Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

318 Phase equilibrium and phase diagrams
mirabilite dehydrates to thenardite plus vapor, may be reached, and perhaps crossed. In
this case mirabilite will be partially or totally replaced by thenardite. Under conditions
hotter than the invariant point, such as T
2
, evaporite formation occurs by crystallization of
thenardite only and mirabilite never forms. If, however, the climate becomes cooler and
the (L) curve is reached from the right, then mirabilite would crystallize at the expense of
thenardite. Note that in every case the label “H
2
O” on the phase boundaries indicates the
direction in which this component is being exchanged between system (evaporite basin)
and environment (atmosphere).
There are four paragenetic sequences which, at least in principle, can be identified in
evaporite sequences and used as paleoclimatic indicators: mirabilite only, thenardite only,
mirabilite followed by thenardite, and thenardite followed by mirabilite. Of course, real
evaporites are much more complex than this, but some of the key thermodynamic principles
that govern their formation can be seen in this example.
6.3.2 Slopes of pseudo-univariant phase boundaries
Phase diagrams in which the coordinates are chemical potentials are also known as chemical
potential diagrams. There are simple equations that yield the slopes of phase boundaries
in them, analogous to Clapeyron’s equation for P–T phase diagrams. Let A and B be phase
assemblages in an open system, such that they are at equilibrium along a pseudo-univariant
curve by exchanging species X and Y with the environment, according to the following
balanced chemical reaction:
A +ν
x
X = B +ν
y
Y . (6.25)
The stoichiometric coefficients of X and Y are ν
x
and ν
y
, whereas those of the phases
that constitute the open system are subsumed in the symbols A and B. We can write the
equilibrium condition for this reaction as follows:
r
G
phases
+ν
y
µ
y
−ν
x
µ
x
=0, (6.26)
where
r
G
phases
is the difference in Gibbs free energy between assemblages B and A.We
seek the response of the system to infinitesimal changes in pressure, temperature and the
chemical potentials of X and Y . In order for equilibrium to be maintained between the
system and its environment the following identity must hold:
∂
r
G
phases
∂T
+ν
y
∂µ
y
∂T
−ν
x
∂µ
x
∂T
dT
+
∂
r
G
phases
∂P
+ν
y
∂µ
y
∂P
−ν
x
∂µ
x
∂P
dP
+ν
y
dµ
y
−ν
x
dµ
x
=0. (6.27)
Because the chemical potentials of X and Y are controlled externally they can be
varied independently of temperature and pressure, which requires that we include the
differentials of these chemical potentials in the last line of the equation. The terms
in parentheses in the first two lines of (6.27) are simply the temperature and pres-
sure derivatives of the Gibbs free energy change for the complete chemical reac-
tion (6.25), which we will symbolize by
r
