Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

368 Critical phase transitions
0 0.2 0.4 0.6 0.8 1 1.2
0
0.2
0.4
0.6
0.8
φ
1
0 0.2 0.4 0.6 0.8 1 1.
2
–0.8
–0.6
–0.4
–0.2
0
T/T
λ
T/T
λ
G(φ)/RT
λ
G(0)
ordered phase stable
disordered
phase stable
(a)
(b)
G(φ)
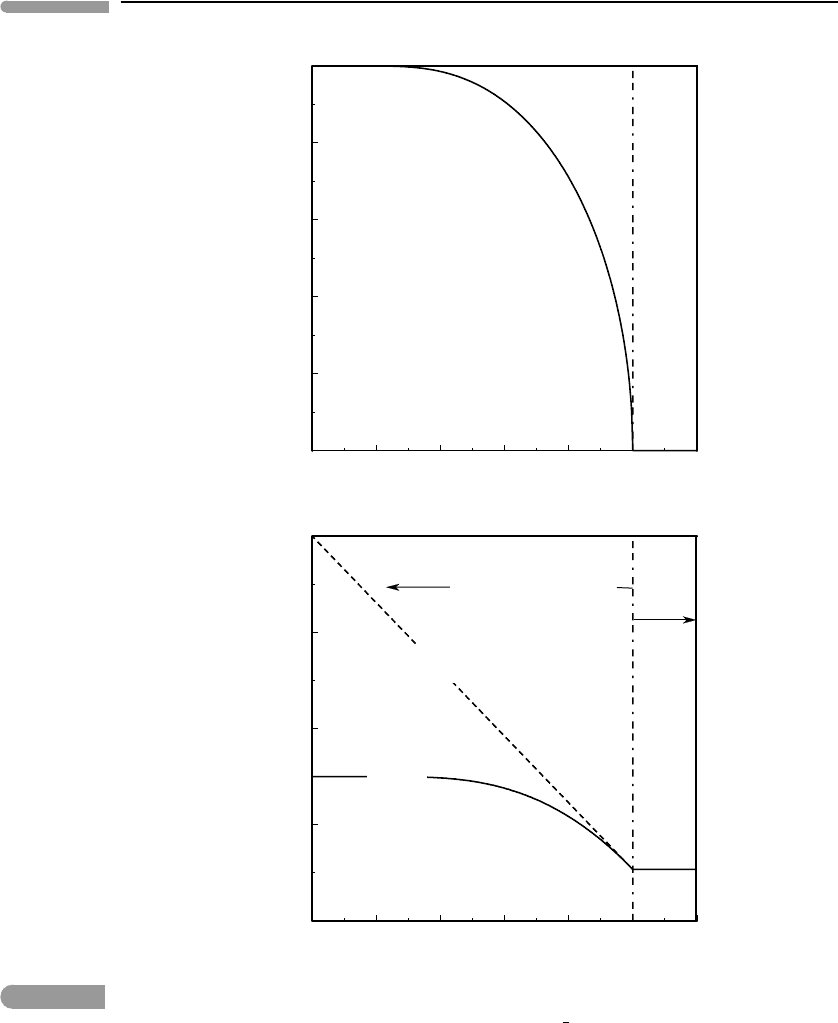
Fig. 7.6
(Top) Order parameter vs. non-dimensional temperature for a phase in which two types of atoms, A and B, occupy
two crystallographic sites, α and β, such that X
A
=X
B
=X
α
=X
β
=
1
2
and that there is A–B affinity. (Bottom)
Ordering contribution to the Gibbs free energy of the phase, shown in non-dimensional units. G(0) is the Gibbs free
energy of the disordered phase, which for T < T
λ
is metastable relative to the ordered phase, shown by G(φ).
