Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

338 Phase equilibrium and phase diagrams
What would happen if the bulk proportion of ethane in Titan’s hydrocarbons was between
60% and 70 %, i.e. in the interval between the freezing and vaporization curves? There would
still be liquid hydrocarbons on the surface, but in equilibrium with a lower hydrocarbon
partial pressure in the atmosphere. This is shown in the right panel of Fig. 6.20, which shows
isothermal phase relations at 95 K, calculated with equations (6.69). The bounding curve of
the vapor field (called the vaporus, by analogy to solidus and liquidus; see Ricci, 1966)isso
steep in this region that atmospheric hydrocarbons would still be essentially pure methane,
even if lakes were composed of up to 70% ethane. At approximately this composition the
freezing curve is intersected, such that for bulk compositions with more than 70% ethane
one would find hydrocarbon icefields and frozen lakes in equilibrium with atmospheric
hydrocarbons that would still be dominated by methane, unless the bulk composition was
almost pure ethane. The solid–vapor curves are shown (schematically) in the figure, and
could be precisely calculated with (6.69) if the vapor pressure of pure solid ethane at 95 K
were accurately known, which as far as I can tell is not the case.
6.6.2 Complete immiscibility in the low temperature or high pressure phase
We now turn to the case in which there is complete miscibility between the two compo-
nents on one side of the phase transition, typically in a liquid or gas phase, and complete
immiscibility on the other side, typically between two solid phases. We will assume that
the high-entropy and high-volume phase, labeled 2 as before, is the one in which A and B
mix without restrictions. Phase 1 is replaced by two distinct phases in the same aggrega-
tion state, a and b, composed of pure A and B, respectively. As in the previous case, the
phase transitions for pure end-member phases are such that, at constant pressure T
A
<T
B
and at constant temperature P
A
>P
B
. Consider the isobaric relations for a phase transi-
tion between condensed phases first. This is almost always a melting reaction, although in
principle it could also take place between two immiscible solids with different crystalline
structures on one side and a third solid on the other. Since now a and b are pure phases the
activities of A and B in them are unity and equations (6.63) become:
X
A
2
=α
(
T
)
1
γ
A
2
X
B
2
=β
(
T
)
1
γ
B
2
. (6.70)
We have only one mol fraction equation, for the high-entropy phase:
X
A
2
+X
B
2
=1. (6.71)
The situation is rather different from the one that arises if there is full miscibility in both
phases. We cannot choose any arbitrary temperature and solve for the composition of
phase 2. Rather, 6.70 and 6.71 constitute a system of three equations in three unknowns:
the two mol fractions in phase 2 and temperature. The system of equations has zero degrees
of freedom, meaning that there is only one temperature at which the three phases co-exist
at equilibrium. This is of course the phase rule result: three phases in a binary system have
one degree of freedom, that is taken up by pressure, which we fix arbitrarily. Temperature
is therefore fixed by the stable co-existence of the three phases. This temperature, which is
339 6.6 Discontinuous phase transitions
the solution to the system of simultaneous equations (6.70) and (6.71), is called the eutectic
temperature, T
e
.
In this case the parameters α(T) and β(T) equal the activities of each of the components
in phase 2, because the phases a and b remain at their standard states (equation (6.70)).
Therefore, if phase 2 is a stable solution (we will return to this in the next chapter), then
by equation (5.31) both α(T) and β(T) must be less than one (see also Fig. 5.7). Given that
phase 2, typically a liquid or gas, is the high entropy phase,
r
H is always positive. It then
follows from equation (6.51) that the eutectic temperature must be lower than both of the
end-member phase transition temperatures: T
e
<T
A
<T
B
. This relationship applies most
commonly to melting. In order for melting to occur at equilibrium at a point other than
the eutectic, one of the solid phases must disappear – the phase rule assures us of this.
Say that the phase that disappears is a. Then the chemical potential of A in the liquid must
decrease relative to that at the eutectic and, by equation 5.31, the chemical potential of B
must increase (Fig. 5.7). If the temperature did not change then the chemical potential of B
would be lower in the solid than in the liquid. Because the liquid is the high-entropy phase
equilibrium between solid and liquid can be restored only by increasing the temperature.
The algebraic expression of this is that, when one of the solids disappears, we are left with
only one of the equations in (6.70), which represents equilibrium between liquid and the
other solid phase. This equation can be solved for the mol fraction of one component, A
or B, in the liquid, in equilibrium with its pure solid, a or b, for any temperature between
T
e
and T
A
or T
B
, respectively. There are two physical solutions for T
e
<T <T
A
, and only
one for T
A
<T <T
B
. The liquid must disappear at T<T
e
. In contrast to the binary loops
discussed in the previous section there is no analytical solution for this system of equations,
because temperature appears in an exponential function, equation (6.51) (i.e. α(T) and β(T)
in (6.70)). It is, however, very easy to write a Maple routine that solves for the eutectic
temperature and the composition of phase 2 at the eutectic (Software Box 6.1).
Software Box 6.1 Calculation of melting loop and eutectic melting and vaporization
The worksheet phasediags1.mw contains two Maple procedures.
Ibin_Tloop: Calculates a melting loop assuming full miscibility and ideal one-site
solution behavior in the two coexisting phases. It solves the system of equations
(6.63) and (6.65). Note that these calculations can also be implemented in a
spreadsheet.
Ibin_eutec: Calculates eutectic melting relations assuming complete immiscibil-
ity in the solid and full miscibility and ideal solution in the liquid. It first solves
equations (6.70) and (6.71) for the eutectic temperature, and then calculates each
branch of the liquidus with the corresponding equation from (6.70). Each branch
of the liquidus is stored in a separate file, with “a” or “b” appended at the end
of the name. Both branches are stored in terms of mol fraction of species A.