G. Note very carefully that this is not
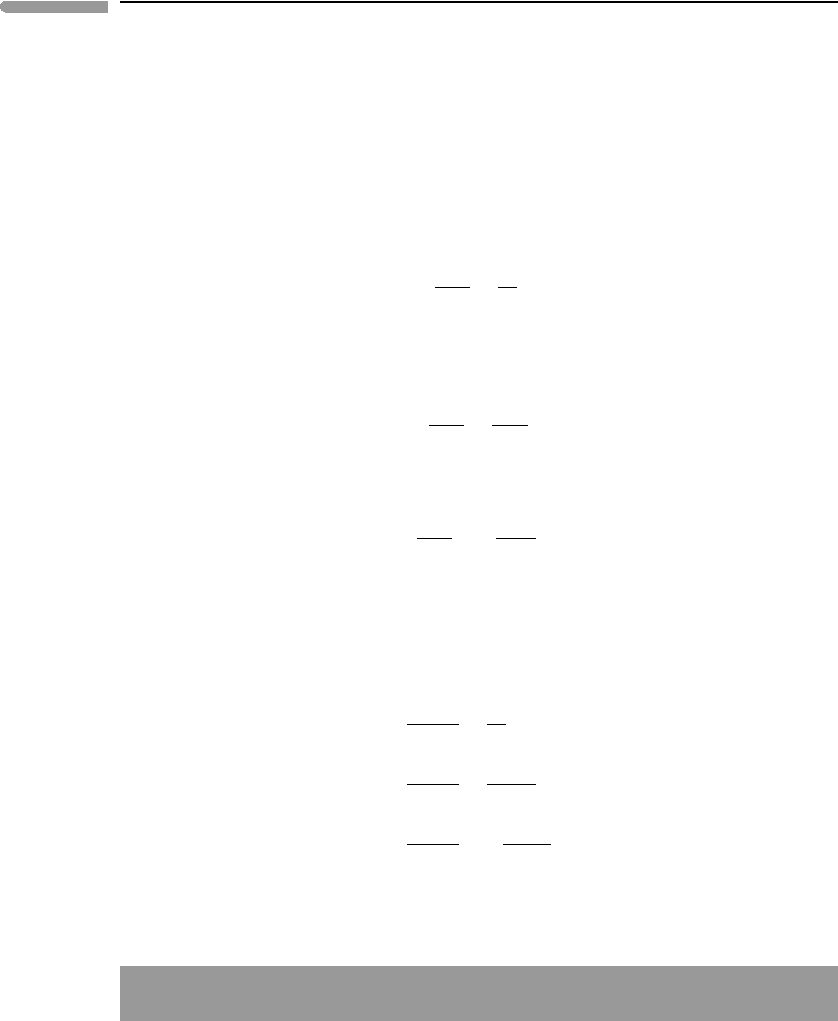
319 6.3 Phase diagrams in open systems
r
G
0
, as the phases may not be in their standard states. We can then simplify equation
(6.27) to:
−
r
SdT +
r
VdP +ν
y
dµ
y
−ν
x
dµ
x
=0. (6.28)
The slopes of phase boundaries in various types of diagrams follow immediately from this
equation. First, setting dµ
y
= dµ
x
= 0 we recover Clapeyron’s equation (5.6). For an
isobaric and isothermal phase diagram we have:
dµ
y
dµ
x
=
ν
x
ν
y
, (6.29)
i.e. the slope is the ratio between the stoichiometric coefficients of the externally-controlled
species. For a T −µ
y
diagram at constant P and µ
x
:
dµ
y
dT
=
r
S
ν
y
(6.30)
and for a P −µ
y
diagram at constant T and µ
x
:
dµ
y
dP
=−
r
V
ν
y
. (6.31)
The signs in equations (6.30) and (6.31) are of course reversed if we interchange µ
y
and µ
x
.
It is sometimes convenient to plot phase relations in terms of activities (or concentrations, or
fugacities, see Chapter 9) of externally controlled species. Differentiating equation (5.45)
and applying the chain rule equations (6.29)to(6.31) become:
d ln a
y
d ln a
x
=
ν
x
ν
y
(6.32)
d ln a
y
dT
=
r
S
RT ν
y
(6.33)
d ln a
y
dP
=−
r
V
RT ν
y
(6.34)
where, again, it must be kept in mind that the entropy and volume of reaction in these
equations are generally not the standard state values (more on this later).
Worked Example 6.7 Equilibrium among iron compounds in different oxidation states: a key to
early terrestrial environments
Iron, one of the most abundant elements in terrestrial planets, has three oxidation states: Fe
0
(metallic iron), Fe
2+
(ferrous iron) and Fe
3+
(ferric iron). As a consequence there is a wide
range of phase relations involving iron compounds that play important roles in the evolution
of rocky planetary bodies. We begin with a simple example, which focuses on equilibria
among the three phases: hematite (Fe
2
O
3
), magnetite (Fe
3
O
4
) and siderite (FeCO
3
).
These three phases constitute a divariant assemblage in the ternary system: FeO–Fe
2
O
3
–
CO
2
(we could have also chosen the system components as Fe–O
2
–CO
2
, it makes no

320 Phase equilibrium and phase diagrams
difference). We can think of situations in which the chemical potentials of O
2
and CO
2
are
controlled externally, for instance, if an assemblage of oxides and carbonates equilibrates
with a planet’s atmosphere, or with groundwater, or with hydrothermal fluids. The two
chemical potentials (v = 2) allow us to map the divariant assemblage onto a pseudo-invariant
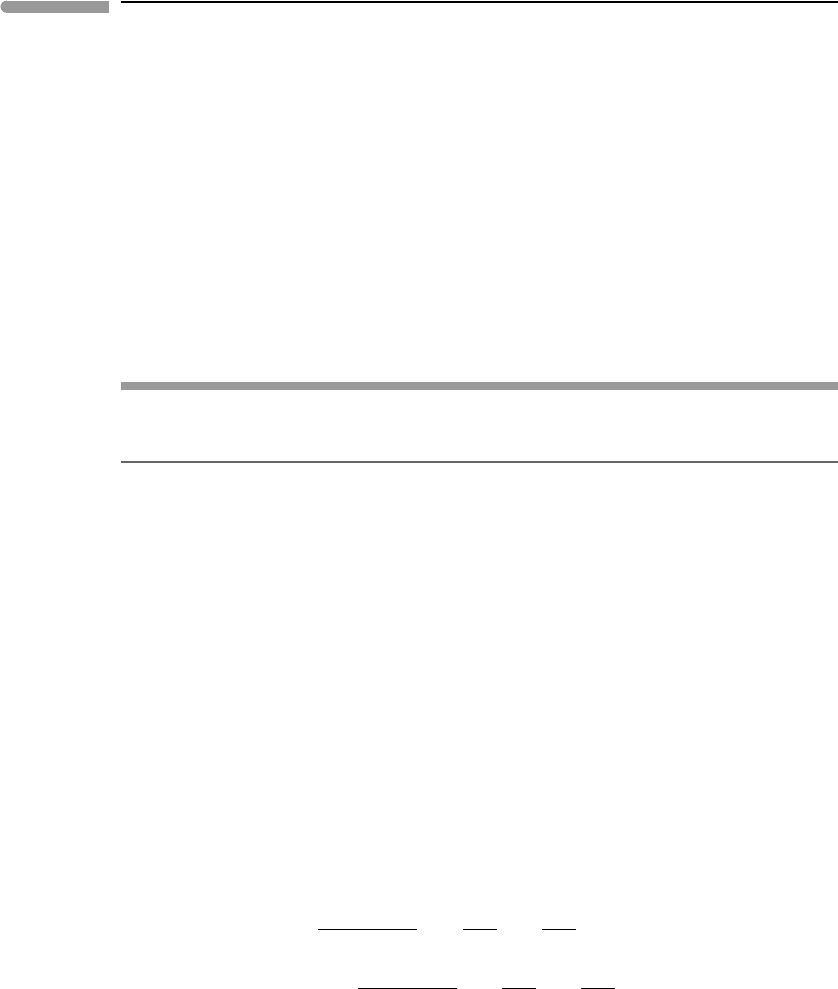
point in an isobaric and isothermal phase diagram. Such diagram is shown in the top panel
of Fig. 6.14. The three pseudo-univariant curves that radiate from the pseudo-invariant point
correspond to the following three reactions:
4mt +O
2
6hm (sd) (6.35)
4sd +O
2
2hm +4CO
2
(mt) (6.36)
6sd +O
2
2mt +6CO
2
(hm). (6.37)
