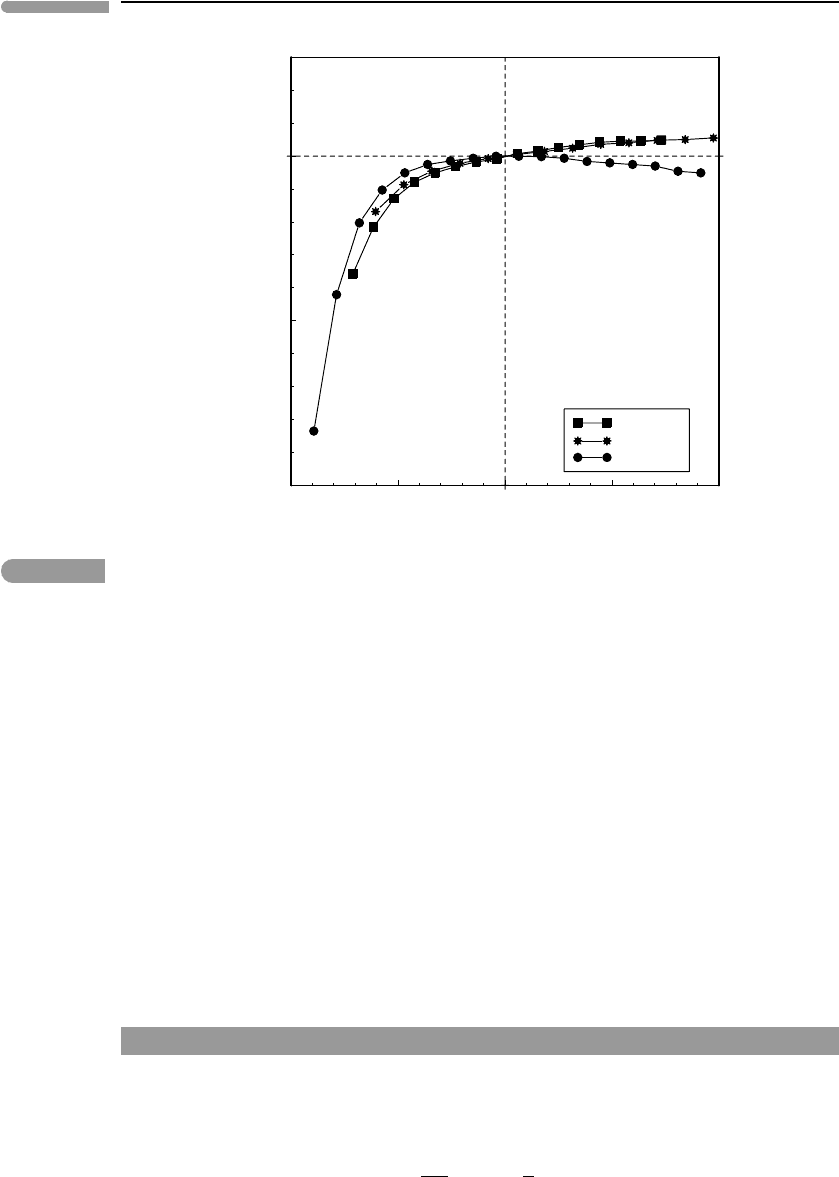
borne out by experimental measurements. Fig. 8.7 shows non-dimensional plots of αK
T
normalized to the value of this product at the Debye temperature, versus non-dimensional
temperature, T/θ
D
, for the same three minerals as in Figs. 8.2 and 8.3 (data from Anderson
et al., 1992). Although in detail it is necessary to account for the small deviations observed
above θ
D
(seeAnderson, 1995), assuming that αK
T
is constant above the Debye temperature
is commonly an excellent approximation.
For T>θ
D
, which is the case for planetary interiors at depths greater than the top few
kilometers, we can break up (8.62) as follows:
P
th
=
(
αK
T
)
D
(
T −θ
D
)
+
θ
D
0
(
αK
T
)
dT , (8.63)
where (αK
T
)
D
is the approximately constant high temperature value of αK
T
, for example
measured at some T above the Debye temperature. If the regular non-dimensional behavior
depicted in Fig. 8.7 is truly universal (or at least applicable to the chief minerals in deep
planetary interiors) then an additional simplification is possible. Let A
D
be the area under
the (nearly identical) curves in Fig. 8.7 in the interval [0,1], given by:
A
D
=
1
0
(
αK
T
)
(
αK
T
)
D
d
T
θ
D
=
1
θ
D
(
αK
T
)
D
θ
D
0
(
αK
T
)
dT . (8.64)
409 8.4 Thermal pressure
0 0.5 1 1.5
2
0
0.5
1
αK
T
/αK
T
(θ
D
)
Al
2
O
3
Forsterite
T/θ
D
MgO
Fig. 8.7
Variation of the product αK
T
, normalized to the values of the product at the Debye temperatures, relative to
non-dimensional temperature. Data from Anderson et al. (1992). The area under the curves between 0 and 1 is
approximately 0.77.