The eutectic behavior for vaporization reactions is obtained in the same way, by solving
the system of equations:
X
A
2
=
α
(
T
)
P
X
B
2
=
β
(
T
)
P
(6.72)
340 Phase equilibrium and phase diagrams
together with equation (6.71), and recalling that in this case α(T ) and β(T ) are given by
equation (6.60) (see Software Box 6.1).
Of considerable interest are isothermal phase transitions involving eutectic vaporization
or condensation at low pressure. In this case the system of equations consists of two versions
of (6.62):
X
A
2
=
p
A,0
P
X
B
2
=
p
B,0
P
(6.73)
together with equation (6.71). The total pressure P is the combined partial pressure of the
two chemical species. An analytical solution is in this case trivial, with the pressure at the
eutectic given by:
P
e
=p
A,0
+p
B,0
. (6.74)
The eutectic composition follows immediately from substitution of P
e
in (6.73). In this case
the combined partial pressure at the eutectic is greater than the partial pressures at the end-
member phase transitions. The gas compositions in equilibrium with each of the condensed
phases at any pressure P ,p
B,0
<P <P
e
or p
A,0
<P <P
e
is also obtained directly from
(6.73).
Isothermal phase relations for condensed phases are obtained by substituting α(P) and
β(P) – see equations (6.55) and (6.56) – for α(T) and β(T)in(6.70). They also predict a
eutectic pressure, P
e
, which must be higher than the phase transition pressures for the two
end-members, i.e. P
e
>P
A
>P
B
(exercise left for the reader).
The key aspect of eutectic phase relations is that the melting and boiling points of an
assemblage of immiscible phases are lower than the corresponding values for each of the
phases in isolation, and the vapor pressure of the assemblage is higher than those of either of
the isolated phases. An assemblage of immiscible phases is less refractory and more volatile
than each of the phases by themselves. Equally important is the fact that the composition of
the liquid or gas that forms at the eutectic point (minimum temperature or maximum vapor
pressure) is fixed, and is independent of the bulk composition of the system. These simple
facts underlie many fundamental planetary processes. To name just two, they are the reason
why terrestrial planets have basaltic crusts (the Earth too, or at least 70% of it) and why