The slopes of these reactions in the isobaric and isothermal phase diagram (also called a
chemical potential diagram)are given by the stoichiometric coefficients of the two externally
controlled chemical potentials (equation (6.29)). Thus, the (sd) reaction is parallel to the
µ
CO
2
axis, and the (mt) and (hm) reactions have ∂µ
O
2
/∂µ
CO
2
slopes equal to 4 and 6,
respectively. The units on the coordinate axes in Fig. 6.14 are arbitrary, as it shows only
the relative position of the reactions and not their absolute locations (we will get to this in
Chapter 11). The relative slopes scale with a change in coordinates, however, and could
also have been derived from Schreinemakers’ rule, as the figure shows. It is important to
remember that slopes derived from equation (6.29) must be consistent with Schreinemakers’
rule – if they are not then you made a mistake somewhere. The mirror orientation of the
phase diagram can be obtained in several different ways, for example, from the fact that
hematite is the most oxidized phase, or that siderite must become stable with increasing
µ
CO
2
. Each pseudo-divariant field contains a single phase which, at fixed pressure and
temperature, is stable over a µ
O
2
–µ
CO
2
region (of course, a single phase in a ternary system
has four degrees of freedom, but two of these are “used up” by fixing P and T ). The diagram
confirms what we should intuitively expect: that hematite forms by oxidation of magnetite,
that siderite forms by carbonation of the oxides, or the oxides by oxidation of siderite, and
that reduction of ferric iron in the oxides to ferrous iron in siderite requires higher µ
CO
2
the higher µ
O
2
is. It may seem that we have not gained much information that we did not
already know, and this is indeed a very simple example. But a schematic phase diagram
such as Fig. 6.14 is the starting point for construction of the rigorous quantitative version,
which we will do in Chapter 11.
We can choose to fix different subsets of the four externally controlled intensive
variables. The phase diagrams at the bottom of Fig. 6.14 show two possibilities: µ
O
2
–T and
µ
O2
–P diagrams. Although the slopes can be derived from equations (6.30) and (6.31), this
is not always as straightforward as for the µ–µ diagram. Only one gas species participates
in the (sd) reaction, which immediately identifies the high-entropy and high-volume side
of the reaction, and the signs of
r
S and
r
V in (6.30) and (6.31). In the (mt) and (hm)
reactions there are four and six times more gas, respectively, on one side of the reaction
than on the other. These relationships define the signs of
r
S
0
and
r
V
0
but not of
r
S
and
r
V, as the gas species are generally not in their standard states. Schreinemakers’ rule
would allow for the three lines to have positive slopes in the µ
O
2
–T diagram.The justification
for the negative slopes of the (mt) and (hm) reactions will be given in Chapter 9. The