Numerical integration of the curves in Fig. 8.7 yields A
D
≈0.77, so:
θ
D
0
(
αK
T
)
dT ≈ 0.77θ
D
(
αK
T
)
D
(8.65)
and, substituting in (8.63):
P
th
≈
(
αK
T
)
D
(
T −0.23θ
D
)
, T ≥ θ
D
. (8.66)
Other thermodynamic approximations to thermal pressure may be found, for example, in
Anderson (1995) and Jackson and Rigden (1996), but my approximation, equation (8.66),
appears to work reasonably well. Thermal pressures for T <θ
D
can be calculated in a
similar manner, by integrating numerically (8.64) to the desired temperature.
Worked Example 8.3 Thermal pressure in planetary mantles
Let us compare the relative magnitudes of thermal pressure and elastic pressure (see equation
(8.2)) along mantle adiabats. We re-write the condition of hydrostatic equilibrium (equation
(2.34)) as a function of depth, z, as follows:
dP
dz
=gρ =
g
v
, (8.67)
410 Equations of state for solids
where v is specific volume (i.e. volume per unit of mass). We will first calculate zero-
temperature pressure as a function of depth inside a planet by integrating this equation as
follows:
z −z
r
=
1
g
P
P
r
VdP, (8.68)
where P
r
is the pressure at some reference depth, z
r
. If we choose the reference depth as the
surface of the planet then z
r
=P
r
=0, but as we shall see we cannot always do this. Whereas
in previous discussions we considered density to be a constant, we will now incorporate the
variation of density with pressure (at constant T =0). This requires a function V = V(P),
i.e. an isothermal EOS, which for this example I choose to be the Born–Mie EOS, equation
(8.56). In order to use a pressure-explicit EOS such as this one we must integrate by parts, so
we substitute (8.45)in(8.68) and, accounting for thenon-zero lower integration limit, obtain:
z = z
r
+
1
g
PV
P
−P
r
V
P
r
+
V
P
r
V
0
PdV −
V
P
V
0
PdV
. (8.69)
In this equation V
0
is the zero-pressure volume, as usual, V
P
is the volume at the pressure of
depth z, and V
Pr
is the volume at the pressure of the reference depth, P
r
, all taken at the same
temperature, 0 K in this case. The integral is easily implemented in Maple (see Software
Box 8.3), but there is an important point that I want to stress here: equation (8.69) is a function
of the form z =z(V ). Thus, the calculation procedure requires that we choose a value of V
P
,
use this value to calculate P from the EOS (e.g. equation (8.56) if we choose the Born–Mie
EOS), and then evaluate the integral in (8.69). In other words, the independent variable in
the calculations is volume (or density), and both pressure and depth are functions of V. This
may sound odd, but it is required by the pressure-explicit nature of the equations of state.
Software Box 8.3 Calculation of zero-temperature pressures in solid planets
The Maple worksheet Z_P.mw contains two procedures that generate tables of zero-
temperature pressure and density as a function of depth, by calling on the procedures in
the S_EOS package. The two procedures, ZvsP_BoMi and ZvsP_BiMu are identical,
except that they use the Born–Mie or Birch–Murnaghan EOS, respectively. The proce-
dures use density as the independent variable, convert density to specific volume, and
calculate pressure from the equation of state and depth from equation (8.69). In order to
apply these procedures to planets with phase transitions (e.g. Fig. 8.9) the procedure is
run separately for each layer, using the pressure and depth at the bottom of each layer as
the starting point for the next layer. The material properties for each phase, molar vol-
ume and bulk modulus, are always the zero-pressure values (see discussion in Worked
Example 8.3).
If we knew nothing about phase transitions in the Earth’s mantle, or chose to ignore them,
then we could start with olivine at the Earth’s surface and solve the equations (the EOS for
P and 8.69 for z) all the way to the core–mantle boundary. Using this approach we have
V
0
=3.006×10
−4
m
3
kg
−1
(corresponding to ρ
0
=3300 kg m
−3
, which is the zero-pressure
density of Fo
90
olivine), K
0
= 1.3 Mbar = 1.3 × 10
11
Pa, K
= 4 and z
r
=P
r
= 0.
411 8.4 Thermal pressure
The resulting zero-temperature pressure vs. depth curve calculated with the Born-Mie EOS
and assuming a constant value of g = 9.8ms
−2
(see Chapter 2 for a justification of why
this is an acceptable approximation) is shown by the thin P
0
curve in Fig. 8.8. This curve
can’t be much better than an order of magnitude approximation, however, as both density
and bulk modulus change at mantle phase transitions.
The correct procedure is, of course, to calculate the curve in various segments, changing
the material properties at each phase transition to those of the incoming phase, and setting
z
r
equal to the depth of the phase transition and P
r
equal to the pressure at that depth. Note
very carefully, however, that the material properties of the incoming phase that are used to
solve the EOS and the depth equation, (8.69), are always the zero-pressure properties. If
you are not clear on why this is so you should derive equation (8.69) and convince yourself
that this is indeed the case.