369 7.5 Analogies with other phase transitions
0
0.2 0.4 0.6 0.8
–0.3
–0.2
–0.1
0
0.1
0.2
0.3
T/T
λ
=10
-(G(φ) - G(0))/G(0)
φ
T/T
λ
=0.6
T/T
λ
=0.8
T/T
λ
=0.9
T/T
λ
=1
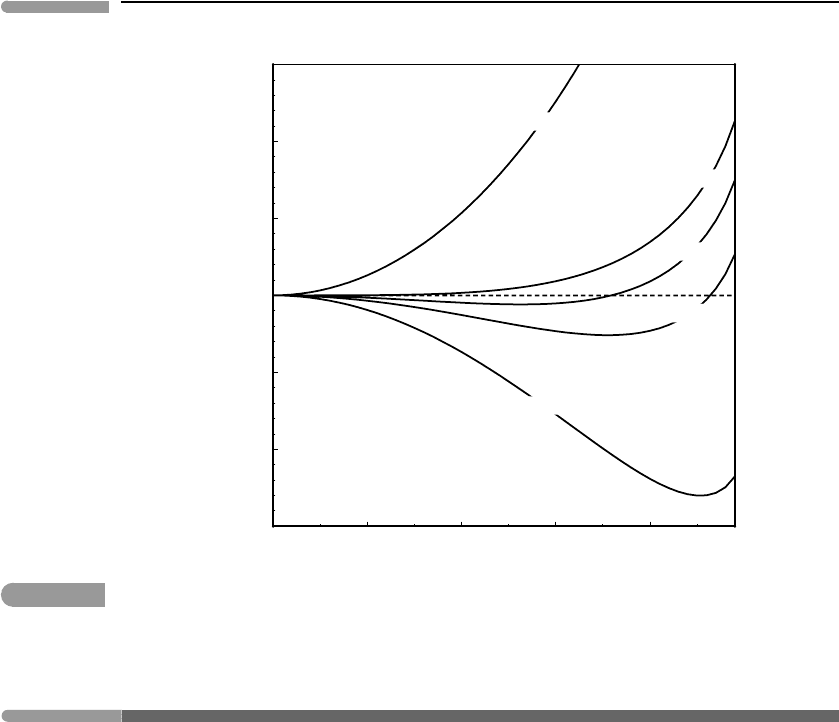
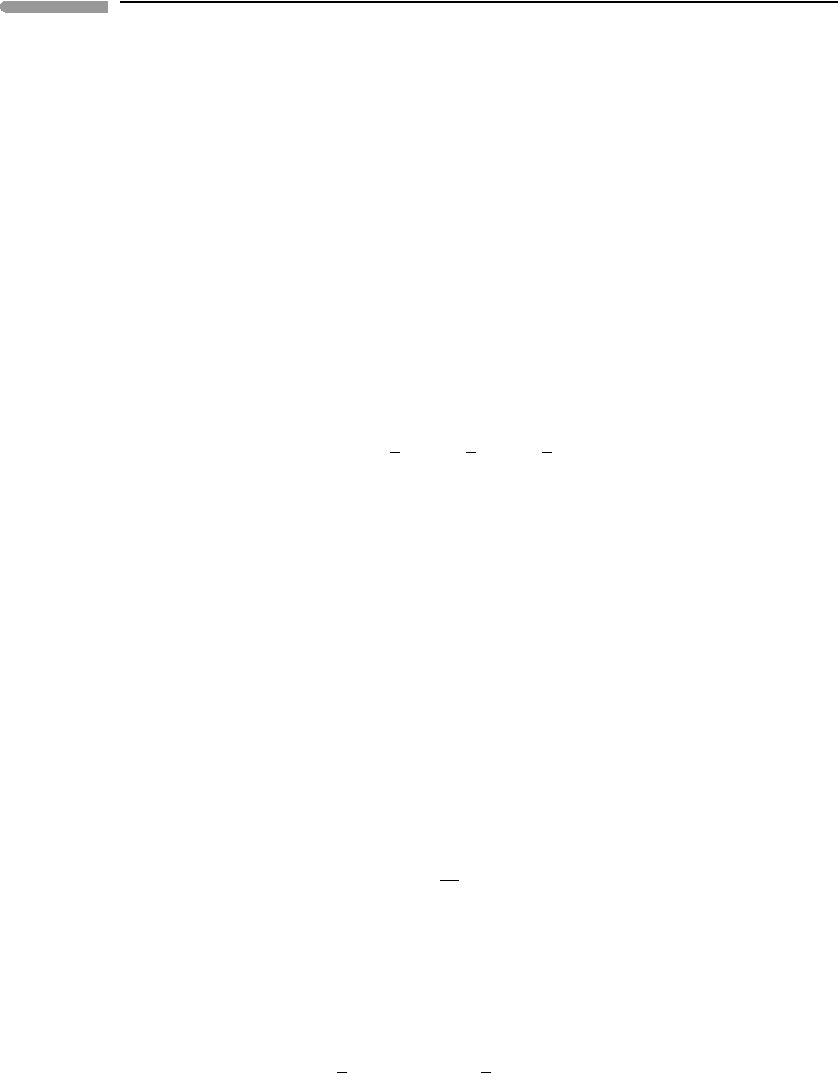
Fig. 7.7
G(φ) as a function of φ at constant temperature. The value of φ at each minimum corresponds to the value of φ at
that temperature in Fig. 7.6, with φ = 0 for T ≥ T
λ
.
7.5 Analogies with other phase transitions
There are systems that display behaviors that are remarkably similar to order–disorder
phase transitions, even though at first sight they do not appear to bear close similarities
to the atomic ordering processes that we discussed in the previous section. The key is to
identify a variable in the system that can serve as an order parameter. Figure 7.8 shows
some examples. The top panel is a calculated lambda phase transition for atomic ordering
– it is in fact the same curve as in Fig 7.6 (top), scaled so as to make comparisons with
the other examples easier. The second panel shows the difference in composition between
the two branches of a calculated symmetric solvus (e.g. from Figs. 7.1 or 7.5), plotted
against the ratio T/T
c
, where T
c
is the critical mixing temperature. The vertical coordinate
is X
A
(α) −X
B
(β). This variable takes values between 0 and 1 (see Fig. 7.1) and it can serve
as the order parameter. The curve is identical to that in the top panel, but note that the two
curves describe different phenomena. In one case it is atomic ordering in a single phase at
constant composition. In the other it is separation of two phases of different composition
from a single homogeneous supercritical phase. A way to think of this is as “ordering” of
the components A and B into the phases α and β, with perfect order (pure A and pure B)
attained only at T = 0, but already approached very closely at ∼0.5T
c
(see Fig. 7.5).