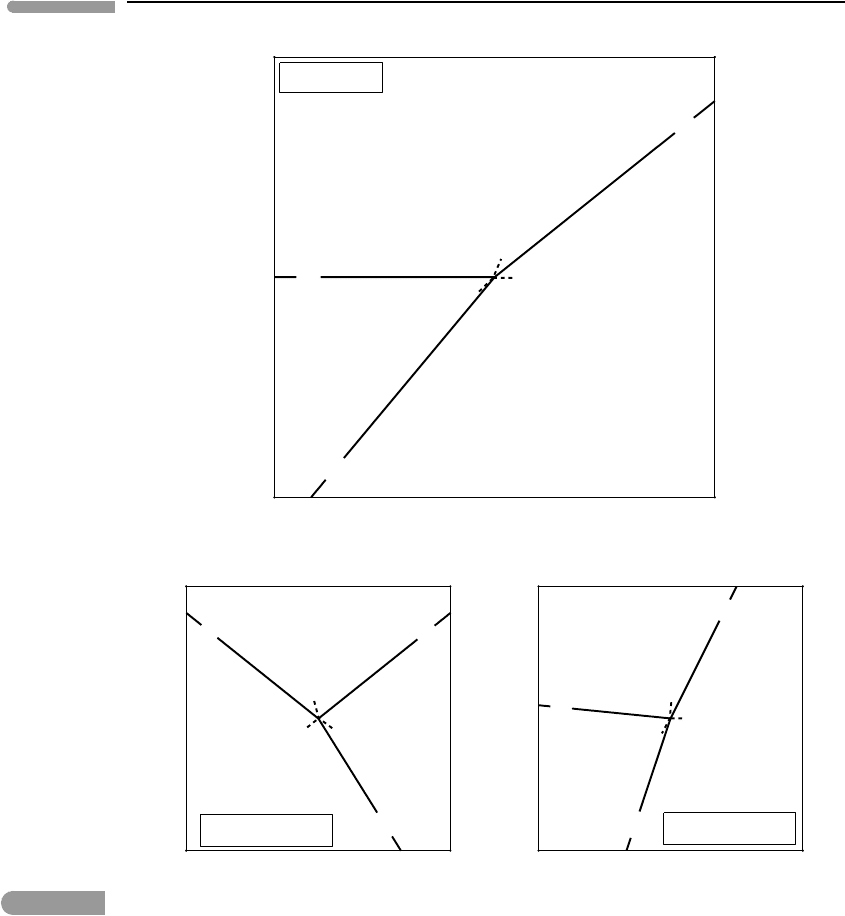
321 6.3 Phase diagrams in open systems
CO
2
O
2
T
O
2
P
O
2
(sd)
(mt)
(hm)
mt
+
CO
2
sd +
O
2
hm
+
CO
2
sd
+
O
2
hm
mt + O
2
siderite
hematite
magnetite
P, T constant
(hm)
mt +
CO
2
sd
+
O
2
(mt)
hm
+
CO
2
sd
+
O
2
(sd)
mt
+
O
2
hm
siderite
hematite
magnetite
(sd)
mt
+
O
2
hm
(mt)
hm
+
CO
2
sd
+
O
2
(hm)
mt + CO
2
sd
+O
2
siderite
hematite
magnetite
P,
CO
2
constant
T,
CO
2
constant
µ
µ
µ
µ
µ
µ
Fig. 6.14 Magnetite–hematite–siderite phase relations in three different projections. See text for discussion, but note that
Schreinemakers’ rule is followed in all three projections.
µ
O
2
–T and µ
O
2
–P phase diagrams show that siderite breaks down to iron oxide plus CO
2
with increasing temperature, and that the resulting oxide is either hematite or magnetite,
depending on the chemical potential of oxygen. They also tell us something that may not
have been so obvious: that at constant µ
O
2
hematite is the low-temperature and high-
pressure phase relative to magnetite. In fact, the positive slope of the hematite–magnetite
phase boundary in the isobaric µ
O
2
–T diagram is characteristic of all oxidation reactions
(Chapter 9).

322 Phase equilibrium and phase diagrams
We can now ask some additional questions. For instance: (i) under what conditions can
an aqueous solution contain a significant concentration of dissolved iron (we shall see in
Chapter 11 that the relevant aqueous ion is Fe
2+
) and (ii) what are the identities of the iron
phases that crystallize from such solutions under different conditions? Such questions are
relevant to understanding changes in the oxidation state of the early terrestrial atmosphere, as
well as the possible conditions under which liquid water may have existed on the Martian
surface in the geological past. In order to address them we can consider the quaternary
system Fe–O
2
–CO
2
–H
2
O, and four phases in this system: siderite, magnetite, hematite
and an aqueous liquid phase that may or may not contain a significant concentration of
Fe
2+
(I will clarify the meaning of “significant” in the next paragraph). Subsets of three of
these phases constitute trivariant assemblages in the quaternary system, that we can map to
pseudo-invariant points by finding three externally controlled chemical potentials (v = 3),
and fixing any three of the resulting five independent intensive variables (P, T and the three
µs). Two of the chemical potentials are µ
O
2
and µ
CO
2
, as before. The third one is the
chemical potential of the hydrogen ion, which can be measured in terms of the pH of the
aqueous solution. Recalling that pH is the negative of the logarithm of the H
+
concentration
(Chapter 11), we see that we can apply equations (6.32)–(6.34) with the signs switched to
determine the slopes of phase boundaries in diagrams in which pH is one of the coordinates.