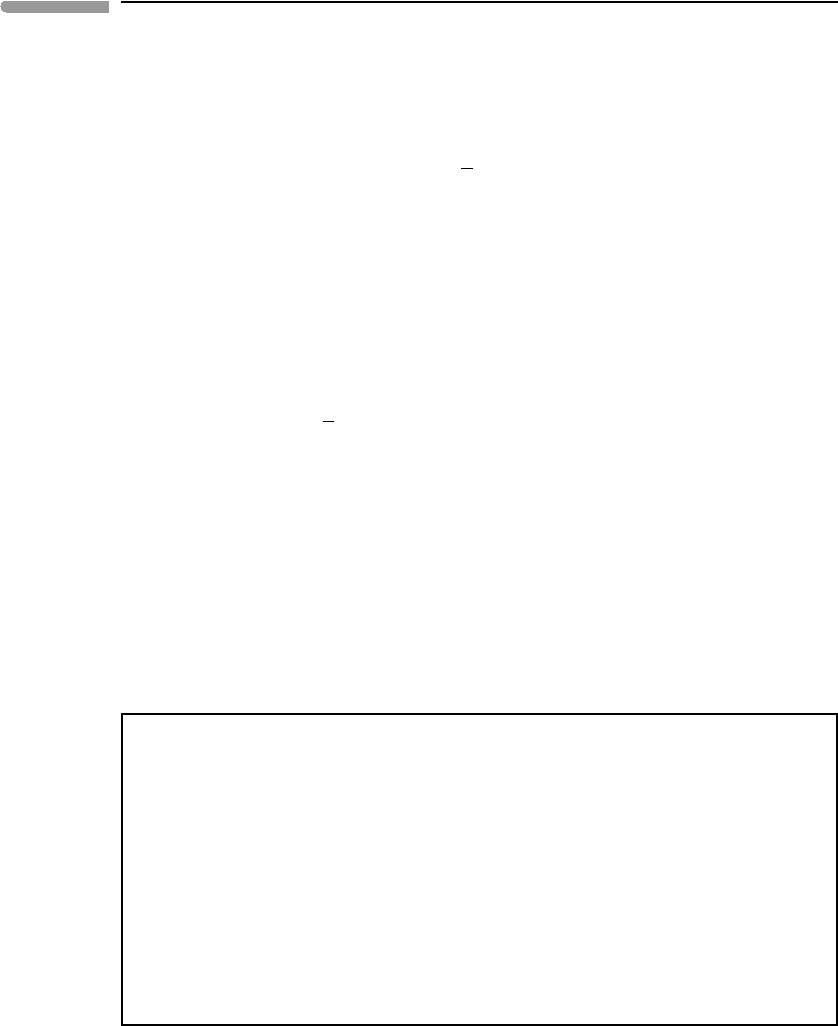
500 1000 1500 2000 2500
0
500
1000
Depth (km)
Pressure (kbar)
PREM
P
0
(Born-Mie)
P
0
(Born-Mie - no phase transitions)
P
th
∆P
th
∆P
th
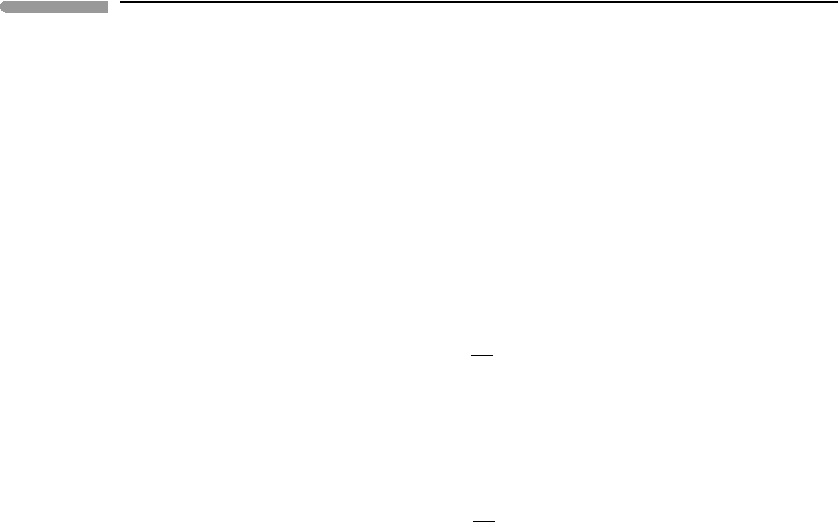
Pressure in the Earth’s mantle
Fig. 8.8 Calculated pressures in the Earth’s mantle compared to pressures on the Preliminary Reference Earth Model (PREM) of
Dziewonski and Anderson (1981). Chemical composition of the mantle is assumed to be Mg
1.8
Fe
0.2
SiO
4
. P
0
are
zero-temperature pressures calculated with the Born-Mie EOS, either ignoring olivine phase transitions (thin curve),
or assuming two phase transitions, at depths of 500 km (olivine to ringwoodite) and 670 km (ringwoodite to
perovskite + ferropericlase), see also Figure 8.9. P
th
is the thermal pressure calculated with equation 8.66.
Thermodynamic calculation of thermal pressure assumes heating at constant volume (see Figure 8.1). This is not
realizable in the Earth’s upper mantle and transition zone, because self-compression of the mantle is not sufficient to
counter thermal expansion. The actual thermal pressure in the Earth’s mantle may be better represented by the
difference between PREM and the calculated zero-temperature pressure, shown by the curve labeled P
th
.
412 Equations of state for solids
The thick P
0
curve in Fig. 8.8 was calculated in this way, assuming that olivine transforms
to ringwoodite at 500 km depth, and ringwoodite transforms to perovskite at 660 km depth.