Some minerals acquire higher symmetry with increasing temperature. For example, a
mineral may be orthorhombic at low temperature but, owing to anisotropic thermal expan-
sion, the lengths of its a and b crystallographic axes become progressively closer to one
370 Critical phase transitions
0.5 0.6 0.7 0.8 0.9 1
0
0.2
0.4
0.6
0.8
1
NaMgF
3
perovskite
unit cell dimensions
Zhao et al., 1993
symmetric solvus
(calculated)
H
2
O liquid–vapor
phase transition
lambda transition
(calculated)
0.5 0.6 0.7 0.8 0.9 1
0
0.2
0.4
0.6
0.8
1
X
A
( X-)
A
( )
0.8 0.85 0.9 0.95 1
0
0.2
0.4
0.6
0.8
1
T/T
c
T/T
c
T/T
λ
T/T
λ
V
(
ropav
V
-
iuqil d
/)
V
ropav
0.4 0.6 0.8 1
0
0.05
0.1
-b(aA)
φ
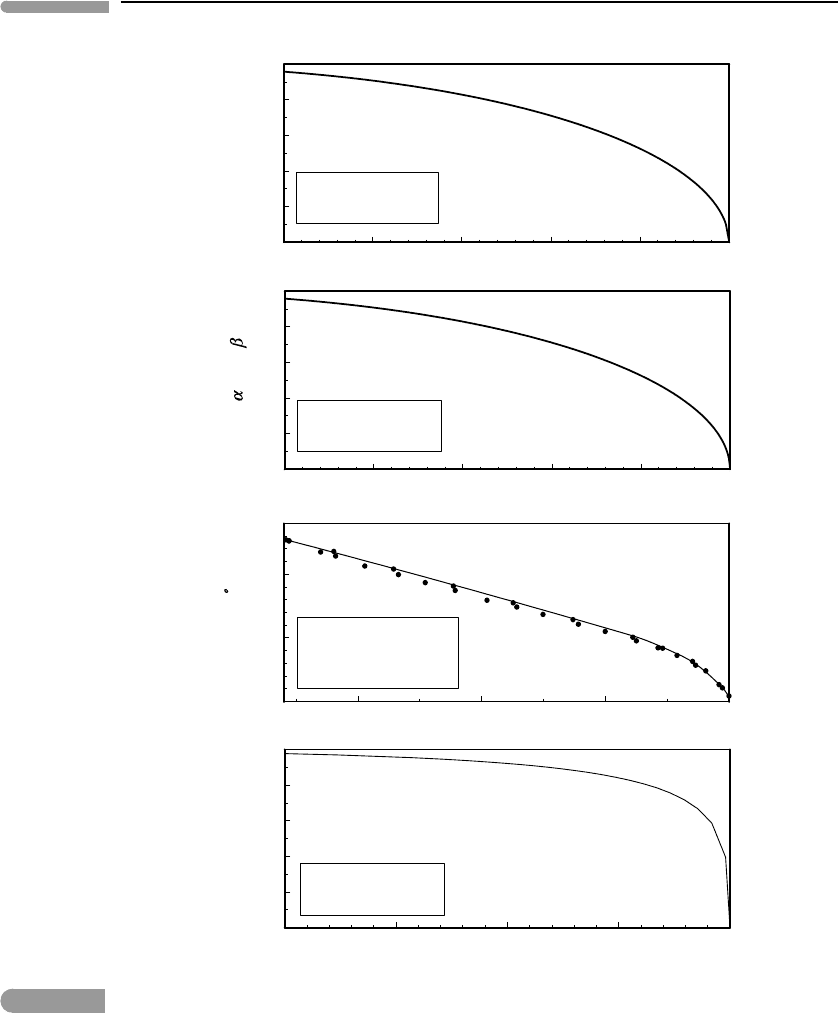
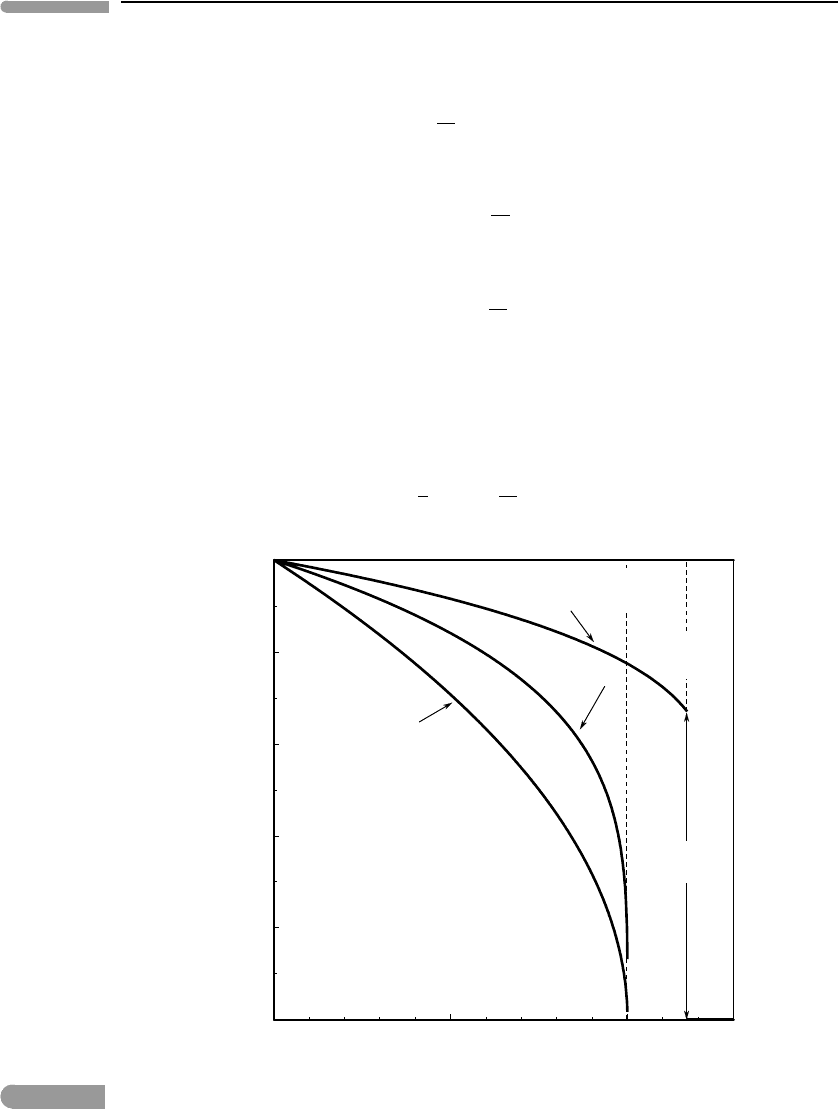
Fig. 7.8
Order parameter vs. non-dimensional temperature. Measured values of crystallographic axes of NaMgF
3
perovskite
from Zhao et al. (1993). The other three curves are calculated and correspond to: the hypothetical phase in Figs. 7.6
and 7.7, the symmetric solvus in Fig. 7.1, and liquid–vapor equilibrium in H
2
O (Chapter 9).
another with increasing temperature, until at a well-defined temperature a and b become
equal. When this happens the symmetry changes from orthorhombic to tetragonal. It can
also happen that all three axes become equal at a single temperature, and in such case the
symmetry of the crystal changes from orthorhombic to cubic. The third panel in Fig. 7.8

371 7.5 Analogies with other phase transitions
shows data for a solid, NaMgF
3
with perovskite structure, that behaves in this way. It
is orthorhombic at low temperature, but as temperature increases the lengths of its three
crystallographic axes approach each other and the solid undergoes a lambda-type phase
transition at T
λ
≈ 1050 K, where it takes on cubic symmetry. The figure shows the dif-
ference (in Å) between the lengths of the a and b crystallographic axes (data from Zhao
et al., 1993). This difference serves as the order parameter, and could be converted, if one
wishes, to a non-dimensional variable that takes the value 1 at T = 0 by dividing by a −b
at 0 K. Even without doing this, however, the figure shows clearly that a −b goes to 0 at an
accelerating rate as T
λ
is approached, just as in the case of an order–disorder transition. In