granitic rocks have a well-defined and restricted compositional range. We will examine
these and other applications of eutectics in later chapters. At this point it is important
to build an understanding of how eutectics work, including what are the thermodynamic
parameters that determine the magnitude of the displacement of the eutectic temperature or
vapor pressure.
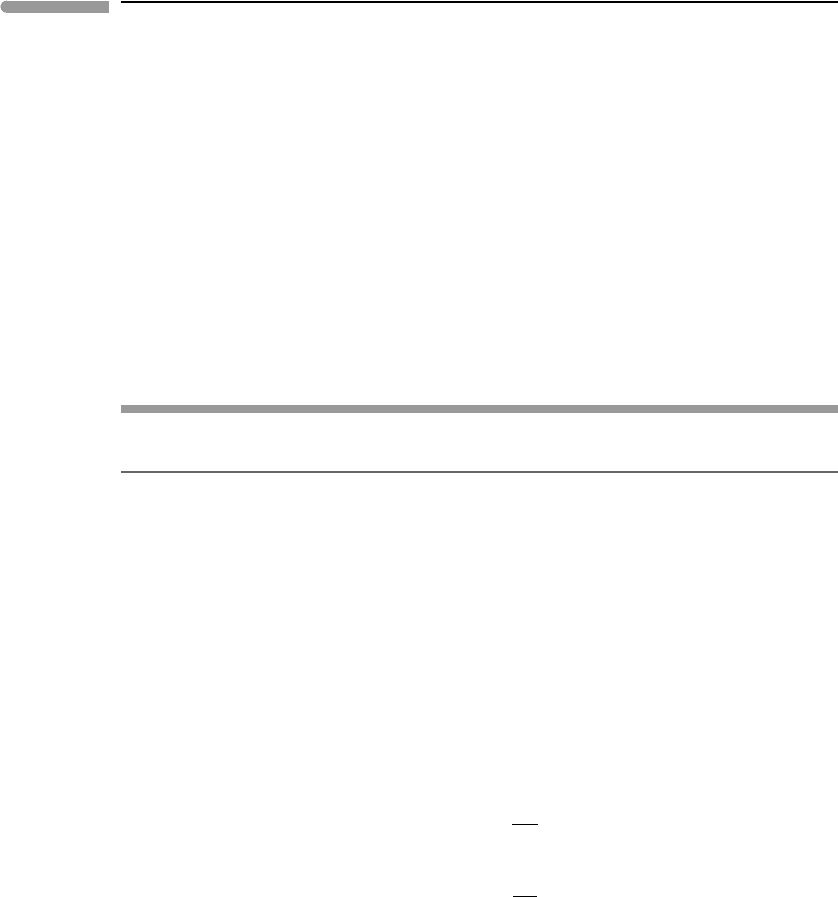
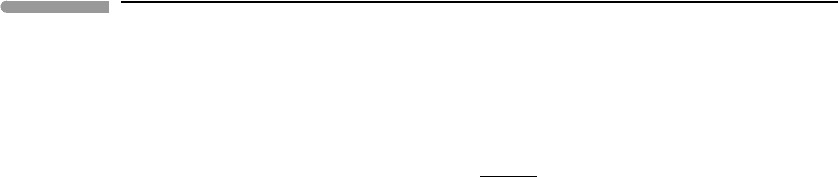
Figure 6.21 shows three isobaric phase diagrams for eutectic melting of hypothetical
substances that are reasonable models for materials abundant in terrestrial planets. In all
cases the pure solids have the same melting point: a (=pure component A) melts at 1600 K,
and b (pure B) melts at 1950 K. They also have the same enthalpy of fusion, but this differs
among the three diagrams: 100 kJ mol
−1
in the top panel, 30 kJ mol
−1
in the middle and
10 kJ mol
−1
in the bottom. Silicate minerals typically fall in the range between the top and
middle panels, and metals between the middle and bottom panels (see Worked Example
6.8). The magnitude of the melting point depression is a strong inverse function of the
enthalpy of melting, and it is easy to see why. Fixing the composition of the liquid at any
341 6.6 Discontinuous phase transitions
0 0.2 0.4 0.6 0.8 1
1000
1500
2000
X (A)
T (K) T (K)
T (K)
T
B
T
A
f
H
A
=
f
H
B
= 100 kJ mol
–1
f
H
A
=
f
H
B
=30 kJ mol
–1
0 0.2 0.4 0.6 0.8 1
1000
1500
2000
X(A)
0 0.2 0.4 0.6 0.8 1
1000
1500
2000
X(A)
T
B
T
A
T
B
T
A
b + melt
melt
a + melt
a + b
a + b
b + melt
melt
a +
melt
a+b
b + melt
melt
a + melt
T
e
T
e
T
e
X
I
T
I
X
I
X
I
T
I
T
I
f
H
A
=
f
H
B
=10 kJ mol
–1
Fig. 6.21
Eutectic melting at constant pressure in three hypothetical systems in which the solids are fully immiscible and the
melt is an ideal solution. Enthalpy of melting decreases from top to bottom, causing increased depression of the
eutectic temperature.
342 Phase equilibrium and phase diagrams
arbitrary value we find from equation (6.49) that the depression of the melting point for a
given liquid composition, T
m
, varies approximately as the square of the melting point of
the end-member and as the inverse of the enthalpy of melting, i.e.:
T
m
∼
T
A
2
m
H
0
A
. (6.75)
The figure also shows that the composition of the eutectic phase shifts towards that of
the refractory phase as the eutectic temperature decreases. This also follows from (6.49)