This is still a simplification, as it ignores other phase transitions in the mantle transition
zone (olivine transforms to wadsleyite at the top of the transition zone, ∼410 km deep), but
it is much better than assuming that olivine is stable throughout the entire mantle. Pressures
up to 500 km depth were calculated with the olivine properties given above. This yields
a pressure of 163 kbar at 500 km depth, and these values are set equal to P
r
and z
r
for
the ringwoodite layer. For ringwoodite we have ρ
0
= 3700 kg m
−3
, K
0
= 1.9 Mbar =
1.9 × 10
11
Pa, and K
=4. With these values we get P
r
=222 kbar at z
r
=660 km, which
is the top of the perovskite layer, and then use the zero pressure properties of perovskite,
ρ
0
= 4200 kg m
−3
, K
0
= 2.5 Mbar = 2.5 × 10
11
Pa, and K
= 4 to calculate pressures
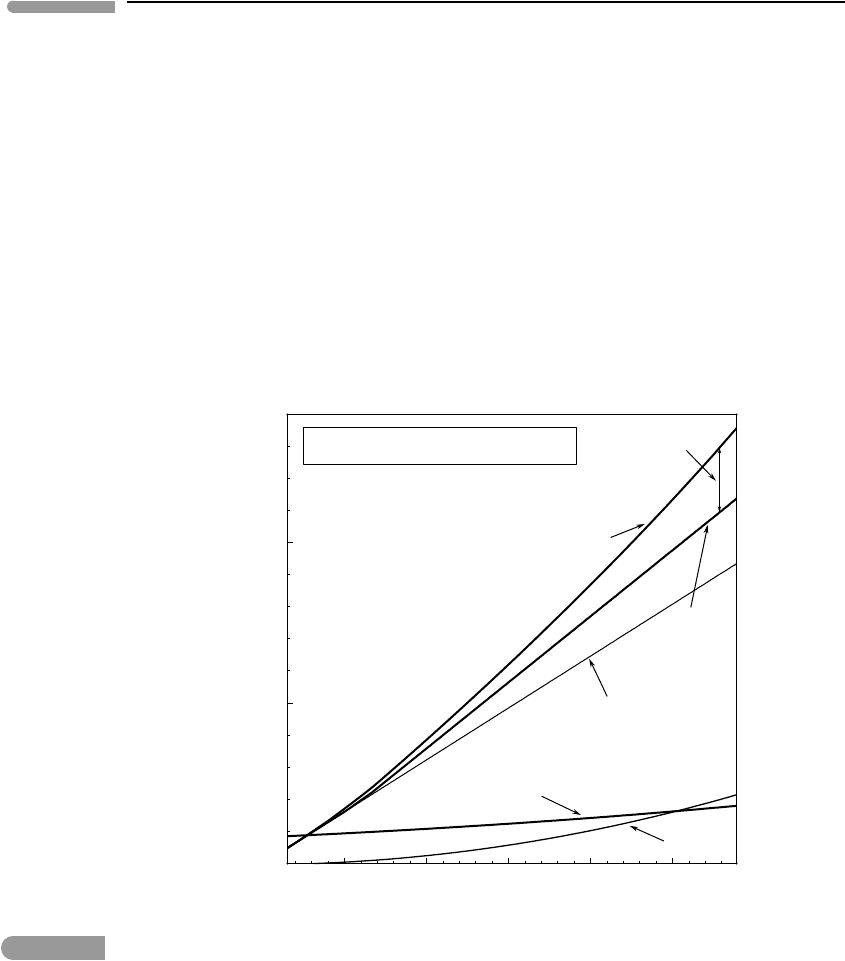
all the way to the core–mantle boundary. Figure 8.9 shows zero-temperature densities as a
function of depth calculated with this model, as well as the (hypothetical) density of olivine
if phase transitions are ignored (i.e. along the thin P
0
curve in Fig. 8.8). Exercise 8.5 asks
you to verify these results.
Figure 8.8 shows that ignoring mantle phase transitions underestimates the pressure at
the core–mantle boundary by ∼200 kbar, or roughly 20% relative. This difference reflects
the fact that there are significant density jumps at the phase transitions, as shown by Fig. 8.9.
Also shown in Figs. 8.8 and 8.9 are curves labeled PREM. These curves show pressure and
density along the Preliminary Reference Earth Model of Dziewonski and Anderson (1981).
Details of how these values were calculated are complex and beyond the space available
here (see, for example, Anderson, 1989). Suffice it to say that the PREM results are not
500 1000 1500 2000 2500
3500
4000
4500
5000
5500
Depth (km)
Density (kg m
–3
)
Density in the Earth’s mantle
Zero-T density
(Born-Mie- no phase transitions)
Zero-T density
(Born-Mie)
PREM
olivine
ringwoodite
perovskite
Fig. 8.9 Densities as a function of depth in the Earth’s mantle calculated assuming zero-temperature compression of
Mg
1.8
Fe
0.2
SiO
4
, with or without phase transitions as in Fig. 8.8. Densities along PREM shown for comparison; note the
much more complicated pattern in the upper mantle and transition zone, corresponding to mineral reactions that I
ignored in the simplified calculations.
413 8.4 Thermal pressure
altogether independent of a specific choice of EOS, but they are generally accepted as a
good approximation to actual pressures and densities inside the Earth.
