Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

438 Thermodynamics of planetary volatiles
0.5 1 1.5
φ
π
2 2.5
0.5
1
1.5
1
1.1
1.2
1.5
0.9
5
0.85
0.75
liquid + vapor
supercritical fluid
1.05
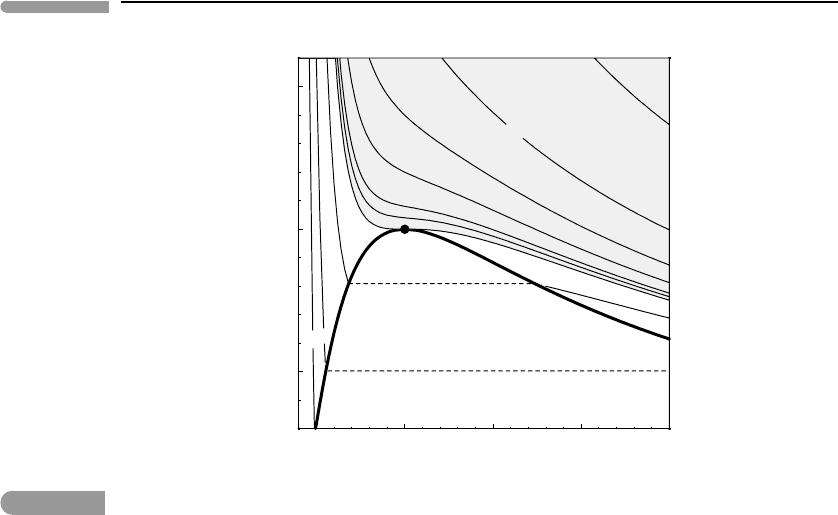
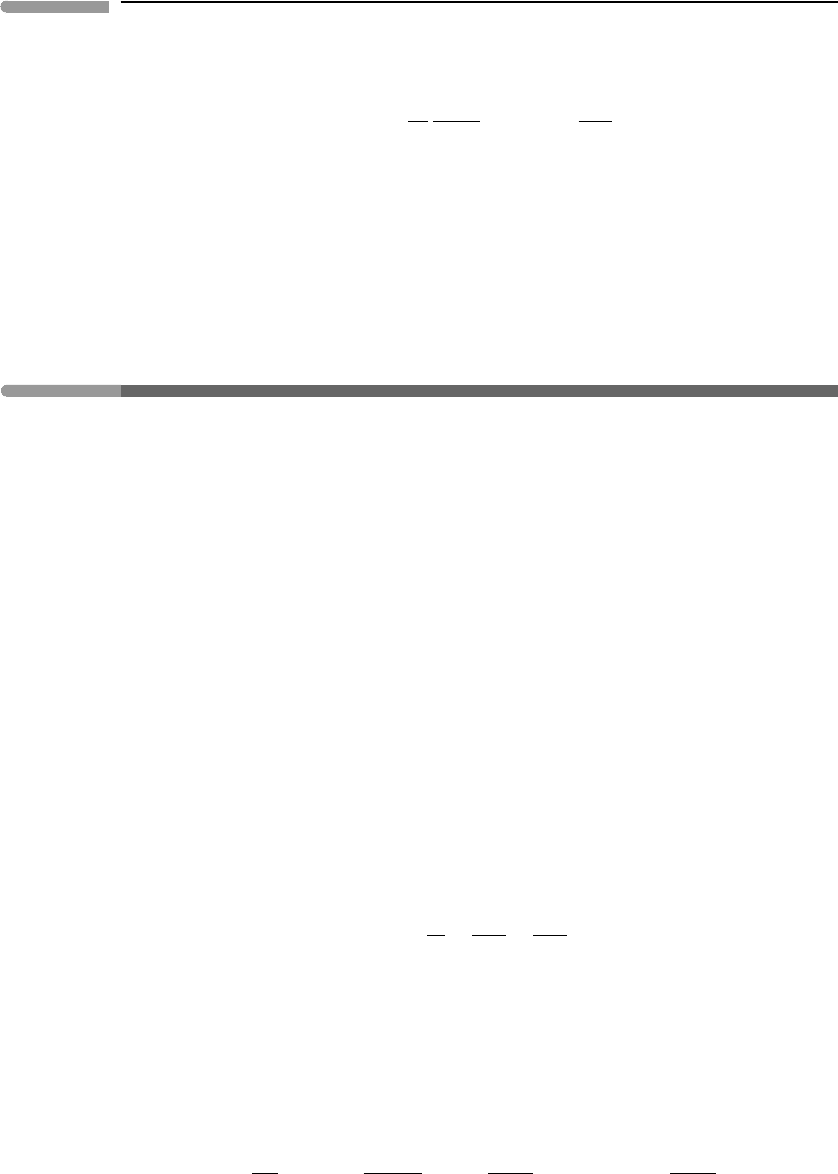
Fig. 9.7
Non-dimensional pressure–volume diagram for a van der Waals fluid. The contours are isotherms (τ given on the
right edge of the diagram). The critical isotherm is tangent to the vapor–liquid loop at the critical point. In a
supercritical fluid there is no discontinuous phase transition separating “low density fluids” (=gases) from “high
density fluids” (=liquids). A discontinuous phase transition between the two phases always exists at temperatures
below the critical temperature.