(exercise left to the reader).
It is customary to label eutectic phase diagrams as shown in Fig. 6.21. The labels identify
the phases that are stable for any combination of temperature and bulk composition inside
each field. Below the eutectic temperature, T
e
, only the solid assemblage is stable. Liquid
first appears at T
e
, and the composition of this liquid is fixed at X
e
, for all bulk compositions
between A and B, except for the two degenerate cases, pure A and pure B. The solidus
of the non-degenerate system (and of a third degenerate system, of composition X
e
)is
thus the point (X
e
, T
e
). Depending on whether the bulk composition of the system lies
to the left or to the right of X
e
the first phase to disappear as the system absorbs heat
will be a or b, respectively. For example, a will be consumed first for bulk composition
X
I
. If this happens then the system gains one degree of freedom allowing its temperature
to increase, so that it enters the field labeled b + liquid. For any point inside this field
the composition of the liquid in equilibrium with pure b is given by the intersection of
the temperature coordinate with the bounding curve, which is the liquidus and is also the
geometric representation of the second equation (6.70) – the other curve emanating from the
eutectic is the representation of the first equation. Liquidus and solidus coincide for the three
degenerate compositions, A, B and X
e
. A closed system of composition X
I
becomes entirely
molten at T
I
. Students of earth sciences are well acquainted with these relations, commonly
exemplified by important eutectic systems such as anorthite–diopside and albite–quartz.
Note that whereas the liquidus curves in those systems are convex up, as in the top panel
in Fig. 6.21, the curvature vanishes and eventually changes sign as enthalpy of melting
decreases.
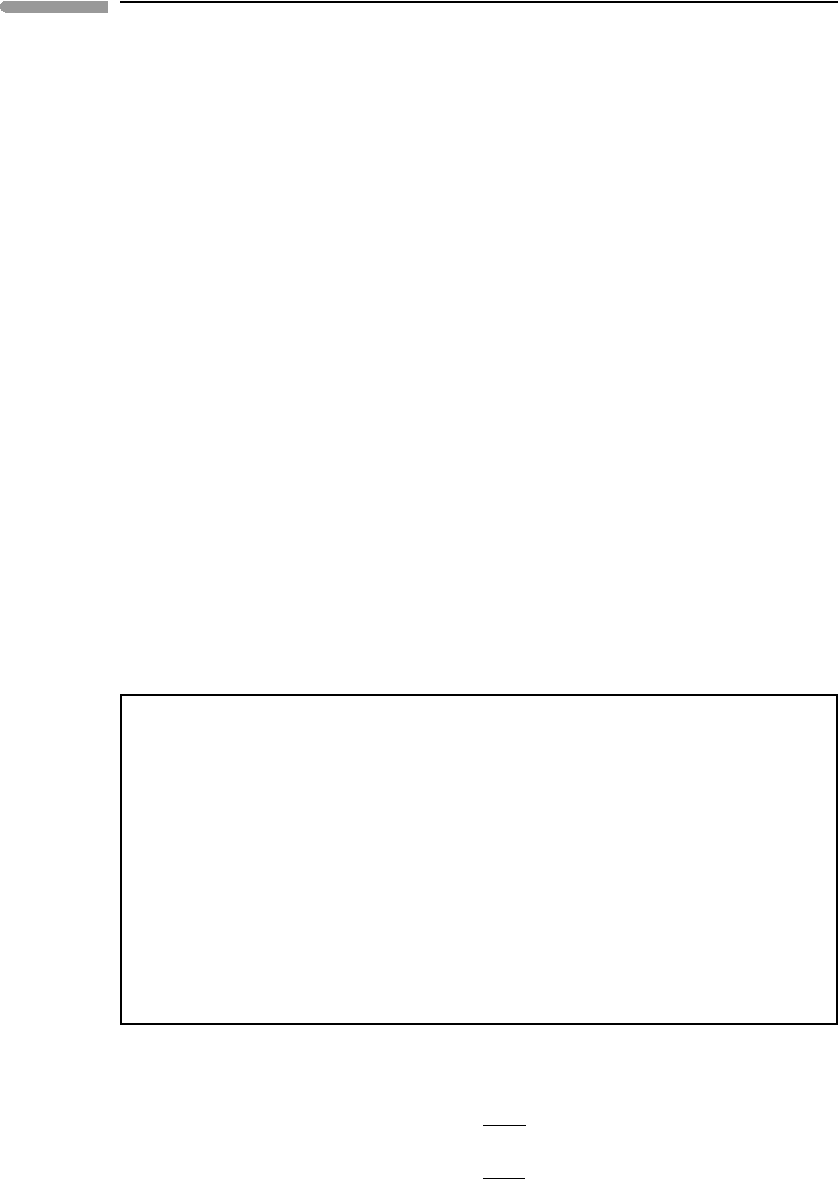
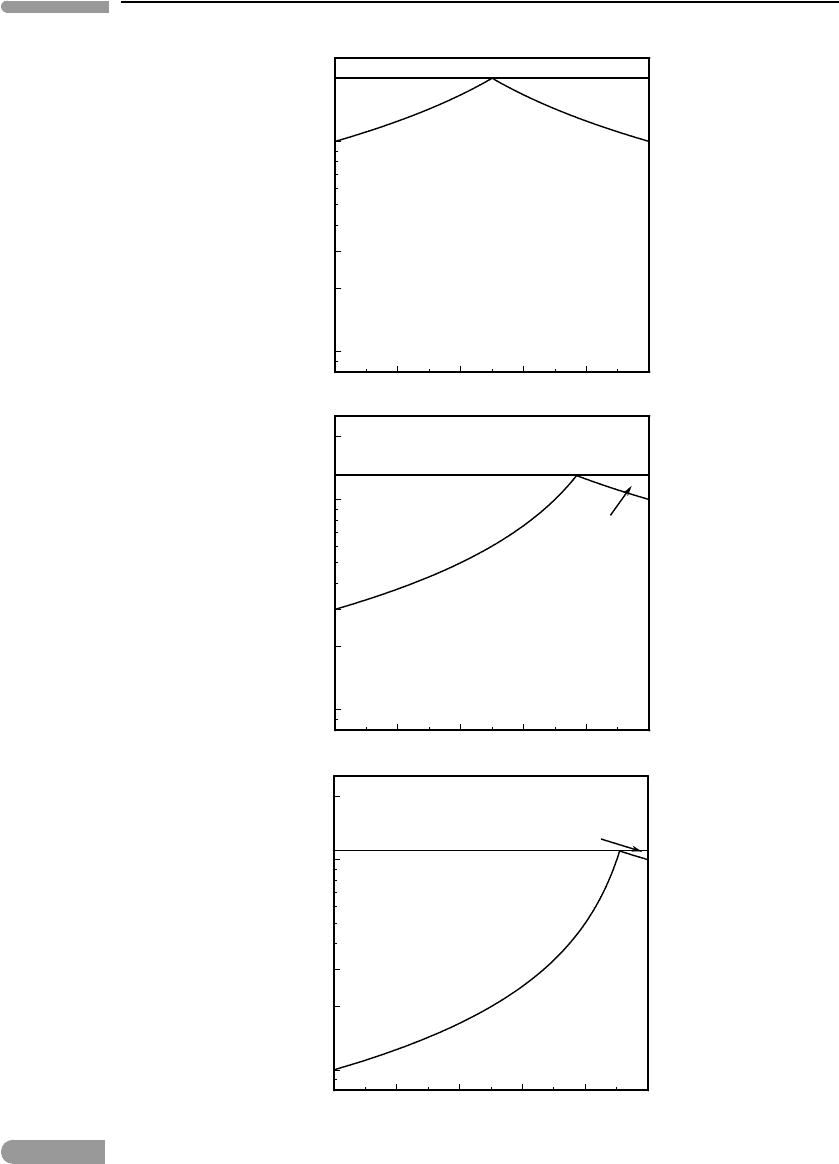
Perhaps less familiar to geologists is the effect of pressure on eutectic phase relations.
These are exemplified in Fig. 6.22, for equilibrium between gas and, say, two solids, a and
b. The geometry of the phase diagram in this case is determined exclusively by the relative
values of the vapor pressures of the two end-member solids, as shown by equations (6.73)
and (6.74).