The phase diagram in Figure 6.14 is valid in the quaternary system, with the proviso
that: (a) we indicate that there are three intensive variables that are kept constant: P, T
and pH , and (b) we indicate that one of the phases, the aqueous solution, is absent at the
pseudo-invariant point and along all reactions that emanate from it. A common convention
is to indicate the phase that is absent at an invariant point by enclosing it in square brackets.
The pseudo-invariant point in Fig. 6.14 can then be labeled [aq], and the three pseudo-
univariant phase boundaries could be re-labeled (sd, aq), (mt, aq) and (hm, aq). Take now
the pseudo-invariant point [hm]. The following three pseudo-univariant reactions radiate
from it:
2mt +12H
+
6Fe
2+
+6H
2
O +O
2
(sd,hm) (6.38)
sd +2H
+
Fe
2+
+H
2
O +CO
2
(mt,hm) (6.39)
6sd +O
2
2mt +6CO
2
(hm, aq). (6.40)
The aqueous solution phase appears in these reactions as the combination (Fe
2+
+H
2
O),
but what exactly does this mean? A liquid H
2
O phase is typically always present on both
sides of reactions such as (6.38) and (6.39). The “appearance” or “disappearance” of the
aqueous Fe
2+
species is more accurately described as a change in the concentration of
Fe
2+
. In order to plot these reactions as pseudo-univariant phase boundaries it is necessary
to (arbitrarily) fix some concentration of Fe
2+
that we will use as the boundary between
“dissolved Fe” and “precipitated Fe”. This is what I meant by “significant concentration
of Fe
2+
”. If, in addition, we note that the chemical potential of H
2
O stays approximately
constant (because its mol fraction is very close to 1, regardless of how much Fe is dissolved
in it), then we see that reactions (6.38) and (6.39) have one degree of freedom (µ
O
2
or µ
CO
2
,
respectively) at constant P, T and pH . With these restrictions it is possible to treat them as
pseudo-univariant reactions, and Schreinemakers’ rule applies. In Chapter 11 we will see
how to deal with aqueous solutions in a less restrictive way.
Reactions (6.40) and (6.37) are the same one, so this reaction must be the one that joins
the [hm] and [aq] pseudo-invariant points (see Worked Example 6.5). Because hematite is

323 6.3 Phase diagrams in open systems
not stable at the [hm] point this must be located on the stable side of reaction (6.37). The
resulting phase diagram is shown in Fig. 6.15a. The labels of the pseudo-univariant lines
have been omitted for clarity but you should convince yourself that their relative locations
abide by Schreinemakers’ rule. Just as there is a hematite-absent pseudo-invariant point,
there is also a magnetite-absent pseudo-invariant point, [mt], where the following three
reactions meet:
2hm +8H
+
4Fe
2+
+4H
2
O +O
2
(sd,mt) (6.41)
sd +2H
+
Fe
2+
+H
2
O +CO
2
(hm, mt) (6.42)
4sd +O
2
2hm +4CO
2
(mt,aq). (6.43)
Reaction (6.43) is now the same as (6.36), so this must be the reaction that joins the [mt]
and [aq] pseudo-invariant points. Since magnetite is not stable at [mt], this point must be
located on the stable side of reaction (6.36). The only way in which this is possible is if
both the [hm] and [aq] pseudo-invariant points are located on the metastable side of the
(aq, mt) reaction, as shown in Fig. 6.15b. The two phase diagrams in Fig. 6.15 represent
two different sets of phase relations for the same system. We will get to that in a moment,
but first note that there is an important difference between the two sets of phase relations.
When the [mt] point becomes stable magnetite is never stable and both the [hm] and [aq]
pseudo-invariant points are metastable. In contrast, when the [hm] point is stable hematite
exists around the stable [aq] pseudo-invariant point.
How can two different sets of phase relations be possible for the same system? Because
each of the phase diagrams in Fig. 6.15 is valid for a different combination of the three
intensive variables that are being held constant, P, T and pH. In particular, they may corre-
spond to different pH values, labeled pH
I
and pH
II
in the figure, at the same pressure and
temperature.