Close-ups of the neighborhood of the critical point are shown in Figs. 9.7–9.9. The first of
these figures shows the pressure–volume projection, with temperature depicted by isotherms
(the numbers on the right-hand side are the values of τ , the two missing values are 1.01
and 1.02). Figure 9.8shows the temperature–volume projection, with pressure depicted by
isobars (showing values of π ), and Fig. 9.9shows the pressure–temperature projection with
contoured isochores (showing values of φ). The fat dot in all figures is the critical point. The
univariant phase boundary in Fig. 9.9maps the location of the liquid–vapor discontinuous
phase transition, i.e. it tracks the P –T path of the liquid–vapor loop that appears in the
other two projections. The density jump across the first-order phase transition decreases
towards the critical point, and disappears at τ = π = 1. Therefore, the univariant phase
boundary necessarily terminates at the critical point, which is a singular point on the curve,
not an invariant point (see Box 7.1). No discontinuous phase transition is possible beyond
the termination of the univariant phase boundary, but note that the critical isochore (φ =1)
is the continuation of the univariant phase boundary (Exercise 9.6).
The distinction between condensed and non-condensed fluids, expressed, for example,
by their different densities and compressibilities, is discontinuous below the critical point.
The density of the liquid varies relatively little along the vapor–liquid coexistence curve,
but the density of the vapor decreases rapidly with decreasing pressure. Above, but in the
neighborhood of, the critical point there is a sharp decrease in the compressibility as density
increases, but no discontinuity between condensed and non-condensed phases. Where one
chooses to place the boundary between condensed and non-condensed fluid is conventional