The figure shows three hypothetical isothermal phase diagrams. Phase a is the same in all
of them, composed of pure A and with a vapor pressure of 0.1 bar. Component B and phase
b are different in each diagram, with vapor pressures of 0.1, 0.03 and 0.01 bar from top to
bottom. The labeling of the various fields and the meaning of the various curves are the same
as in the more familiar temperature–composition melting phase diagram of Fig. 6.21, except
that in this case the curves extending from the eutectic composition to the vapor pressures
of each of the end-members represents the composition of the gas phase and are called
vaporus, rather than liquidus. The diagrams make clear what equation (6.74) says: that a
mixture of two immiscible solids (or liquids) is more volatile, i.e. has higher vapor pressure,
than each condensed phase in isolation. If the two condensed phases have very different
vapor pressures the effect is particularly strong for the less volatile substance (e.g. B in the
bottom panel of the figure). With increasing disparity in volatilities the composition of the
343 6.6 Discontinuous phase transitions
0 0.2 0.4 0.6 0.8 1
0.01
X (A)
P (bar)
p
B,0
p
A,
0
0 0.2 0.4 0.6 0.8 1
0.01
0.1
X (A)
P (bar)
0 0.2 0.4 0.6 0.8 1
0.1
X (A)
P (bar)
p
B,0
p
A,0
p
B,0
p
A,0
a+b
b + vapor
vapor
a+b
b + vapor
vapor
a + vapor
a+b
b + vapor
vapor
a + vapor
P
e
P
e
P
e
a + vapor
Fig. 6.22
Eutectic vaporization of immiscible solids or liquids at constant temperature. An assemblage of immiscible condensed
phases is more volatile (i.e. has a higher saturation vapor pressure) than each phase individually. Note logarithmic
scale on pressure axis.

344 Phase equilibrium and phase diagrams
“eutectic” gas shifts in the direction of the more volatile substance. These may have been
important factors in the condensation of solids from nebular gas during formation of the
Solar System. For example, a solid of relatively low volatility such as B in the bottom panel
of Fig. 6.22 may have been prevented from condensing by the presence of more volatile
components in the nebular gas, such as A in the figure, until the nebular gas attained a
pressure considerably higher than what would have been needed to condense the pure solid.
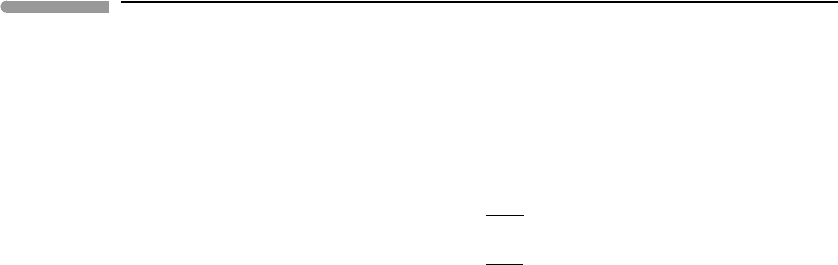
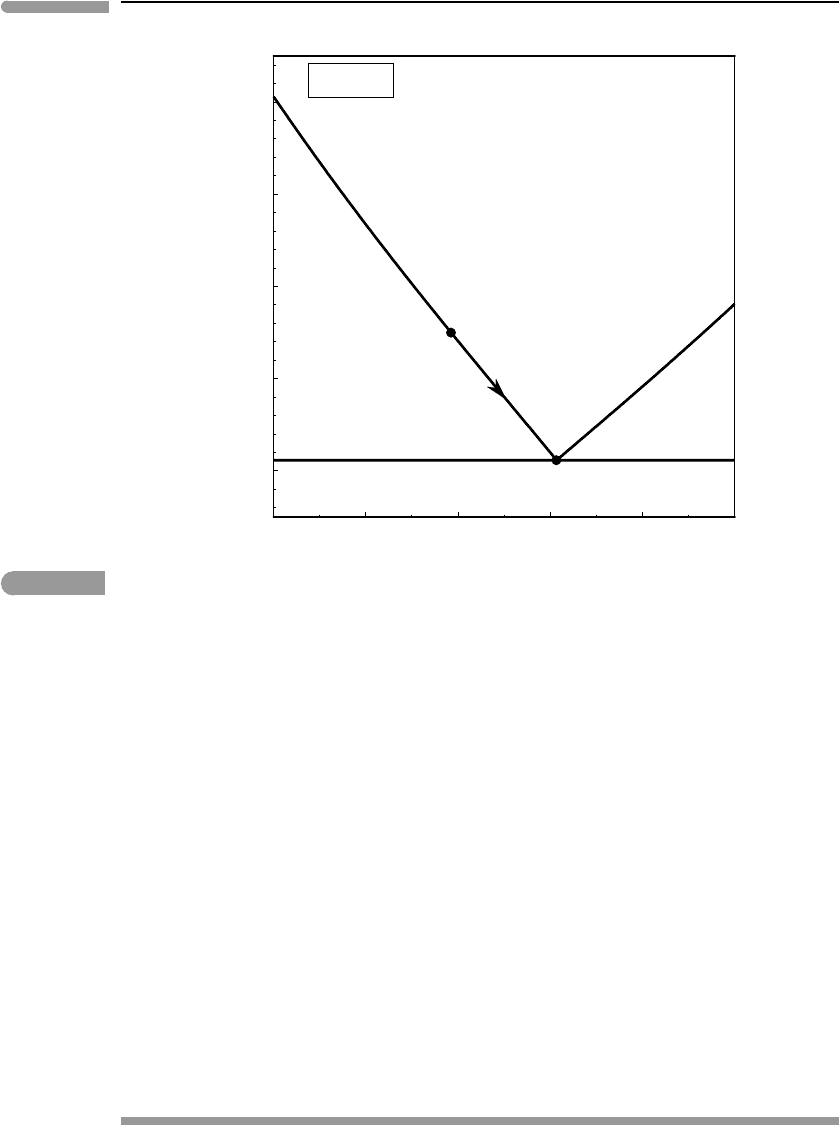
Worked Example 6.10 Cryolavas in Triton
One of the biggest surprises to emerge from the flyby of the Neptune system by Voyager 2
in 1989 was the discovery that its large satellite Triton has a tenuous atmosphere and shows
signs of current, or at least recent, geological activity. There are stunning pictures of what
are almost certainly volcanic calderas and associated lava flows. Icy worlds such as Triton
undergo volcanic processes (and, necessarily, plutonic ones) that may resemble those in
terrestrial planets in many ways except one: the composition of the magmatic liquids. The
temperatures and compositions of icy crusts do not allow the existence of silicate liquids,
but melts composed of mixtures of C–H–O–N species, known as cryolavas if they are
erupted, are certainly possible. Melting in the binary system CH
4
–CO is a simple example
of cryomagmatism that may be applicable to Triton (Fig. 6.23). Actual icy magmas are no
doubt more complex and varied than this. Using this phase diagram as an introduction to
cryomagmatism is akin to introducing the study of basaltic magmatism with the system
diopside–anorthite – an informative simplification.