We first note that although PREM densities show more “texture” than the ones calcu-
lated in this example, reflecting the real phase transitions in the Earth’s mantle, they are
in generally good agreement with the simple model. PREM pressures and calculated zero
temperature pressures are virtually indistinguishable to a depth of ∼500 km, but diverge
smoothly as depth increases beyond this value. At the base of the Earth’s mantle the cal-
culated zero-temperature pressure is ∼220 kbar lower than the PREM pressure. Clearly,
thermal pressure must have a role in this discrepancy, but what exactly is this role? In order
to answer this question we calculate thermal pressure along the mantle adiabat.
Temperature along an adiabat is obtained by integrating equation (3.35), as follows:
T = T
L
exp
αg
c
P
(
z −z
L
)
, (8.70)
where T
L
is the temperature at the base of the lithosphere (top of the convective layer)
and z
L
is the depth to the base of the lithosphere. For Earth we can take T
L
= 1650 K
and z
L
= 150 km. Substituting in (8.66) we obtain an approximate expression for thermal
pressure along a mantle adiabat:
P
th
≈
(
αK
T
)
D
T
L
exp
αg
c
P
(
z −z
L
)
−0.23θ
D
. (8.71)
We now need to choose characteristic values for the parameters in this equation. For many
minerals θ
D
is of the order of 1000 K and the high-temperature (i.e. above θ
D
) values of
α and c
P
are approximately 3 × 10
−5
K
−1
and 1.2 kJ K
−1
kg
−1
, respectively. The high-
temperature value of αK
T
for closely-packed minerals is of the order of 60 bar K
−1
. Using
these parameter values equation (8.71) generates the curve labeled P
th
in Fig. 8.8.
A “blind” application of equation (8.2) would lead us to add P
th
to P
0
in order to obtain
the total pressure. Figure 8.8 shows that, whereas this would yield a value comparable to the
PREM pressure at the core–mantle boundary, pressures calculated in this way would become
progressively more erroneous with decreasing depth, and would be gross overestimates in
the upper mantle. What is going on here? Quite simply, that if we had applied equation
(8.2) “blindly” we would have been careless with how we applied thermodynamics to
the real world. The definition of thermal pressure (equations (8.57)or(8.59)) requires
that volume be kept constant as the material is heated, yet the Earth does not behave as
a perfectly rigid container. At shallow depth, where the zero-temperature pressure and
the (calculated) thermal pressure are of comparable magnitudes, compression of the mantle
under its own weight (often called self-compression) cannot keep the mantle from expanding
as its temperature increases. The zero-temperature (elastic) pressure corresponds to the load
that is available to keep the volume of the “container” fixed and it is not enough to counteract
the thermal expansion of the mantle. In other words, the isochoric heating leg in Fig. 8.1
is not realizable in the Earth’s upper mantle, because the upper mantle does not behave as
a rigid vessel. In particular, Fig. 8.8 suggests that at depths less than 300 km or so thermal
pressure would be higher than zero temperature pressure, which is physically impossible
(the material would shatter).
If the material is allowed to expand freely then there can be no thermal pressure. The
increase in atomic vibrational energy that occurs with increasing temperature is in such
case expressed macroscopically as thermal expansion rather than thermal pressure. The
coincidence between PREM and P
0
suggests that this is the case in the Earth’s upper mantle
414 Equations of state for solids
800 1000 1200 1400 160
0
0
50
100
150
200
250
Depth (km)
P
0
P
th
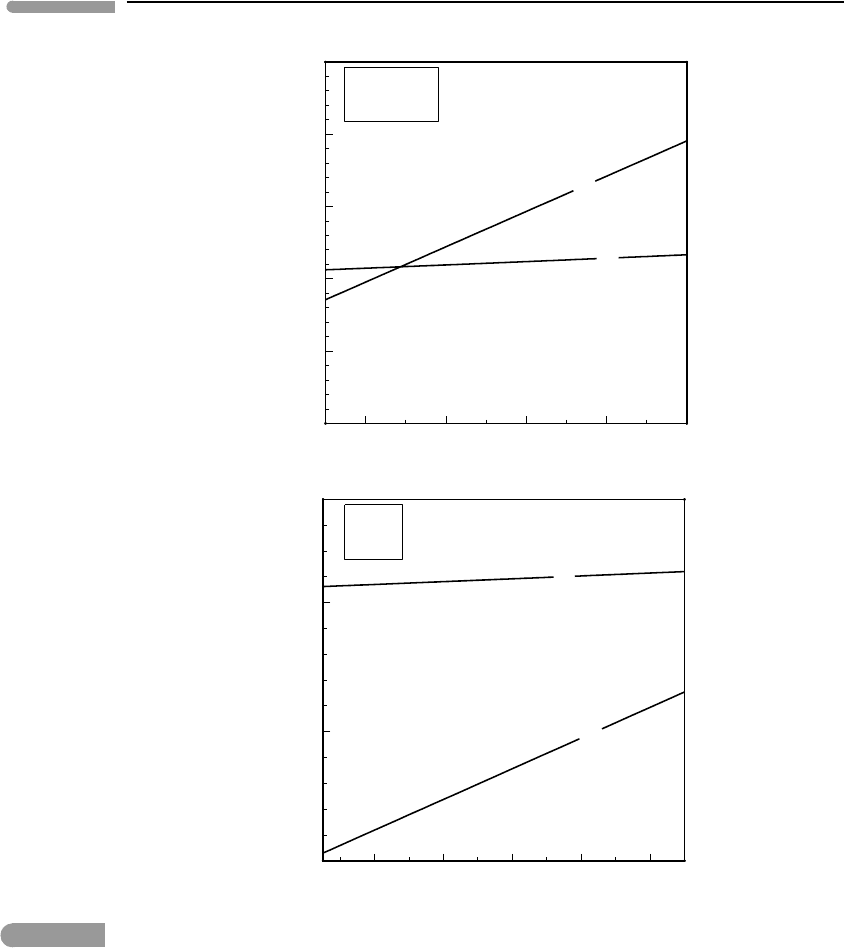
Mars
200 400 600 800 1000
0
50
100
Depth (km)
Pressure (kbar)
Pressure (kbar)
P
th
P
0
Io
Fig. 8.10 Zero-temperature pressures and thermal pressures (calculated with the constant-volume assumption, Fig. 8.1)in
Mars and Io. Thermal pressure is likely to be vanishingly small in Io, and almost negligible in the Martian mantle.