(or unimportant): in the supercritical region, which is shaded in Fig. 9.7and 9.8, there is
no discontinuous phase transition between the two states. Supercritical fluid means that the
439 9.2 Liquid–vapor equilibrium
0.5 1 1.5
2
τ
φ
2.5 3
0.7
0.8
0.9
1
1.1
1.2
1.3
2
1.1
1.2
1.5
0.8
0.6
5
liquid + vapor
supercritical fluid
1.05
0.4
1
5
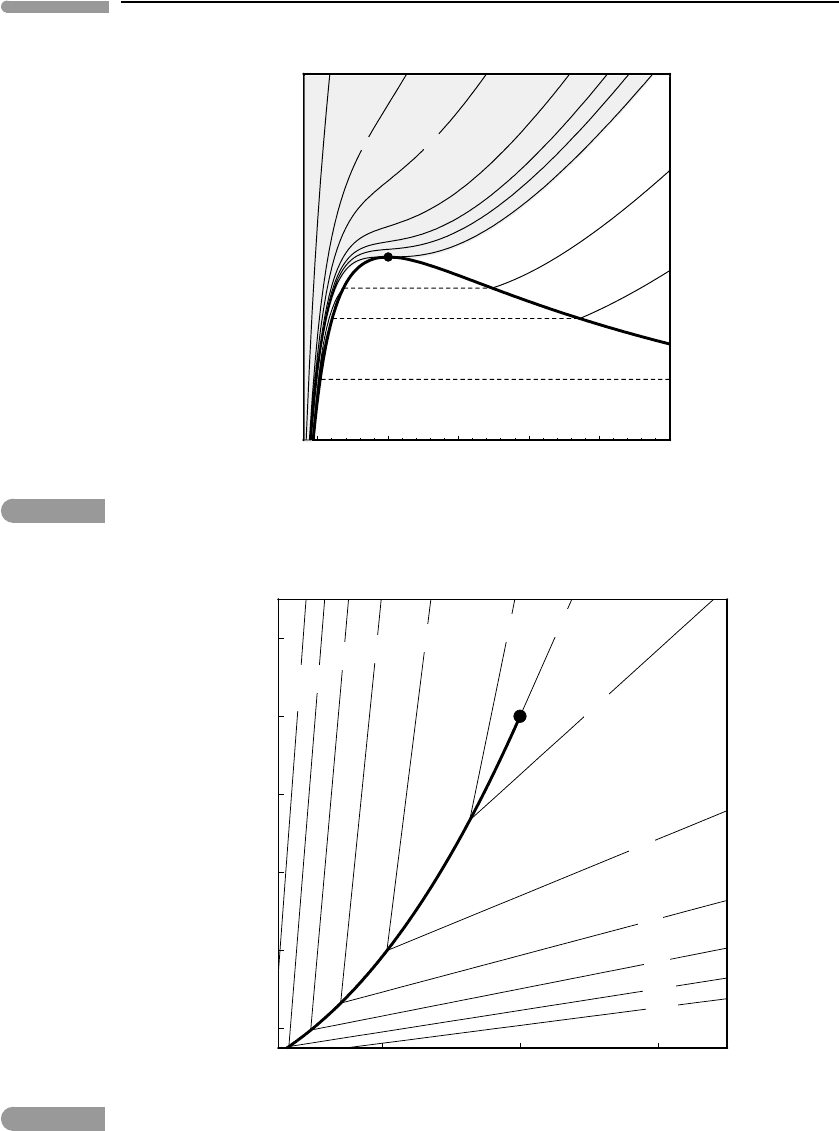
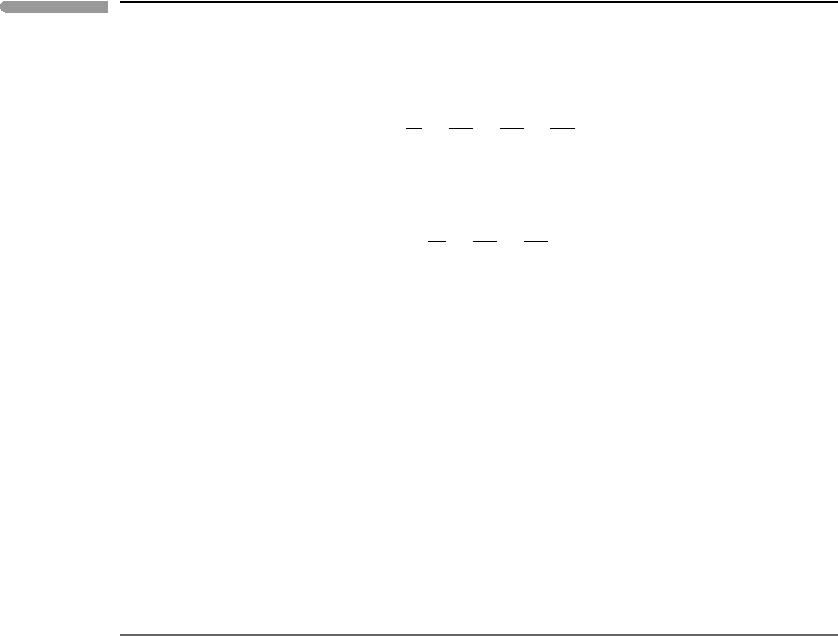
Fig. 9.8
Non-dimensional temperature–volume diagram for a van der Waals fluid. The contours are isobars (π given along
the top and right edges). The critical isobar is tangent to the vapor–liquid loop at the critical point. Liquid–vapor
unmixing at a discontinuous phase transition is possible only if the pressure is less than the critical pressure.
0.8 1 1.2
0.2
0.4
0.6
0.8
1
1.2
1
2
4
6
8
10
12
0.65
0.52
0.45
0.48
0.47
0.44
τ
π
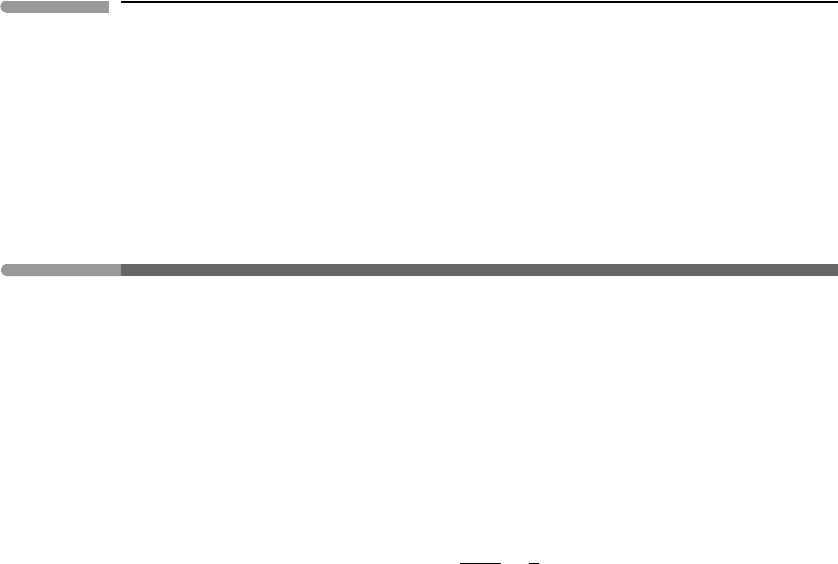
Fig. 9.9
Non-dimensional pressure–temperature diagram for a van der Waals fluid. The contours are isochores. The univariant
phase boundary, i.e. the discontinuous phase transition mapped by the liquid–vapor loop in Figs. 9.7 and 9.8, ends at
the critical point, which is not an invariant point (Box 7.1). The density jump across the discontinuous phase transition
vanishes at the critical point, and the critical isochore is continuous with the univariant phase boundary.
440 Thermodynamics of planetary volatiles
fluid’s properties change continuously from those of a gas to those of a liquid, but carries
no density connotation. For example, given that the critical temperature and pressure for
nitrogen and oxygen are approximately 126 K, 34 bar and 154 K, 50 bar, respectively, air
cannot be made to condense at a discontinuous phase transition by compressing it at room
temperature – it must be cooled below its critical temperature.