The melting points of pure CH
4
and CO at 1 bar are 90.6 K and 68.1 K, respectively.
These values would of course be different at the higher pressures at which the cryomagmas
would form in Triton’s interior, but we will ignore that. The enthalpies of melting are 0.93
and 0.84 kJ mol
−1
for CH
4
and CO, respectively. Assuming that the two species crystallize
as pure immiscible solids and that they mix ideally in the liquid phase we use the system of
equations (6.70) and (6.71) to calculate the phase diagram in Fig. 6.23 (see Software Box
6.1). The system melts at a eutectic temperature of ∼51 K, and the eutectic melt consists of
approximately 60 mol% carbon monoxide and 40 mol% methane.
Magmas on Earth seldom reach the surface at temperatures significantly above their
liquidus (Chapter 10). If this is also the case in icy satellites then one could expect cryolavas
to have temperature–composition combinations along one of the two liquidus curves on
Fig. 6.23, and perhaps to be saturated with phenocrysts of either methane ice or carbon
monoxide ice, depending on the bulk composition. Say that a cryolava of composition L
(Fig. 6.23) is erupted at a temperature of ∼65 K on Triton’s surface, where the ambient
temperature is about 38 K. The saturation vapor pressures of liquid CH
4
and CO at 65 K
are about 1 and 94 mbar, respectively, which are orders of magnitude higher than Triton’s
atmospheric pressures (tens of µbar, see Worked Example 6.11). The lava will therefore
boil upon eruption, but boiling will increase its cooling rate, so that a quenched crust
(microcrystalline? glassy?) is likely to form on the surface of the lava flow. Say that this
crust cools instantaneously to the ambient 38 K. The saturation vapor pressures of solid CH
4
and CO at 38 K are, respectively, ∼0.006 and 2.6 µbar, i.e., below atmospheric pressure.
The solid crust is therefore stable against sublimation in Triton’s atmosphere (but see also
next Example). If the lava flow is thick enough, or the liquid collects in a lava lake, cooling
and crystallization of the interior of the flow may progress slowly enough that the liquid
composition will move down the liquidus curve, crystallizing methane ice, as shown by the
arrow in Fig. 6.23. An interesting question is what happens to these crystals – do they float
or sink? This depends on how the density of methane–carbon monoxide liquids varies with
345 6.6 Discontinuous phase transitions
0 0.2 0.4 0.6 0.8 1
50
60
70
80
90
XCO
T (K)
CH
4
+COices
CH
4
ice + melt
melt
P = 1bar
L
E
CO ice
+
melt
Fig. 6.23 Eutectic melting of a mixture of methane and carbon monoxide ices, assuming ideal mixing in the melt, as a
hypothetical model for cryomagmas in Triton. The eutectic temperature (∼51 K) is slightly higher than Triton’s
average surface temperature (∼38 K), but the saturation vapor pressures of liquid CH
4
and CO at these temperatures
are orders of magnitude higher than Triton’s atmospheric pressure, suggesting that cryolavas are likely to boil upon
eruption.
composition and temperature. In any event, when the liquid reaches the eutectic, point E in
the figure, carbon monoxide ice joins the crystallizing assemblage and no further changes in
liquid composition nor temperature will take place until solidification is complete. If there
was efficient segregation of the liquid from the early-formed methane ice crystals then crys-
tallization of the last pools of liquid may give rise to pods in which the two ices are present
in eutectic proportions, perhaps not unlike terrestrial minimum-temperature granites.
An interesting aspect of this model for Tritonian cryomagmatism is that the solidus (eutec-
tic temperature) is only 10–15 K higher than the satellite’s surface temperature, making it
possible for Triton to be geologically active even if its source of internal heat is feeble.
Cryomagmas in H
2
O-rich icy satellites such as Titan and Ganymede are likely to be
composed of H
2
O + NH
3
± CH
4
mixtures. This introduces a few complications relative
to the simple system that we considered here. First, although water–ammonia mixtures do
melt eutectically, several intermediate compounds form (ammonia hydrates) and the system
has at least three different eutectics between H
2
O and NH
3
. Second, for a range of liquid
compositions starting at the H
2
O end-member the liquid is denser than the solid, so that
magmatic ascent and extrusion may be impossible. We return to these issues in Chapter 10.