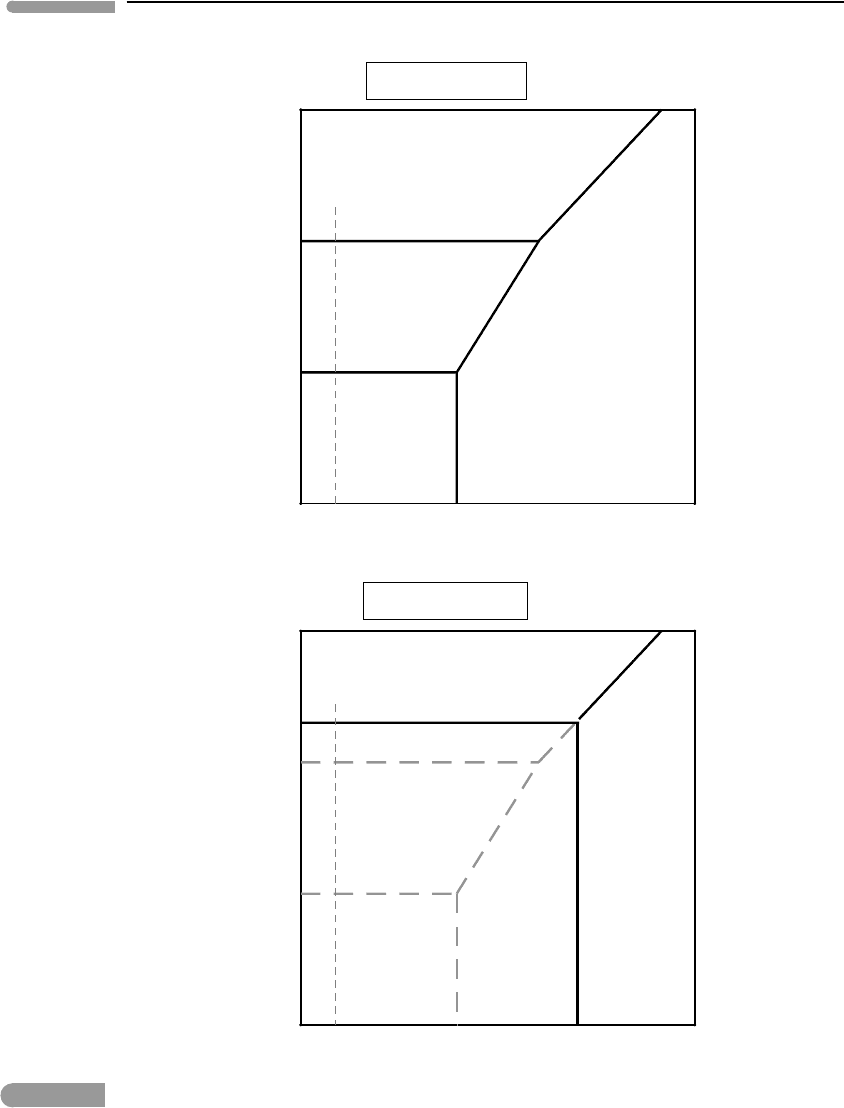
We can determine the relative values of pH
I
and pH
II
by drawing the phase relations on
the µ
O
2
–pH plane at constant P , T and µ
CO
2
. These are shown in Fig. 6.16 – you should
demonstrate to yourself that this is the correct diagram. We can now see that magnetite
becomes unstable with increasing acidity (decreasing pH ). Let the two pH values be as
shown in Fig 6.16 and recall that µ
CO
2
is now being held constant, for example at the value
µ
c
in Figure 6.15. Under less acidic conditions, pH
I
, reduction of hematite at constant µ
CO
2
produces magnetite first and dissolved Fe
2+
at lower µ
O
2
, as in Fig. 6.15a. If conditions
are more acidic, pH
II
, then hematite dissolves in response to a decrease in µ
O
2
without
forming magnetite as an intermediate product, as in Fig. 6.15a.
Banded iron formations (BIF) are chemical sediments that are found in Earth’s strati-
graphic record beginning in the Early Archaean, reaching their maximum extent in the
interval 2.5–2.0 Ga, and tapering off after that. Their major development coincides in time
with what is known as the Great Oxidation Event, when µ
O
2
in the terrestrial atmosphere
increased sharply and relatively rapidly (geologically speaking). The most likely explana-
tion for the origin of BIFs is that they formed by precipitation of Fe
2+
dissolved in seawater.
What was the ultimate source of this iron is a different, and controversial, question. There is
considerable variability in the mineralogy of BIFs but some consistent patterns exist. Some
are dominated by hematite, which in some cases is accompanied by subordinate magnetite.
Siderite is rare in hematite-dominated BIFs. Other BIFs are dominated by magnetite, and
these commonly also contain large quantities of siderite. Yet a third kind is composed
predominantly of siderite, with no oxides. All BIFs contain chert and/or iron silicates.
324 Phase equilibrium and phase diagrams
CO
2
O
2
siderite
magnetite
hematite
O
2
siderite
hematite
aq
P, T, pH
I
constant
P, T, pH
II
constant
[hm]
[mt]
[aq]
[hm]
c
(a)
(b)
[aq]
aq
µ
µ
µ
µ
CO
2
c
µ
µ
Fig. 6.15 Phase relations of hematite–magnetite–siderite in equilibrium with an aqueous phase at two different values of pH.
The boundaries of the aqueous phase field correspond to an arbitrarily chosen concentration of dissolved Fe
2+
, not to
the disappearance of the phase (see text and also Chapter 11). The phase absent at each invariant point is enclosed in
square brackets. The phase boundaries shown in grey broken lines in the bottom diagram are metastable relative to
dissolved Fe
2+
. Hence, magnetite is not stable at pH
II
. Note that µ
c
is the chemical potential of CO
2
in Fig. 6.16.