9.3 The principle of corresponding states
It is an empirical observation, which was put on solid footing largely by the work of
Guggenheim (for example, 1967, pp. 135–140), that some gases follow the same behavior
if their pressure, temperature and volume are expressed as the non-dimensional reduced
quantities π, τ and φ. This is known as the principle of corresponding states. It suggests that
a universal equation of state may exist, but it does not tell us what form the universal EOS
has, nor, for that matter, whether an analytic function with the properties of a universal EOS
even exists. We can quickly discard the non-dimensional van der Waals EOS, however. If
gases followed this equation as a universal EOS then their critical compressibility factor,
Z
c
, would be (using (9.31)–(9.33)):
Z
c
=
P
c
V
c
RT
c
=
3
8
. (9.41)
A compilation of critical parameters for eighteen gases of interest in planetary sciences
(Table 9.1) shows that this is not the case, and that Z
c
is in every case significantly less than
0.375. The gases in Table 9.1are listed in order of decreasing Z
c
. There is a group of gases for
which Z
c
is ∼0.3. These gases are sometimes called simple gases and follow the principle of
corresponding states more or less closely. As we shall see, a simple EOS exists which, if not
completely accurate, is at least acceptable as a first order semi-quantitative approximation
to their behavior.At the other extreme there are substances such as H
2
O, NH
3
, HCN and HF
for which Z
c
is much less than 0.3 and, more importantly, significantly different among the
various gases. This suggests that a universal EOS for these substances does not exist. These
gases do not obey the principle of corresponding states. What distinguishes these substances
from the “simple gases” is that their molecules are strongly polar. In between there are some
gases, most notably CO
2
and SO
2
, which depart significantly from the behavior of simple
gases but not as much as, say, H
2
O. This emphasizes the fact, pointed out by Guggenheim,
that there is no sharp boundary that one can draw between gases that follow the principle
of corresponding states and those that don’t.
One important consequence of the principle of corresponding states is that, if a sufficiently
simple universal EOS can be found, then the adjustable parameters of the EOS can be
calculated from the critical parameters of the gas. To exemplify this, let us assume for the
sake of argument that the van der Waals equation is the universal EOS. We could then pick
any two of the equations (9.31)–(9.33) and solve for a and b in terms of any two of the
critical parameters. If the gas followed the van der Waals EOS exactly then we would get
the same values for a and b regardless of whether we chose to solve in terms of {T
c
,P
c
},
{T
c
,V
c
} or {P
c
,V
c
}, as the three critical parameters would be related by (9.41). For real
gases this is not the case, however, as Z
c
=0.375. Because the pressure and temperature of
the critical point are easier to measure accurately than the critical volume, it is customary
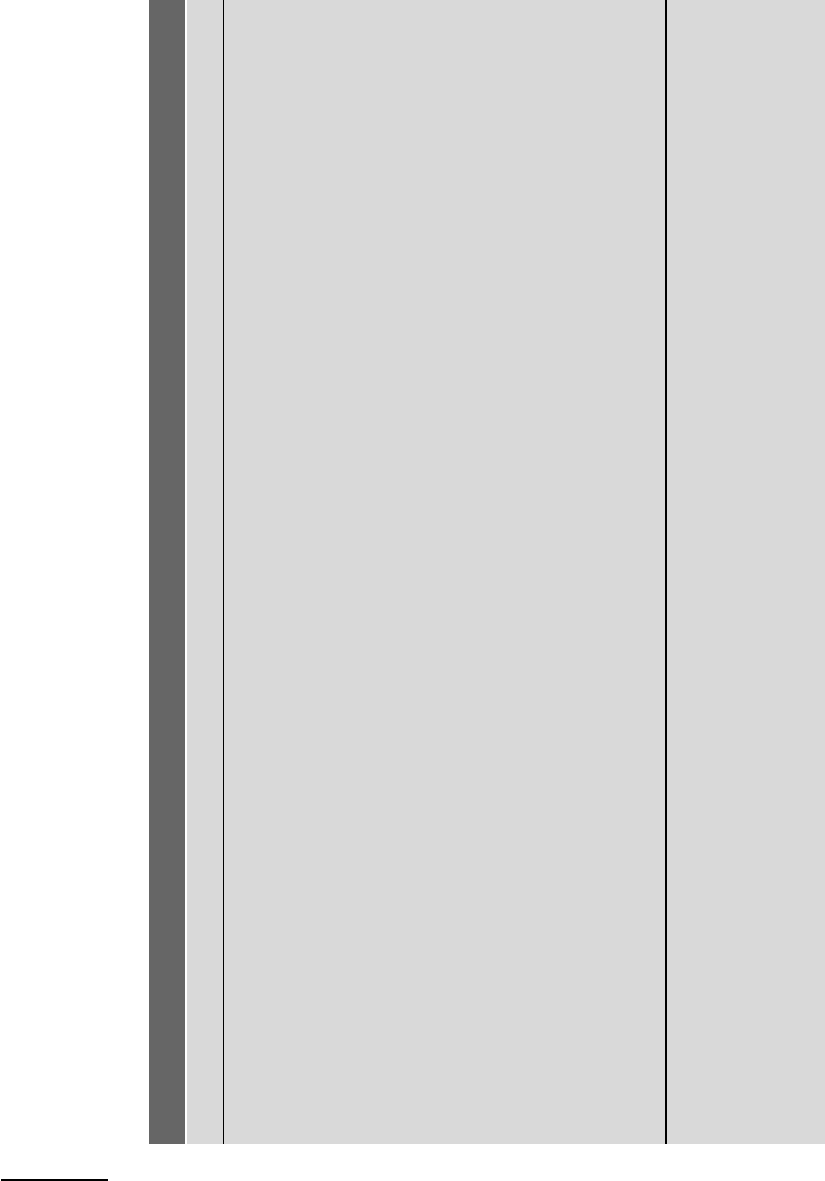
Table 9.1 Critical properties and Redlich–Kwong parameters of some planetary fluids
Species T
c
k P
c
bar V
c
cm
3
mol
−1
ρ
c
kg m
−3
n
c
m
−3
Z
c
a (R–K) b (R–K) ρ
Moho
kg m
−3
ρ
Moho
/ρ
c
Ne 44.4 27.6 42.00 519.0 1.4 ×10
28
0.3140 1.51 ×10
6
12.0
H
2
33.0 12.9 65.00 30.8 9.3 ×10
27
0.3066 1.81 ×10
6
15.3
He 5.2 2.3 57.00 70.2 1.1 ×10
28
0.2998 1.05 ×10
6
11.1
CO 132.9 35.0 93.10 300.8 6.5 ×10
27
0.2948 1.72 ×10
7
27.4 809.1 2.7
Ar 150.9 49.0 75.00 532.6 8.0 ×10
27
0.2929 1.69 ×10
7
22.2
N
2
126.2 34.0 89.20 313.9 6.7 ×10
27
0.2890 1.56 ×10
7
26.7
O
2
154.6 50.5 73.40 436.0 8.2 ×10
27
0.2884 1.74 ×10
7
22.1
CH
4
190.5 46.0 98.40 162.6 6.1 ×10
27
0.2856 3.22 ×10
7
29.9 432.4 2.7
F
2
144.1 51.7 66.00 575.8 9.1 ×10
27
0.2849 1.43 ×10
7
20.1
H
2