346 Phase equilibrium and phase diagrams
Worked Example 6.11 A simple model for the atmosphere of Triton and other icy bodies in the
outermost Solar System
The dominant species in Triton’s atmosphere is N
2
, and nitrogen ice is also abundant on
its surface. Triton is thus an example of a planetary body in which the chief atmospheric
component condenses on the surface. This is distinct from Earth and Titan, in both of which
N
2
is also the chief atmospheric gas but the surface temperature is above the boiling point
of N
2
(∼78 K). The dominant component of the Martian atmosphere also condenses on the
planetary surface, but whereas on Mars there are only relatively minor amounts of frozen
CO
2
in the planet’s ice caps, much of Triton’s surface appears to be composed of frozen
nitrogen. We can consider Triton’s surface to be a collapsed atmosphere (a situation vividly
described in Dan Simmons’ Rise of Endymion). This may be the norm in the outermost
bodies of the Solar System, such as Pluto, Charon and other Kuiper Belt objects.
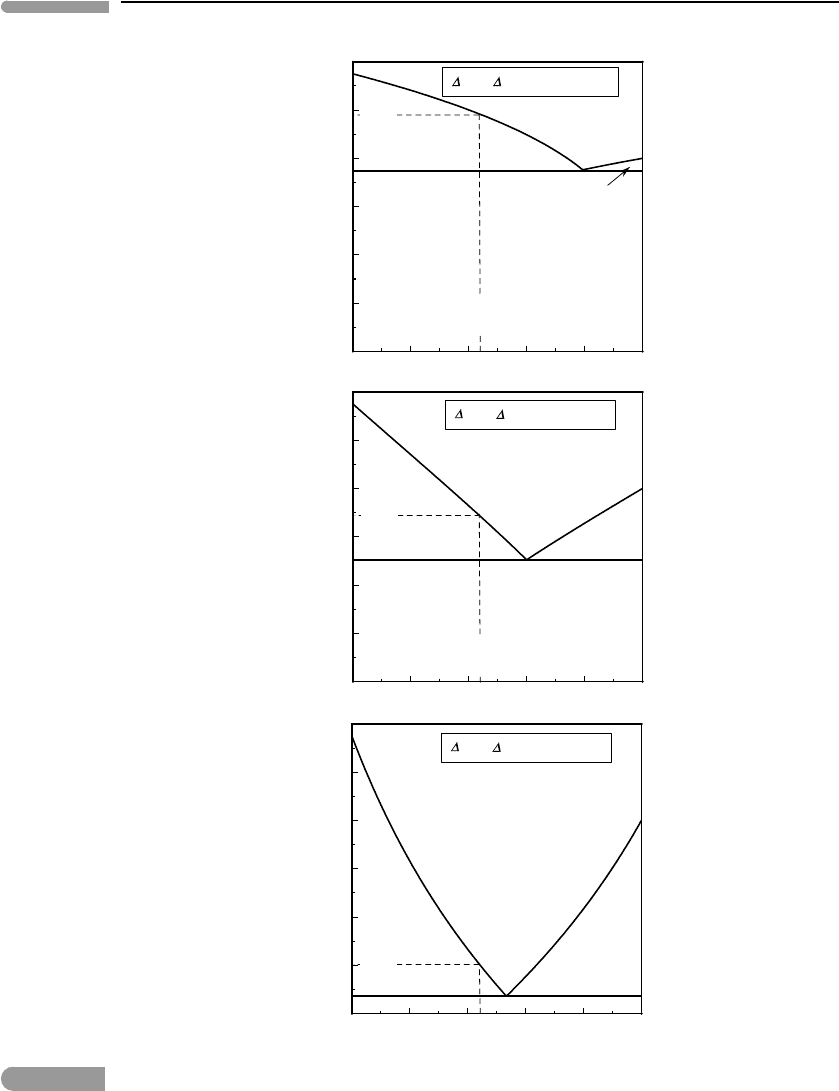
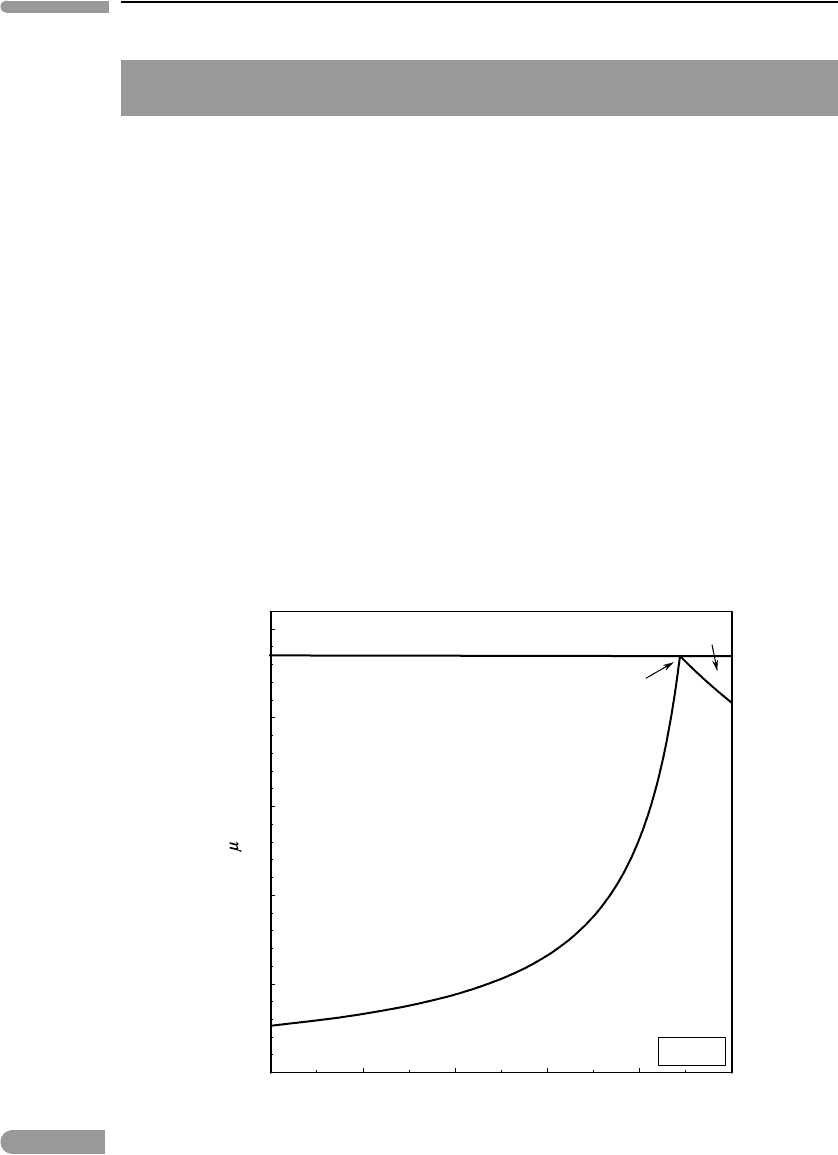
The following is a simple thermodynamic model for this type of atmosphere. Suppose
that the surface of Triton consists of frozen nitrogen and carbon monoxide, two immiscible
solids. Triton’s surface temperature is ∼38 K, the lowest surface temperature so far
measured for any body in the Solar System. From equation (6.60) (a and b parameters from
Lodders & Fegley, 1998) we calculate vapor pressures at 38 K of ∼20.8 and 2.6 µbar for N
2
and CO, respectively. We can then construct the phase diagram in Fig. 6.24, using the system
of equations (6.73) and (6.74). The combined vapor pressure of CO and N
2
in equilibrium
with the mixture of pure solids at 38 K is ∼23.5 µbar. If there are no other components in
0 0.2 0.4 0.6 0.8 1
0
5
10
15
20
25
XN
2
P( bar)
T = 38K
N
2
+ CO ices
N
2
ice +
vapor
CO ice + vapor
vapor
Possible surface conditions
on Triton
Fig. 6.24 Sublimation of a mixture of nitrogen and carbon monoxide ices at the temperature of Triton’s surface. If the surface of
the moon consists chiefly of N–C–O ices in equilibrium with the atmosphere then atmospheric pressure and
composition should correspond approximately to the eutectic composition.

347 Exercises for Chapter 6
Triton’s atmosphere then this is also the total pressure of the gas, i.e. atmospheric pressure
at Triton’s surface. The composition of Triton’s atmosphere also follows from the phase
diagram: it is the composition of the eutectic gas, ∼89 mol% N
2
and 11 mol% CO.
Note what we have done here. Beginning with a simple physical model for Triton’s atmo-
sphere and the observed surface temperature and composition we calculated atmospheric