fact, the change in crystal symmetry can be understood as an order–disorder transition by
considering rotations and distortions in the crystalline framework that affect the entropy of
the crystal (see, for example, Putnis, 1992).
The bottom panel in Fig. 7.8 shows the difference between the molar volumes of water
vapor and liquid water coexisting at equilibrium (obviously, pressure cannot be constant
along this curve – why?), normalized to the volume of the vapor. The molar volumes
become equal at the critical temperature (∼647.3 K) and a single supercritical phase exists
at T>T
c
. Two phases exist below T
c
, and their volumes diverge rapidly immediately below
this temperature. We shall return to critical phenomena in fluids in Chapter 9. There are
other important critical phenomena that we will not discuss in this book. Examples include:
the para-ferromagnetic transition at the Curie temperature, responsible, for example, for
the preservation of thermal remanent magnetization in igneous rocks; the appearance of
superconductivity in some materials at low temperature; and the existence of a superfluid
liquid-helium phase near absolute zero. What is common to all of these processes is that in
all of them it is possible to define an order parameter that goes to zero continuously and at
an accelerating rate as the critical temperature is approached from below, and that stays at
zero above the critical temperature.
It has been known for a long time that systems that behave in this way display anomalies
in the second derivatives of the Gibbs free energy in the neighborhood of the critical
temperature. This is true of heat capacity, compressibility and thermal expansion (equations
(4.135)to(4.137)), but for now we will focus on c
P
only. At a first-order phase transition
these three quantities become infinite, because as long as a univariant assemblage is present
energy transfer (either thermal or mechanical) causes changes in entropy and volume at
constant temperature and pressure, by forming some phases at the expense of others. The
“infinite heat capacity” occurs only at the first order phase transition, at which S and V are
discontinuous, but c
P
(and the other second derivatives) behave normally everywhere else.