S 373.2 89.4 98.50 345.2 6.1 ×10
27
0.2838 8.89 ×10
7
30.1 925.1 2.7
Cl
2
416.9 79.9 123.00 576.4 4.9 ×10
27
0.2836 1.31 ×10
8
37.6
CO
2
304.2 73.8 94.43 466.0 6.4 ×10
27
0.2756 6.46 ×10
7
29.7 1257.7 2.7
SO
2
430.8 78.8 122.00 524.6 4.9 ×10
27
0.2684 1.33 ×10
8
37.4 1390.0 2.6
HCl 324.6 83.1 81.00 450.6 7.4 ×10
27
0.2494 6.75 ×10
7
28.1 1047.3 2.3
NH
3
405.6 112.8 72.50 234.5 8.3 ×10
27
0.2425 8.68 ×10
7
25.9 525.0 2.2
H
2
O 647.1 220.5 56.00 321.4 1.1 ×10
28
0.2295 8.80 ×10
7
14.6 944.1 2.9
HCN 456.7 53.9 139.00 194.2 4.3 ×10
27
0.1973 2.44 ×10
8
61.0 398.2 2.0
HF 461.0 64.8 69.00 289.9 8.7 ×10
27
0.1167 7.80 ×10
7
12.8 345.4 1.2
Critical temperature, pressure and density from Mathews (1972), checked for recent corrections against NIST Chemistry WebBook.
Critical volume and particle number calculated from critical density.
Z
c
calculated from P
c
, V
c
and T
c
(equation (9.41)).
Redlich–Kwong a and b parameters calculated from T
c
and P
c
(equation (9.50)), except values in italics taken from Holloway (1987, Table 1). Units are:
bar cm
6
K
1/2
for a,cm
3
for b.
Estimated density at the Moho, ρ
Moho
, calculated with R–K EOS at 950 K, 10.7 kbar.
441
442 Thermodynamics of planetary volatiles
to solve for a and b in terms of these variables and then we have, from (9.32) and (9.33):
a
vdW
=
27
64
R
2
T
2
c
P
c
, b
vdW
=
RT
c
8P
c
. (9.42)
Equations (9.42) are of interest primarily for historical and didactic reasons, as the van
de Waals EOS, even if qualitatively correct, does not provide an accurate quantitative
representation of the behavior of any real gas. For any EOS with two adjustable parameters,
however, the same methodology can be appliedin order to derive the values of the parameters
from measured values of T
c
and P
c
.
9.4 Equations of state for real fluids at P–T conditions typical of the
crusts and upper mantles of the terrestrial planets
There are two basic approaches to constructing EOS applicable over a wide range of tem-
peratures and pressures. The first one, exemplified by the van der Waals equation, is to
start from the ideal gas EOS and add adjustable parameters that account for repulsion and
attraction between molecules, and for the ways in which these forces vary with temperature
and density. These are empirical equations that are cubic in volume and are thus called
cubic equations of state. We will discuss two equations of this type, in addition to the
van der Waals EOS: the Redlich–Kwong EOS (Redlich & Kwong, 1949) and a successful
modification to this equation due to Kerrick and Jacobs (1981).
The other approach is philosophically more satisfying because it can be shown to have
physical fundamentation, but it unfortunately results in an equation of state that performs
very poorly and that can only be improved by empirical tweaks not unlike those used in cubic
EOS. As we did for some of the equations of state for solids, we begin with an expression
for the Helmholtz free energy of the fluid. In this case we consider two contributions to F,
one corresponding to the Helmholtz free energy of the ideal gas and a second one, called
the residual free energy, F
res
, that encapsulates all of the energetic effects that arise from
intermolecular interactions:
F = F
ideal
+F
res
. (9.43)
We now write the residual term as a power series in density or, equivalently, in the inverse
of volume:
F
res
=RT
a
1
V
+
a
2
2V
2
+
a
3
3V
3
+···
, (9.44)
where the coefficients a
i
, called the virial coefficients, are functions of temperature. The
virial coefficients can be shown to represent the energetic effects arising from interactions
between two molecules (a
1
), three molecules (a
2
) and so on (see, for example, Mason &
Spurling, 1969). The name derives from the fact that the distribution of molecular energies
in terms of kinetic and potential energy terms is described by the virial theorem that we
discussed in Chapter 2.