(depths less than ∼650 km). The picture at greater depths becomes more blurred. If PREM
is an accurate representation of actual pressures inside the Earth and P
0
is the correct zero
temperature pressure then the difference between the two values must be thermal pressure.
This difference, let us call it the inferred thermal pressure, is shown by the curve labeled
P
th
in Fig. 8.8. The inferred thermal pressure only becomes comparable to the calculated
thermal pressure at depths in excess of 1500 km or so. This suggests that at such depths
it becomes reasonable to consider the mantle as a “constant volume” vessel, and that
a direct application of equation (8.2) to calculate pressure is likely acceptable for the Earth’s
deep mantle and core. As we move towards the surface there is not enough load to keep

415 8.4 Thermal pressure
the volume perfectly constant, but neither is free expansion possible, so that some thermal
pressure builds up. This decreases with decreasing depth, until thermal pressure essentially
vanishes in the upper mantle. There is no well-defined ratio between P
0
and calculated P
th
at which the actual thermal pressure becomes significant, but Figure 8.8 suggests that when
P
th
is less than about 1/3P
0
, the constant volume approximation, and hence equation (8.2),
may become reasonably accurate.
What about application to other planets, for which we do not have PREM as a benchmark?
Figure 8.10 shows P
o
and P
th
calculated for the mantles of Mars and Io (core–mantle
boundaries assumed to be at 1630 km and 1100 km depth, respectively, after Lodders &
Fegley, 1998). In the case of Mars I assumed an adiabatic (convecting) mantle below
a 700 km lithosphere, with T
L
= 2000 K and g = 3.7ms
−2
. For Io I assumed a thin
lithosphere (z
L
= 50 km), with T
L
= 2000 K and g = 1.8ms
−2
. In both cases I assumed
that olivine does not undergo phase transitions. The case of Io is unambiguous: temperature
is high enough and gravitational acceleration low enough that calculated thermal pressure
is everywhere higher than zero temperature pressure, by a large factor. This means that Io’s
interior must behave as an essentially unconfined solid or, in other words, that pressure in
Io’s interior is well approximated by zero-temperature pressure, with a vanishingly small
thermal component. In the case of Mars we find that at the core–mantle boundary P
th
≈0.6
P
0
. By comparison, a similar relationship occurs in Earth at a depth of ∼600 km, where the
inferred thermal pressure, P
th
,is∼10 kbar, or about 5% of the zero-temperature pressure.
One could tentatively conclude that thermal pressure is generally negligible in the Martian
mantle. Thermal pressures in the Moon and Venus are left as an exercise for the reader.
Worked Example 8.4 The ringwoodite–perovskite phase transition in planetary mantles revisited
In Worked Example 8.2 we calculated phase diagrams for the Mg and Fe end-member
systems of mantle silicates. If we focus on the stishovite-absent phase boundary we see
that, in an Fe-Mg system, we can write three linearly independent heterogeneous equilibria
along this phase boundary:
(i) Mg
2
SiO
4
MgSiO
3
+MgO
(ii) Fe
2
SiO
4
FeSiO
3
+FeO
(iii) MgSiO
3
+FeO FeSiO
3
+MgO. (8.72)
Excess mixing properties for perovksite, ringwoodite and periclase are not well known, and
in any case a set of mixing properties consistent with the standard state properties that I
chose to calculate the phase diagram in Fig. 8.6 does not exist (see Worked Example 5.7
for why this is important). Under these circumstances, and as a first approximation, we will
treat the three phases as being ideal solutions. Assuming that all phases are binary Mg–Fe
solutions we write the equilibrium conditions for the three reactions as follows:
(i) K
(i)
=exp
−
r
G
0,(i)
P,T
RT
=
X
pv
Mg
·X
pc
Mg
X
rw
Mg
2
416 Equations of state for solids
(ii) K
(ii)
=exp
−
r
G
0,(ii)
P,T
RT
=
1 −X
pv
Mg
·
1 −X
pc
Mg
1 −X
rw
Mg
2
(iii) K
(iii)
=exp
−
r
G
0,(iii)
P,T
RT
=
1 −X
pv
Mg
·X
pc
Mg
X
pv
Mg
·
1 −X
pc
. (8.73)
At fixed pressure and temperature these are three equations in three unknowns, so that
we can solve for the compositions of the three phases at equilibrium. It is always a good
idea to check that this is consistent with the phase rule. In the Fe-Mg system we have F =3
and c =3, so f =2 and everything is fine – fixing two intensive variables fully determines
the thermodynamic state of the system.