At a lambda phase transition the second derivatives are anomalous in the neighborhood of
the transition temperature, and diverge strongly (but may or may not become infinite) at the
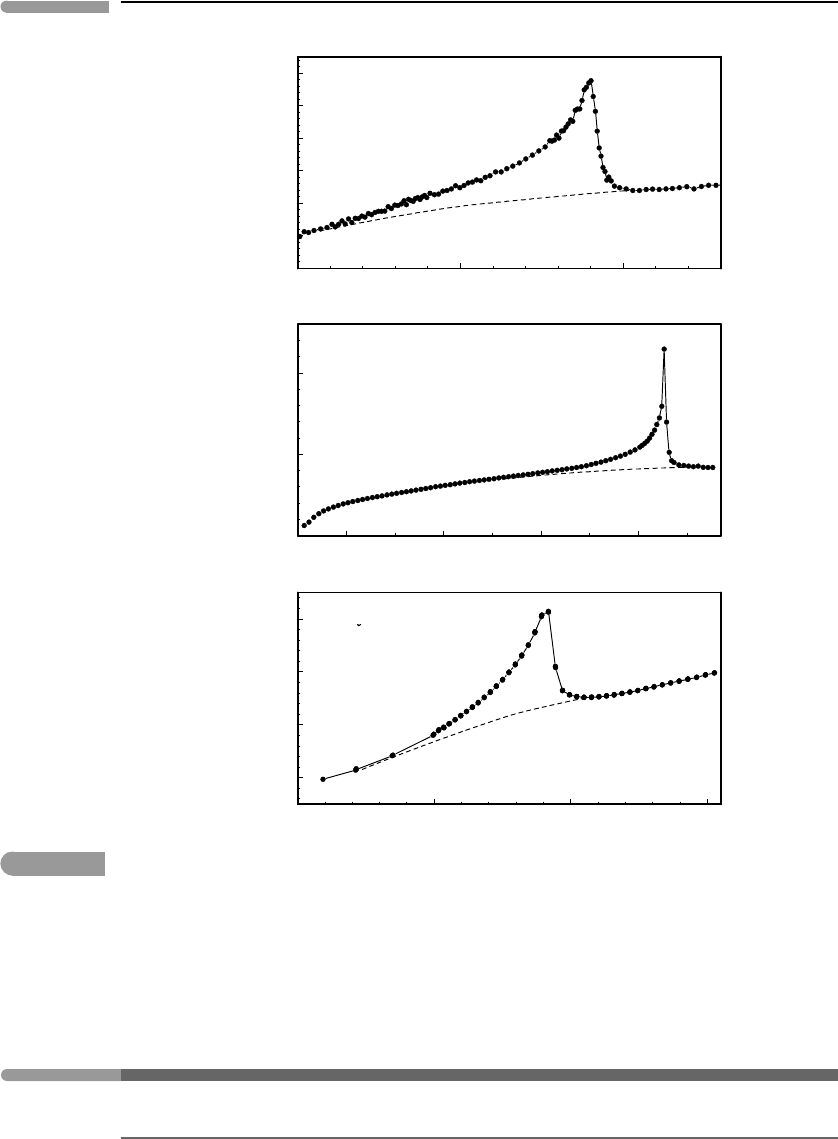
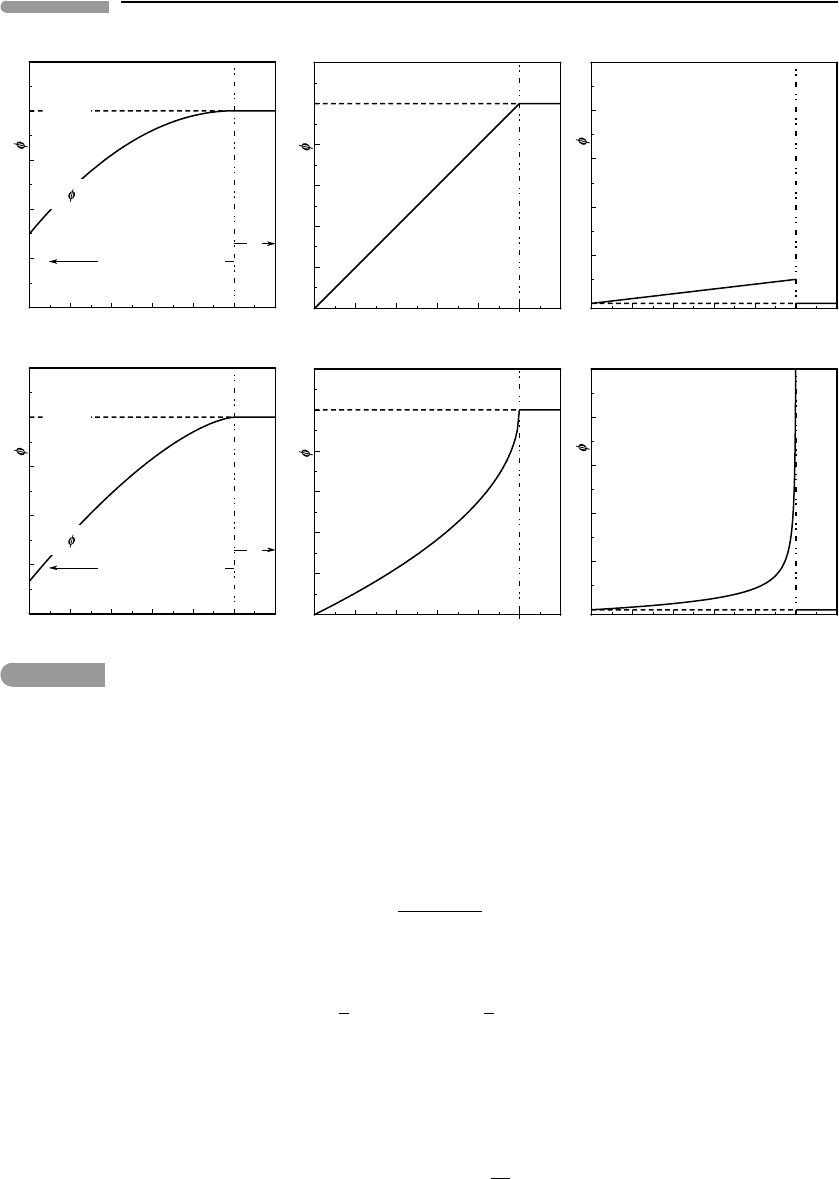
transition temperature itself. Three examples of this behavior are shown in Fig. 7.9. The top
two correspond to crystal symmetry phase transitions, including the example of NaMgF
3
perovskite. The bottom panel in Fig. 7.9 corresponds to an order–disorder transition caused
by alignment of magnetic moments in ferrosilite. In every case the dashed curve shows what
the “normal” heat capacity would be expected to be. The measured values define a strong
and steep positive anomaly relative to these expected values, which is characteristically
lambda-shaped, hence the name for this type of phase transition. An important point is that,
whether or not c
P
becomes infinite at the transition temperature, the function is integrable
across the transition, i.e., the area under the lambda-shaped anomaly is finite. This area needs
to be known in order to be able to calculate the entropy of the solid at T > T
c
(Section 4.7.1).
372 Critical phase transitions
350 400 450
210
220
230
240
250
260
270
T (K)
20 30 40 50
10
20
30
40
T (K)
400 600 800 1000
100
150
200
T (K)
C
P
J(K
–1
lom
–1
)
C
P
J(K
–1
lom
–1
)
C
P
J(K
–1
lom
–1
)
ZrW
2
O
8
Yamamura et al. (2004)
Ferrosilite (Fe
2
Si
2
O
6
)
Cemic and Dachs (2006)
NaMgF
3
perovskite
Topor et al. (1997)
Fig. 7.9
Lambda heat capacity anomalies in three different solid phases. Data from references shown in the figure.
In the simple thermodynamic description of an order–disorder phase transition that we
developed in Section 7.4 there is no indication of the behavior depicted in Fig. 7.9. There
is need for a theoretical framework that reproduces this observed behavior.
7.6 Landau theory of phase transitions
7.6.1 Critical phase transitions
The key idea of Landau theory of phase transitions is to start with equation (7.22), in which
we made G equal to the sum of a function of T and P and a function of φ, and expand the