Differentiating (9.43) we get:
P =−
∂F
∂V
T
=−
∂F
ideal
∂V
T
−
∂F
res
∂V
T
=P
ideal
−
∂F
res
∂V
(9.45)
443 9.4 Equations of state for real fluids
and from (9.44) and the ideal gas EOS:
P = RT
1
V
+
a
1
V
2
+
a
2
V
3
+
a
3
V
4
+···
(9.46)
or, equivalently:
Z = 1 +
a
1
V
+
a
2
V
2
+
a
3
V
3
+···. (9.47)
Equation (9.46), or (9.47), with a
i
=a
i
(T ), is known as the virial equation of state. Despite
its theoretical foundation the equation is unsatisfactory. Many terms are required in order to
represent the behavior of real gases at even moderate pressures, and then at high densities
(small V) the series may diverge, i.e. terms in progressively higher powers of V become
larger rather than smaller. Its one strong point is that it provides an explicit expression for
the Helmholtz free energy (equation (9.44)), which is convenient when calculating other
thermodynamic functions such as fugacity (Section 9.5.1 ). We will discuss an equation of
state (the Pitzer–Sterner EOS) which is constructed following the same idea as the virial
EOS, i.e. beginning from an empirical function for the Helmholtz free energy, but which
is not a virial equation because the function is not a power series in V , and is therefore
not a physical representation of intermolecular potentials. Finally, we will discuss another
empirical EOS (Brodholt–Wood EOS) that combines a cubic EOS with virial-like terms.
9.4.1 Cubic equations: the van der Waals EOS revisited
We begin our discussion with the van der Waals (VDW) EOS, with the sole purpose of
understanding why it fails and what can be done about it. We will compare the predictions
of VDW and three other EOS with the measured density of H
2
O at pressures of 0.1–10 kbar.
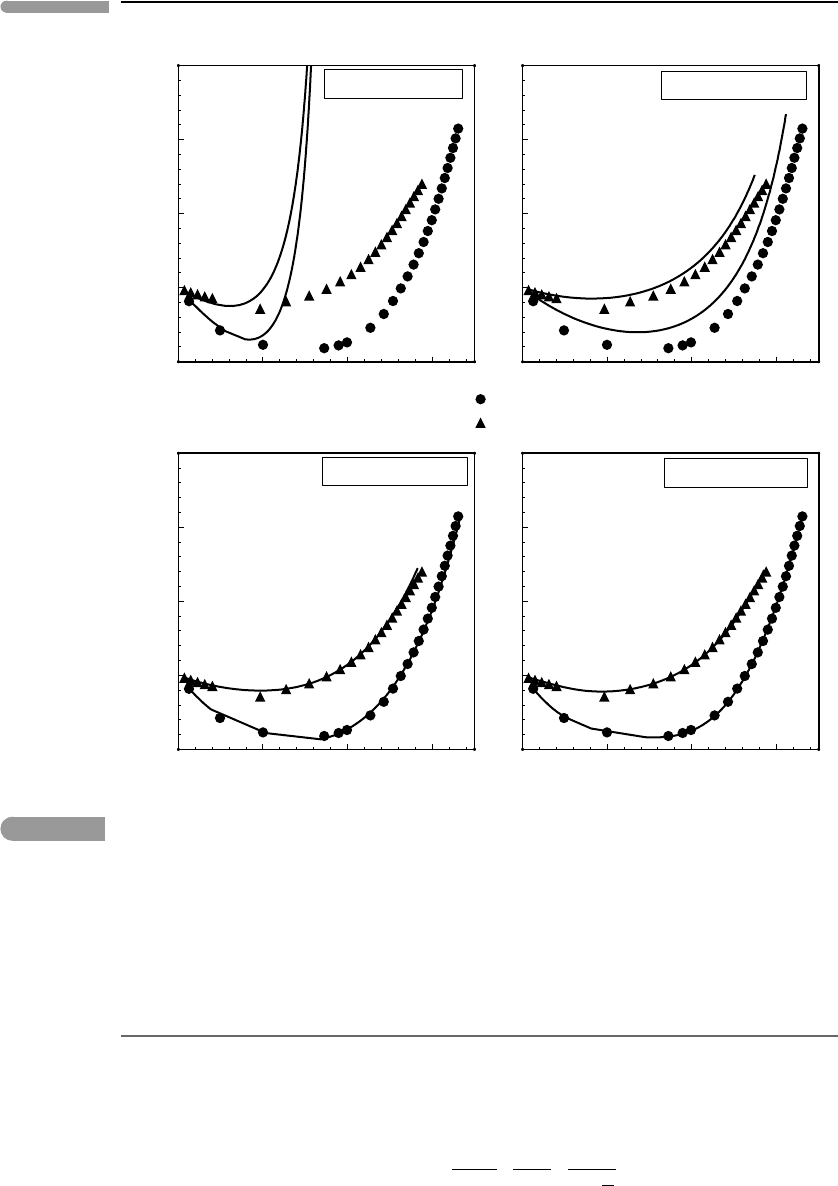
Figure 9.10shows compressibility as a function of reduced density, ρ/ρ
c
=V
c
/V . From the
definition of compressibility (equation 9.22) we also get Z =V/V
ideal
. We can thus interpret
the vertical coordinate either as the ratio of the volume of the gas to that of an ideal gas at
the same pressure, or as the ratio of the pressure acting on a given volume of real gas to
the pressure that would be required to take an ideal gas to the same volume. In any case an
ideal gas would plot in this figure as a horizontal line at Z = 1.