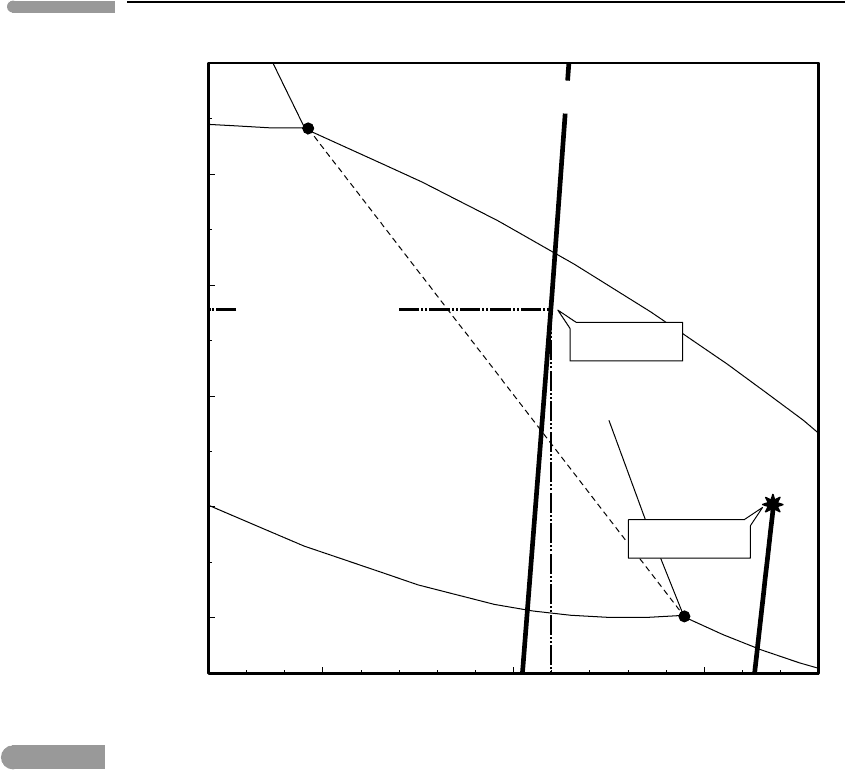
Let us now calculate the composition of the phases at equilibrium at the 670 km discon-
tinuity, and compare the results to the structure and composition of the terrestrial mantle. In
order to do this we use the pressure vs. depth values from PREM (shown in Fig. 8.8), and
combine them with temperatures as a function of depth calculated with (8.70), assuming
T
L
= 1650 K at z
L
= 150 km. This yields a curve of pressure vs. temperature, labeled
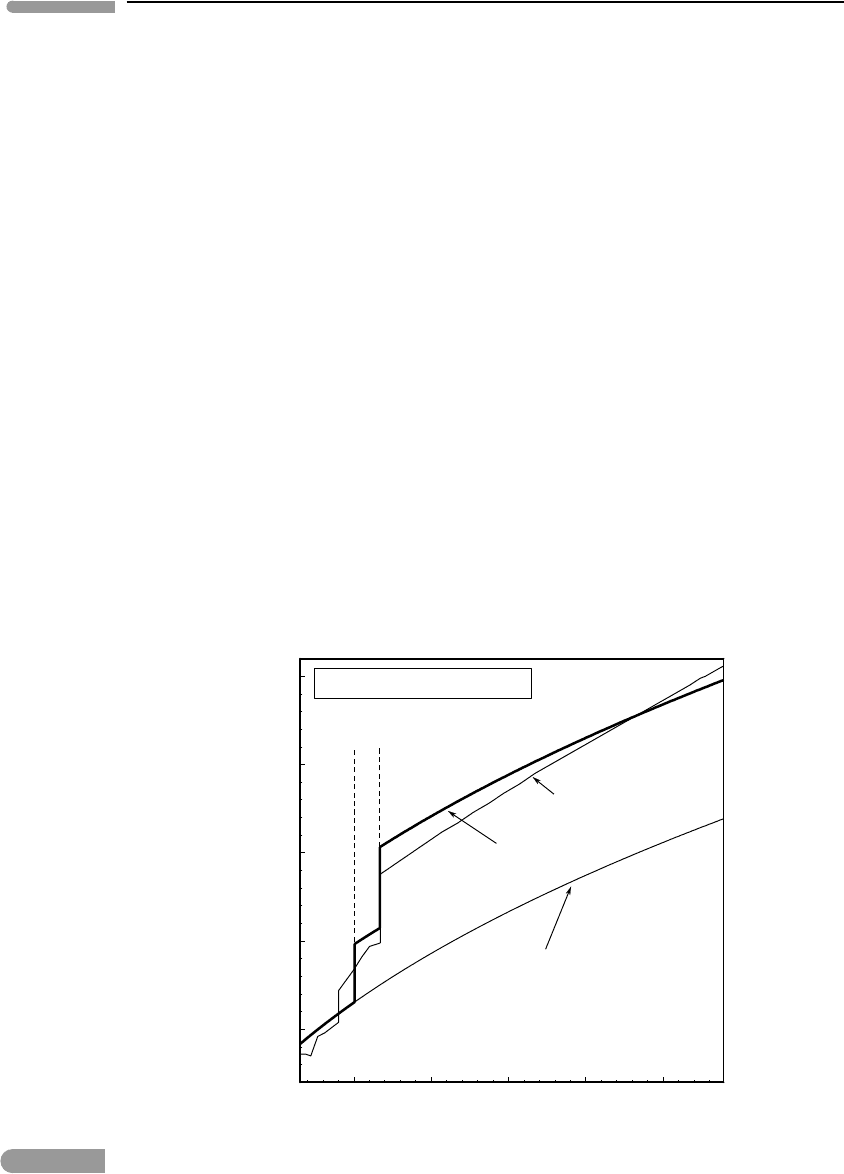
“Terrestrial adiabat” in Fig. 8.11. Calculated conditions at the 670 km discontinuity are
∼238 kbar and ∼1600
◦
C, shown with the dash-dot lines in the figure. Using these values
in the solution of the system of equations (8.73) (Software Box 8.4) yields X
pv
Mg
= 0.86,
X
rw
Mg
= 0.9 and X
pc
Mg
= 0.82, which compares rather favorably with the Mg number of
the terrestrial mantle of ∼90. It is hard to say to what extent this agreement is fortuitous,
but it is encouraging. If correct, it means that for the composition of the Earth’s mantle
the stishovite-absent reaction is displaced relative to the Mg end-member so that it goes
through 238 kbar at 1600
◦
C. The calculation also predicts that Fe partitions into the oxide
phase relative to the silicates, which agrees with experimental findings.
Software Box 8.4 Fe–Mg exchange among ringwoodite, perovskite and ferropericlase
Maple worksheet fe_mg_exch_hiP.mw contains two new procedures that solve the
system of equations in Worked Example 8.4 and generate the P –X loop in Fig. 8.12.
Procedure FMX calls on dgPT (see Software Box 8.2) to solve the system of non-
linear simultaneous equations (8.73), for the three unknowns X
Mg
pv
, X
Mg
rw
and X
Mg
pc
.
Executing the statement block following the procedure runs FMX and outputs the three
mol fractions to the screen. Procedure FMX_LOOP encloses FMX in a pressure loop and
generates a file that lists the three mol fractions as a function of pressure, at constant
temperature. This output is used to construct Fig. 8.12. Remember that the statement
block at the end of the worksheet, where the reaction stoichiometries are defined, must
be executed before the Fe-Mg calculations are attempted, or an error message will result.
The attentive reader should have noticed that, given that ringwoodite–perovskite–