373 7.6 Landau theory of phase transitions
function G(φ) as a power series in φ. When this is done G(φ) is (informally) called the
Landau potential. As before, we seek the values of φ that minimize G(φ), but in this case
we will make no assumptions about the functional forms of H(φ) or S(φ). Rather, we will
derive these, as well as the heat capacity, from the temperature derivatives of the Landau
potential. We require that the order parameter be a non-dimensional variable in the interval
0 ≤ φ ≤ 1 and symmetric relative to 0, so that if we wish to define the order parameter
backwards it would be G(−φ) = G(φ) (this is a minor point, but simplifies the notation).
We also require that at a critical phase transition φ goes to zero as the critical temperature
is approached from below, and remains at zero for T > T
c
. Beyond these constraints we
attach no specific meaning to the order parameter, which gives us flexibility to define it a
posteriori in the most convenient way for each specific application. In fact, the requirement
that the maximum value of φ be 1 can be relaxed trivially, as we shall see.
Symmetry of the order parameter means that we must include only even powers of φ,so
that, including numerical coefficients that will simplify the derivatives, we write the power
series as follows:
G
(
φ
)
=
1
2
g
2
φ
2
+
1
4
g
4
φ
4
+
1
6
g
6
φ
6
+··· (7.42)
The coefficients g
i
are empirical macroscopic parameters, as is the order parameter φ. The
microscopic description that we used to construct (7.32) (number of microstates, energy
of nearest neighbor pairs), or any other kind of a priori microscopic model, is not a part
of Landau theory. Landau theory is an example of a mean field theory. The power of this
approach is that it is applicable to a large class of phenomena which may have only remote
microscopic similarities, and for which our microscopic understanding may be limited. Of
course, there is a drawback too, and that is that it provides little insight about the microscopic
nature of a specific phase transition.