Molar volumes of H
2
O were measured by Burnham et al. (1969) to 8.9 kbar, and extrap-
olated by them to 10 kbar. The symbols in Fig. 9.10represent the density of H
2
O from
0.1–10 kbar along two isotherms, circles for the critical isotherm (T
c
= 647.14 K) and
triangles for 700
◦
C, which corresponds closely to 1.5T
c
. Focusing first on the measured
behavior of H
2
O we see that at low density it is more compressible than an ideal gas
(Z<1), and that it becomes less compressible than an ideal gas as density increases. This is
the behavior that we should expect from a substance with strongly polar molecules: attrac-
tion predominates at low density, but, as the molecules are squeezed more closely together,
repulsion (or the finite size of molecules) takes over. Attraction becomes less important at
high temperature, as thermal agitation tends to swamp intermolecular potentials, explaining
why the data at 1.5T
c
plot at higher Z than data at the critical temperature.
At low densities, generally much lower than the critical density, the VDW EOS is mod-
erately successful, but as density increases it fails spectacularly (Fig. 9.10). This behavior
arises because the repulsive term is a constant. As density increases and V approaches b the
444 Thermodynamics of planetary volatiles
0 1 2 3
0
1
2
3
4
Z
0 1 2 3
0
1
2
3
4
Z
H
2
O, T = T
c
H
2
O, T = 1.5 T
c
0 1 2 3
0
1
2
3
4
Z
0
ρ /ρ
c
ρ /ρ
c
ρ /ρ
c
ρ /ρ
c
1 2 3
0
1
2
3
4
Z
van der Waals EOS
Redlich-Kwong EOS
Kerrick-Jacobs EOS
Pitzer-SternerEOS
Fig. 9.10
Comparison of four equations of state for H
2
O against measured densities at 1 and ∼1.5 T
C
(experimental data from
Burnham et al., 1969).
pressure required to accomplish further compression diverges (equation (9.27)). An impor-
tant reason for the inadequacy of the VDW EOS is its failure to take into account the fact
that molecules are not rigid.
9.4.2 Cubic equations: the Redlich–Kwong EOS
The first successful modification to the VDW EOS was proposed by Redlich and Kwong
in 1949. In this equation the repulsive term (equation (9.24)) is left unchanged, but the
attractive term (equation (9.25)) is modified as follows:
Z
attraction
=
a
RT V
·
1
T
1/2
·
1
1 +
b
V
. (9.48)

445 9.4 Equations of state for real fluids
The additional factors accomplish two things. The first factor causes the attractive force to
fall off faster with increasing temperature than in the VDW equation. The second factor
makes the attractive force less dependent on density. Redlich and Kwong stated that there
is no theoretical justification for these particular correction terms. Rather, they just happen
to vastly improve the agreement between the EOS and measured densities for many fluids,
especially the simple gases discussed in Section 9.3. As in the VDW equation, a and b
in (9.48) are constants with specific values for each gas. Substituting (9.24) and (9.48)in
(9.23) we get the Redlich–Kwong (RK) EOS:
P =
RT
V −b
−
a
T
1/2
V
(
V +b
)
. (9.49)
Because this equation has only two adjustable parameters it is possible to derive their
values from the critical properties of the gas, by using the condition that the second and
third derivatives of the Helmholtz free energy vanish at the critical point. Applying these
conditions to (9.49) we get:
a
RK
=0.4274802337
R
2
T
5/2
c
P
c
, b
RK
=0.08664034997
RT
c
P
c
(9.50)
and Z
c
=1/3. This value for the critical compressibility factor is closer to the value of ∼0.3
measured for the simple gases in Table 9.1. One could then expect that the RK EOS with
parameters calculated from (9.50) may do a reasonable job of representing the properties
of these gases, at least up to moderate pressures. For substances that depart significantly
from corresponding state behavior, however, it is better to obtain the values of the a and b
parameters by fitting the equation to P –V –T measurements spanning as wide a range of
conditions as possible. The parameters for H
2
O in Table 9.1were obtained in this way (data
from Holloway, 1987).
The RK EOS does not solve the problem of the divergence of pressure at a finite volume
that is a characteristic of the VDW EOS. However, comparing (9.50) with (9.42)wesee
that the value of the b parameter in the RK EOS is about half of that in the VDW EOS. This
means that the useful range of the RK EOS can be expected to extend to higher densities
than the VDW EOS. This is borne out by a comparison of its predictions with the measured
density of H
2
O up to 10 kbar (Fig. 9.10). The agreement is far from perfect, and generally
inadequate for accurate thermodynamic calculations, but is much better than for the VDW
EOS. The fit improves with increasing temperature. However, at the highest densities shown
in the diagram the tendency for pressure to diverge clearly insinuates itself. This is a problem
with the EOS, not with H
2
O in particular, which means that its application to simple gases is
also limited to moderate pressures, such that the molar volume remains significantly greater
than b.