periclase equilibrium in the Fe-Mg system is divariant, the three phases must coexist over
a pressure range at constant temperature. This is the behavior depicted in the right hand
panel of Fig. 6.18. By solving the system of equations (8.73) for a range of pressures at a
417 8.4 Thermal pressure
1000
Pressure (kbar)
1500 2000
180
200
220
240
260
280
Temperature (°C)
Mg-rw
Mg-pv
+
Mg-pc
Mg-rw
Mg-pc
+
st
Fe-rw
Fe-pv
+
Fe-pc
[Fe]
[Mg]
Fe-rw
Fe-pc
+
st
Terrestrial adiabat
Martian adiabat
670 km discontinuity
CMB
X
Mg, rw
~
~
0.9
X
Mg, rw
~
~
0.64
Fe-pc
+
st
Fe-pv
Fig. 8.11 Terrestrial and Martian adiabats superimposed on the phase diagram of Fig. 8.6, with some reactions omitted for
clarity. The diagram predicts that stabilization of silicate perovskite in the terrestrial mantle at the 670 km
discontinuity would take place for a bulk composition with X
Mg
≈0.9, which agrees almost perfectly (and perhaps
somewhat fortuitously) with the mantle composition. The Martian adiabat terminates at the conditions of the
core–mantle boundary, shown with a star. The ringwoodite–perovskite phase transition would take place at the
conditions of the Martian core–mantle boundary for a bulk composition with X
Mg
≈0.64, which is more ferroan that
any likely Martian mantle composition. Silicate perovksite is therefore unlikely to be present in the Martian mantle.
Breakdown of ringwoodite to ferropericlase +stishovite is also unlikely if temperatures in the Martian mantle are of
the order of those estimated in Section 3.9, see also Fig. 3.18.
constant temperature (say 1600
◦
C) we can construct a pressure–composition loop such as
that in Fig. 6.18. This is shown in Fig. 8.12, but there are two complications. First, as Fig. 8.6
shows, the stishovite-absent equilibrium must become metastable at a certain X
Mg
value.
Calculation of the exact X
Mg
and P at which this happens is left as an exercise. In Fig.
8.12 I truncate the diagram at 225 kbar, which is close to the conditions at which the
stishovite-absent equilibrium becomes metastable relative to the perovskite-absent one (see
Fig. 8.6). The second complication is that, in contrast to the simple phase transitions dis-
cussed in Section 6.6, the equilibrium described by equations (8.73) has two phases with