pressure and composition. The calculation can be refined by including other solid species
known to be present on Triton’s surface, notably methane (perhaps brought to the surface by
lava flows, as discussed in the previous example) and carbon dioxide. The vapor pressures
of these two solids at 38 K are, however, many orders of magnitude lower than those of N
2
and CO (∼ 10
−9
and 10
−29
bar for CH
4
and CO
2
, respectively), so that they are unlikely to
be present in the atmosphere in any significant concentrations, and the results are not likely
to be much different from those shown in Fig. 6.24.
Exercises for Chapter 6
6.1 Find alternate sets of system components to describe the system spinel–
orthopyroxene–olivine–garnet.
6.2 Modify the Maple worksheets described in Software Boxes 5.2 and 5.4 to solve the
system of five simultaneous equations: (6.14), (6.17), (6.22), (6.23) and (6.24) for the
five unknowns: P,(X
Mg,M1
)
opx
, a
SiO
2
, a
Al
2
O
3
and a
MgO
, as a function of temperature.
Check your results against Figures 5.10 and 6.1.
6.3 Find a set of linearly independent heterogeneous equilibrium equations that fix the
chemical potentials of MgO, SiO
2
and Al
2
O
3
, but different from the set {6.19, 6.20,
6.21}. Repeat Exercise 6.2 and verify that you get the same values of P,(X
Mg,M
1
)
opx
,
a
SiO
2
, a
Al
2
O
3
and a
MgO
, as a function of temperature.
6.4 Compare the phase diagrams of H
2
O and Al
2
SiO
5
with Figs. 6.2 and 6.3. Sketch
G–P and G–T curves for each of the phases in each system, with the correct relative
slopes (recall that the pressure and temperature derivatives G are physically accessible
quantities). Convince yourself that the phase diagrams that you are familiar with
are the only possible one for each of these systems. What is the only significant
difference between the phase diagrams for these two one-component systems? (Hint:
the behaviors of andalusite and liquid H
2
O are opposite to each other.)
6.5 Construct a schematic phase diagram for the phases jadeite–albite–quartz–melt in the
system NaAlSi
2
O
6
–SiO
2
. Assume that the melt is silica-saturated.
6.6 Construct schematic phase diagrams in the neighborhoods of each of the following
invariant points in the ternary system MgO–SiO
2
–Al
2
O
3
:
(i) periclase–forsterite–pyrope–sillimanite–spinel
(ii) forsterite–enstatite–pyrope–sillimanite–spinel
(iii) periclase–forsterite–pyrope–corundum–spinel
(iv) periclase–enstatite–quartz–corundum–spinel
(v) periclase–forsterite–enstatite–quartz–pyrope
(vi) periclase–quartz–corundum–sillimanite–spinel.
Use thermodynamic properties (e.g. from Holland & Powell, 1998) to decide on the
correct orientation of each of the phase diagrams. Compare your phase diagrams and
discuss the differences among them.