9.4.3 Cubic equations: the Kerrick–Jacobs modified Redlich–Kwong EOS
Since Redlich and Kwong’s proposal of equation (9.49) many modifications have been
proposed in order to overcome the limitations of that equation. All of these EOS come under
the general label of modified Redlich–Kwong (MRK) EOS, and they are all empirical fits
of varied complexity. Among the most successful ones for conditions in planetary interiors

446 Thermodynamics of planetary volatiles
is the MRK equation proposed by Kerrick and Jacobs in 1981. This equation leaves the
attractive term in the RK EOS unchanged but: (1) it makes the parameter a a function of
temperature and volume (and hence pressure) and (2) it modifies substantially the repulsive
term. The Kerrick and Jacobs (KJ) EOS is:
P =
RT
1 +y +y
2
−y
3
V
(
1 −y
)
3
−
a
(
V ,T
)
T
1/2
V
(
V +b
)
(9.51)
where:
y =
b
4V
(9.52)
and:
a = a
1
(
T
)
+
a
2
(
T
)
V
+
a
3
(
T
)
V
2
(9.53)
and:
a
i
(
T
)
=c
i,1
+c
i,2
T +c
i,3
T
2
. (9.54)
The KJ EOS has ten adjustable parameters, which obviously makes it much easier for it to
reproduce measured volumes over a wide pressure–temperature range. Figure 9.10shows
that, up to ∼10 kbar, it reproduces the measured behavior of H
2
O almost perfectly, although
at τ = 1.5 there is a hint of the KJ EOS beginning to show divergence in the pressure, as V
approaches b/4.
In contrast to two-parameter equations, it is not possible to estimate the values of the
adjustable parameters of the KJ EOS from the critical properties. The equation can only be
calibrated by fitting it to measured volumes over a pressure–temperature range. Values for
b and the nine c
i,j
parameters are given for H
2
O and CO
2
by Kerrick and Jacobs (1981)
and for CH
4
by Jacobs and Kerrick (1981). Because the KJ EOS is an empirical equation
its performance beyond the range of conditions used to fit the parameters (∼0–10 kbar) is
uncertain, and application to such conditions must be done with caution.
9.4.4 Expansion of the residual Helmholtz free energy: the Pitzer–Sterner EOS
Pitzer and Sterner (1994) proposed an equation of state based on an empirical formulation
for the residual Helmholtz free energy. Their expression is most easily written in terms of
density, ρ = 1/V :
F
res
RT
=a
1
ρ +
1
a
2
+a
3
ρ +a
4
ρ
2
+a
5
ρ
3
+a
6
ρ
4
−
1
a
2
−
a
7
a
8
e
−a
8
ρ
−1
−
a
9
a
10
e
−a
10
ρ
−1
(9.55)

447 9.4 Equations of state for real fluids
where each of the ten adjustable parameters a
i
is a function of temperature parametrized
by the following polynomial:
a
i
(
T
)
=c
i,1
T
−4
+c
i,2
T
−2
+c
i,3
T
−1
+c
i,4
+c
i,5
T +c
i,6
T
2
. (9.56)
The equation has 60 adjustable parameters, although many of them are found to be zero.
Using (9.45) the Pitzer–Sterner (PS) EOS is:
P
RT
=ρ +a
1
ρ
2
−ρ
2
a
3
+2a
4
ρ +3a
5
ρ
2
+4a
6
ρ
3
a
2
+a
3
ρ +a
4
ρ
2
+a
5
ρ
3
+a
6
ρ
4
2
+a
7
ρ
2
e
−a
8
ρ
+a
9
ρ
2
e
−a
10
ρ
.
(9.57)
The equation has no physical justification but is found to reproduce the volumetric proper-
ties of H
2
O and CO
2
to very high pressure with a remarkable degree of accuracy. Figure
9.10shows that the agreement with the measured properties of H
2
O to 10 kbar is essentially
perfect, but the agreement is also excellent to much higher pressures (of order 10
2
–10
3
kbar), for which scattered volume measurements obtained by a variety of experimental
methods are available. The form of equation (9.57) keeps the EOS from blowing up at high
densities, as happens with cubic EOS when the molar volume approaches the value of the
excluded volume, b. The equation is calibrated for H
2
O and CO
2
only. The coefficients for
the two gases are given by Pitzer and Sterner (1994). Given the large number of adjustable
parameters one would expect that the PS EOS would also work well for other fluid species,
but as of this writing, and to the best of my knowledge, no attempt has been made to per-
form the necessary calibrations. It would be interesting to know whether the PS EOS can
be applied successfully to other important fluid species such as CH
4
, CO, H
2
S and NH
3
.
9.4.5 The Brodholt–Wood EOS for H
2
O at high pressure
Brodholt and Wood (1993) performed molecular dynamics simulations of the properties
of H
2
O to 2500 K and 350 kbar. They then fit the following EOS to the results of their
simulations:
P =
RT
V −b
−
a
T
1/2
V
(
V +b
)
+
c
V
+
d
V
2
+
e
V
3
+
f
V
4
(9.58)
with:
a = a
0
+a
1
T +a
2
T
2
+a
3
T
−2
(9.59)
and:
b = b
0
+b
1
V<0 (9.60)
and c,d, e and f constants (values of all coefficients are given by Brodholt & Wood, 1993).
The Brodholt–Wood (BW) EOS has been described as an MRK equation with virial terms,
but I would argue that this is not correct. In the first place, the b term is always negative,