We will initially assume that we can truncate the power series after the second term. As
usual, we require that there be a minimum of G(φ) for φ = 0atT ≥ T
c
, and for a value of
φ>0atT < T
c
. Taking the first and second derivatives of (7.42), these conditions require
the following three relationships (see Exercise 7.2):
g
2
> 0, T>T
c
g
2
< 0, T<T
c
φ
2
=−
g
2
g
4
> 0, T<T
c
.
(7.43)
One solution to (7.43) is to make g
4
a positive constant, and g
2
a linear function of (T −T
c
):
g
2
=α
(
T −T
c
)
(7.44)
with α>0. We then re-write (7.42) as follows:
G
(
φ
)
=
1
2
α
(
T −T
c
)
φ
2
+
1
4
g
4
φ
4
, α>0, g
4
> 0. (7.45)
This function has the same geometric properties as the function G(φ) plotted in Fig. 7.7:a
sharp minimum at φ = 0 for T > T
c
, a “flat” minimum for T = T
c
and a minimum at φ>0
and maximum at φ = 0 for T < T
c
(Exercise 7.3). Perhaps more interestingly, from the last
line in (7.43) we get an equation for φ valid for T<T
c
(we will use only the positive root,
374 Critical phase transitions
because by construction φ is symmetric):
φ =
α
g
4
(
T
c
−T
)
1/2
. (7.46)
If we scale the order parameter so that φ = 1atT = 0, then we get:
T
c
=
g
4
α
(7.47)
and:
φ =
1 −
T
T
c
1/2
. (7.48)
Note that scaling the order parameter to the interval [0,1] is a matter of convenience, not of
necessity. Function (7.48) is plotted in Fig. 7.10 (labeled “critical”). Substituting (7.48)in
(7.45), and recalling that φ = 0 for T ≥ T
c
we get:
G
(
φ
)
=0, T ≥ T
c
G
(
φ
)
=−
1
4
g
4
1 −
T
T
c
2
, T<T
c
(7.49)
0 0.5 1
0
0.2
0.4
0.6
0.8
1
T/T
c
critical
tricritical
first order
T
r
T
c
φ
φ
jump
Fig. 7.10
Order parameter vs. non-dimensional temperature predicted by Landau theory for three types of phase transitions.
The critical phase transition corresponds to the curve in Fig. 7.6 (top), but the curve in that figure was calculated from
a microscopic model of the phase, and the curve in this figure from mean-field Landau theory. The tricritical phase
transition is associated with a lambda-shaped heat capacity anomaly, see Fig. 7.11. The first-order phase transition
occurs discontinuously at T
r
.

375 7.6 Landau theory of phase transitions
and, differentiating (7.49) relative to T:
S
(
φ
)
=0, T ≥ T
c
S
(
φ
)
=−
α
2
1 −
T
T
c
=−
α
2
φ
2
, T<T
c
. (7.50)
We note that at φ =0 both G(φ) and S(φ) are continuous, and G(φ) is smooth, confirming
that these equations describe a continuous, and not a first-order,phase transition. Calculation
of H (φ) is left as an exercise (Exercise 7.3). Note that spontaneous ordering is always
exothermic, so that the entropy of the “universe” increases by a greater amount than the
decrease in the entropy of the system given by (7.50), as required by The second Law (more
on this in Chapter 14).
We will label the contribution of the ordering process to heat capacity C
p
(φ) and, by
analogy with (7.22), we make C
p
= C
p
(P , T)+C
p
(φ). Differentiating (7.50) and using
(4.135):
C
P
(
φ
)
=0, T ≥ T
c
C
P
(
φ
)
=
α
2
T
T
c
=−
α
2
2g
4
T , T<T
c
. (7.51)
Heat capacity is thus discontinuous at the phase transition, and this is in fact the origin
of the name “second order” – entropy (and volume) are not smooth across a continuous
phase transition, and the second derivatives of the free energy are thus discontinuous. The
name is discouraged, though, because in some cases discontinuities show up in higher
order derivatives. “Critical” or “continuous” are preferable names to encompass all “not
first order” phase transitions.
The behaviors of the three thermodynamic functions at a critical phase transition are
shown in the top three panels of Fig. 7.11. Comparison of the free energy plot with that in
Figure 7.6 (bottom) shows what Landau theory accomplishes: it makes G smooth across
the phase transition, while preserving the general relationship between G of the stable
ordered phase and G of the metastable disordered phase below T
c
. If you rotate the curves
to the left of the phase transition in Fig. 7.6 (bottom) counterclockwise until the dashed line
(G of metastable disordered phase) becomes the extension of the solid line (G of the ordered
phase above T
c
) you will get, more or less, the Gibbs free energy diagram in Fig. 7.11.
With this transformation G becomes smooth and S becomes continuous at the phase
transition.