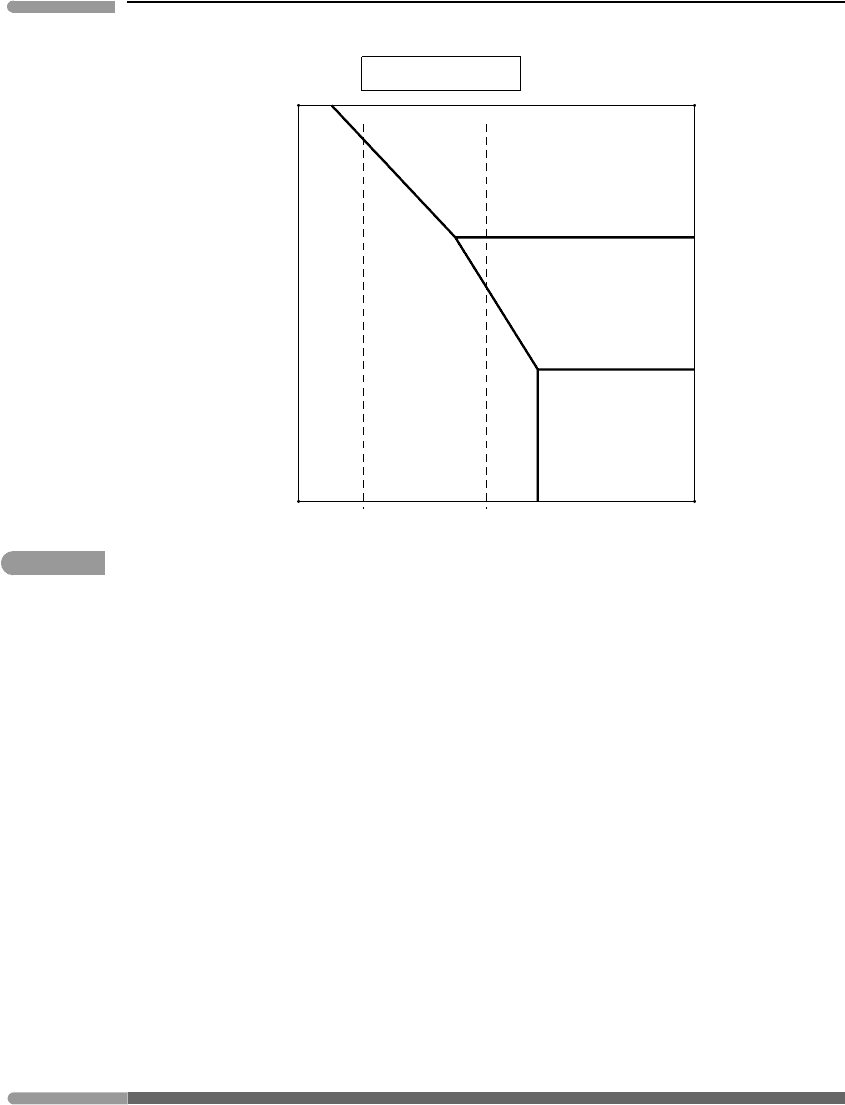
325 6.4 Equilibrium among phases
pH
O
2
siderite
magnetite
hematite
aq
pH
II
pH
I
P, T,
CO
2
constant
[sd]
[hm]
µ
µ
Fig. 6.16 Phase relations for the same system as Fig. 6.15, but at constant µ
CO
2
.
We can construct a qualitative interpretation of these observations on the basis of the
phase relations in Figs. 6.15 and 6.16. Oxide-dominated BIFs are most likely to represent
precipitation of Fe
2+
in response to an increase in µ
O
2
, but precipitation caused by an
increase in the pH of seawater cannot be ruled out. Precipitation of hematite may generally
indicate oxidation under more acidic conditions than precipitation of magnetite, but there
is also a minimum value of the chemical potential of oxygen that is needed to stabilize
hematite. Oxidation under yet less acidic conditions may have caused precipitation of
magnetite + siderite BIFs. Carbonate-dominated BIFs with little or no magnetite, in
contrast, are more likely to represent an increase in pH or µ
CO
2
under relatively reducing
conditions, at which neither of the two oxides is stable. BIFs preserve a priceless record of
the chemical evolution of the atmosphere and ocean in the early Earth, that we will study
in a more quantitative fashion in Chapter 11. Widespread deposits of hematite have also
been identified on the Martian surface, both by remote sensing and in-situ observations
by landers. Magnetite and siderite, in contrast, appear to be absent in Mars, or at the very
least to be far less common than in terrestrial sedimentary iron formations. One possible
explanation is that Martian hematite formed by precipitation from bodies of consistently
acidic water, hinting at an important difference with early terrestrial environments.