7.6.2 Lambda phase transitions
Although heat capacity is discontinuous at T
c
, the ordering contribution to C
P
varies linearly
with temperature at T<T
c
(Fig. 7.11). Thus, although equations (7.45) through (7.51) are
a correct description of a continuous (or critical) phase transition, they do not describe
the behavior observed at lambda phase transitions, where C
P
characteristically diverges
strongly (Fig. 7.9). Can Landau theory be made to account for this? Inspection of equations
(7.51) suggests that, if g
4
were made a function of (T −T
c
), then the heat capacity function
would diverge as T → T
c
. However, it would not have a finite integral, so this solution
is not acceptable. We then do the next best thing, which is to make g
4
= 0, immediately
invalidating (7.45) through (7.51). We note, however, that if g
4
vanishes, then the only way
376 Critical phase transitions
0
–0.8
–0.4
0
G( )
0
–0.8
–0.4
0
S( )
0
0
10
C
P
( )
0
–0.8
–0.4
0
T/T
c
T/T
c
T/T
c
T/T
c
T/T
c
T/T
c
G( )
0
–0.8
–0.4
0
S( )
0
0
10
C
P
( )
G(0)
G( )
ordered phase stable
disordered phase stable
G(0)
G( )
ordered phase stable
disordered phase stable
1
1
1
1
1
1
Fig. 7.11
Gibbs free energy, entropy and heat capacity for systems that undergo a critical phase transition (top) or a tricritical
phase transition (bottom). The Gibbs free energy diagrams are similar to the one in Fig. 7.6 (bottom), rotated
counterclockwise so that the Gibbs free energy function is smooth. The effect of this rotation is to make entropy
continuous, but not smooth, at the phase transition.
of allowing for the ordered phase to be stable at T<T
c
is to have g
6
=0. In effect, we keep
(7.44) and the first two lines in (7.43), and from the first derivative of (7.42) with g
4
= 0
we replace the third line with (see Exercise 7.5):
φ
4
=
α
(
T
c
−T
)
g
6
> 0, T<T
c
, (7.52)
which shows that it must be g
6
> 0. We then replace (7.45) with:
G
(
φ
)
=
1
2
α
(
T −T
c
)
φ
2
+
1
6
g
6
φ
6
, α>0, g
6
> 0 (7.53)
and note that this function generates a family of curves that have the same general char-
acteristics as those obtained from (7.45) (Exercise 7.6). We now obtain a set of equations
that parallel (7.46) through (7.51), but with a crucial difference, as we shall see. Scaling the
order parameter as before so that φ = 1atT = 0, we have:
T
c
=
g
6
α
(7.54)

377 7.6 Landau theory of phase transitions
and, from (7.52):
φ =
1 −
T
T
c
1/4
, T<T
c
. (7.55)
Function (7.55) is labeled “tricritical” in Figure 7.10 (the reason for this name is essentially
historical, and need not concern us; see, for example, Griffiths & Wheeler, 1970; Griffiths,
1973). The Landau potential is now given by:
G
(
φ
)
=0, T ≥ T
c
G
(
φ
)
=−
1
3
g
6
1 −
T
T
c
3/2
, T<T
c
(7.56)
and the contribution of order–disorder to entropy:
S
(
φ
)
=0, T ≥ T
c
S
(
φ
)
=−
α
2
1 −
T
T
c
1/2
=−
α
2
φ
2
, T<T
c
. (7.57)
The crucial difference is in the contribution of ordering to heat capacity, which now becomes:
C
P
(
φ
)
=0, T ≥ T
c
C
P
(
φ
)
=
α
4
T
√
T
c
(
T
c
−T
)
−1/2
, T<T
c
. (7.58)
The bottom panels of Fig. 7.11 show plots of the thermodynamic functions for tricritical
phase transitions (equations (7.56), (7.57) and (7.58)). The heat capacity diverges at T
c
,
but it has a finite integral. Comparison of (7.58) with measured heat capacities (Fig. 7.9)
suggests that a Landau potential given by (7.53) may provide a good approximation to the
behavior of solids that undergo lambda phase transitions.
7.6.3 Discontinuous phase transitions
Beyond the intriguing name, what is a tricritical phase transition? The difference with a
critical phase transition becomes clear once we realize that Landau theory can also describe
discontinuous phase transitions. We have considered the cases g
4
> 0 and g
4
= 0, so it is
natural to be curious about the case g
4
< 0. Let us begin by seeking the equilibrium value
of the Landau potential (with g
4
< 0) at the critical temperature. Taking the first derivative
of (7.42) and equating to zero:
dG
(
φ
)
dφ
=α
(
T −T
c
)
φ +g
4
φ
3
+g
6
φ
5
=0 (7.59)
we find that at T = T
c
the Landau potential has extrema at φ = 0 and φ = (−g
4
/g
6
)
1
2
–
recall that we only use the positive square root. The first non-vanishing derivative for the
solution φ = 0 is the fourth derivative:
d
4
G
(
φ
)
dφ
4
=6g
4
< 0. (7.60)