6.4 Equilibrium among phases of variable composition
When we consider chemical equilibrium among phases of variable composition we are
interested in tracking phase compositions as a function of the intensive variables that control
the system, most commonly temperature and pressure. The following discussion focuses
326 Phase equilibrium and phase diagrams
exclusively on binary systems but the principles are valid, if considerably more cumbersome
to implement, for systems of any number of components. We need to consider two distinct
situations.
In the first case each of the compositional end-members undergoes a discontinuous phase
transition between two phases that have the same chemical composition but different struc-
tural states. This could be solid–liquid, solid–gas, liquid–gas or a phase transition between
two isochemical solids with different crystallographic structures. Although the concept of a
discontinuous phase transition is intuitively easy to grasp, we can give it a precise thermo-
dynamic meaning by noting that they are accompanied by a non-zero enthalpy change (e.g.
the enthalpy of fusion, vaporization, sublimation, etc.) and therefore also a finite entropy
change, as well as a finite volume change. At a discontinuous phase transition
r
G vanishes
but its first derivatives,
r
S and
r
V, do not. Thermodynamic analysis of discontinuous
phase transitions is based on a set of equations that describe the equilibrium of a chemical
species between coexisting phases. These equations are derived in Section 6.5, and applied
to the study of phase transitions in Section 6.6.
The second case consists of equilibrium between two phases of different composition
that are in the same, or very similar, structural states. These could be two liquids or two
isostructural solids (e.g. two feldspars, or two pyroxenes). It is an empirical observation, that
we will also justify from thermodynamic considerations, that the compositions of the two
phases at equilibrium converge with increasing temperature, until the two phases become
indistinguishable at a well-defined temperature called the critical mixing temperature.At
the critical temperature the system undergoes a phase transition between a sub-critical state
in which two phases coexist at equilibrium, and a super-critical state in which only one
phase exists at equilibrium. Such phase transitions are called continuous, or critical, phase
transitions. They are discussed in the next chapter.
6.5 Chemical equilibrium at first-order phase transitions
6.5.1 Condensed phases
Consider a chemical species, A, contained in two condensed phases, 1 and 2, that are
at equilibrium at a discontinuous phase transition. We will follow the convention that
phase 2 has higher entropy and higher volume than phase 1, so that phase 2 occurs on the
high-temperature and low-pressure side of the phase transition, and we will always write
the reaction with the high entropy and high-volume phase as a product. The equilibrium
condition can be written as follows (see equation (5.55)):
ln
a
A
2
a
A
1
=ln K =−
r
G
0
P,T
RT
. (6.44)
If the phases on both sides of the phase transition are condensed phases (i.e., the phase
transition is solid–liquid or solid–solid) then the standard states in (6.44) are taken to be
pure A in phase 1 and pure A in phase 2 at the temperature and pressure of the phase
transition for the pure substance. Equation (6.44) vanishes at the pressure and temperature
at which pure A undergoes the phase transition, but at any other P–T combination it is not
zero. We now wish to find how the ratio of equilibrium activities at the phase transition

327 6.5 Chemical equilibrium
changes with temperature and pressure. Thus, at constant pressure:
∂ ln K
∂T
=−
1
R
∂
∂T
r
G
0
P,T
T
=−
1
R
1
T
∂
r
G
0
P,T
∂T
−
r
G
0
P,T
T
2
=−
1
R
−
r
S
0
P,T
T
−
r
G
0
P,T
T
2
. (6.45)
Now, from the definition of Gibbs free energy (equation (4.128)) we can write:
r
H
0
P,T
T
2
=−
r
G
0
P,T
T
2
−
r
S
0
P,T
T
(6.46)
which, substituting in (6.45), yields:
∂ ln K
∂T
=
r
H
0
P,T
RT
2
. (6.47)
We can now integrate this expression, at constant pressure, between the temperature of the
phase transition for pure A, T
A
, and any other arbitrary temperature T :
ln K
T
=
T
T
A
r
H
0
P,T
RT
2
dT +ln K
T
A
. (6.48)
We will assume that the integration interval is narrow enough that the enthalpy change
associated with the phase transition can be considered to be constant, and equal to that for
the phase transition for pure A at T
A
. Calling this enthalpy change
r
H
0
A
and given that
our choice of standard states makes ln K
T
A
= 0, we get the following expression for the
activity ratio at temperature T:
a
A
2
a
A
1
T
=K
T
=exp
−
r
H
0
A
R
1
T
−
1
T
A
. (6.49)
If we write the chemical formula of species A in such a way that site multiplicity equals
one then the activities are the products of mol fractions times activity coefficients, and we
can recast (6.49) as follows:
X
A
2
X
A
1
=α
(
T
)
γ
A
1
γ
A
2
(6.50)
where, in order to simplify subsequent equations, we have made:
α
(
T
)
=exp
−
r
H
0
A
R
1
T
−
1
T
A
. (6.51)
Equation (6.49), or its equivalent (6.50), is sometimes called the freezing point depression
equation, as it tracks how the melting point T of a substance changes relative to the melting
