Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


348 Phase equilibrium and phase diagrams
6.7 Consider the phases forsterite–enstatite–magnesite–vapor (of composition CO
2
)–
melt (of composition MgCO
3
) in the ternary system MgO–SiO
2
–CO
2
. Construct
a schematic phase diagram and use it to discuss the generation of carbonatite melts
in magnesite-free mantle peridotites. Is it possible, in this simple system, for a Mg-
carbonatite melt to crystallize a silicate mineral assemblage, leaving no traces of the
existence of a carbonate liquid?
6.8 Consider the four-component system MgO–CaO–SiO
2
–CO
2
, and the phases
forsterite, diopside, enstatite, calcite, vapor (of composition CO
2
) and melt (of com-
position CaCO
3
). Construct a schematic phase diagram for this system, and connect
it to the phase diagram in Fig. 6.11 via the multiply-degenerate reaction (di, fo, en,
v). Discuss the similarities and differences between this phase diagram, the one in
Exercise 6.7, and the one in Fig. 6.11.
6.9 Construct a schematic P–µ
O
2
phase diagram for ferroslite–fayalite–magnetite, at con-
stant temperature and µ
SiO
2
. Discuss the implications of your phase diagram for
the effects of pressure and oxidation conditions on the generation of tholeiitic vs.
calc-alkaline igneous differentiation trends.
6.10 Construct schematic T–µ
O
2
and P–µ
O
2
phase diagrams in the neighborhood of the
wustite (FeO)–cohenite (Fe
3
C)–metallic Fe–graphite invariant point in the ternary
system Fe–C–O. Use your diagrams to discuss whether there is more than one possible
explanation for the fact that metallic iron ± cohenite is more characteristic of the
interiors of small planetary bodies (e.g. asteroids and the Moon) than of large bodies
(e.g. Earth, Mars).
6.11 Construct P–T, P–µ
O
2
, T–µ
O
2
and µ
SiO
2
–µ
O
2
phase diagrams showing equilib-
ria among the phases: metallic iron, fayalite, schreibersite (Fe
3
P), whitlockite
(Ca
3
(PO
4
)
2
), perovskite and ilmenite, in the six-component system Fe–Si–P–Ca–
Ti–O. Discuss the various pathways by which whitlockite microcrystals can exsolve
from metal grains in chondritic meteorites.
6.12 In Worked Example 6.9 I calculated a methane–ethane melting loop under the assump-
tion that the two ices are fully miscible. This may not be the case. The opposite
“end-member” possibility is that they are perfectly immiscible, and that the ice mix-
ture exhibits eutectic behavior. Calculate the phase diagram that results from this
assumption, using the Maple worksheet described in Software Box 6.1 (all necessary
data are given in Worked Example 6.9). How does this affect the conclusions about
the possible nature of Titan’s surface hydrocarbons?
6.13 Refine the calculation of Triton’s atmospheric composition by calculating the pressure
and composition of the vapor in equilibrium with the four ices: N
2
, CO, CO
2
and CH
4
.
All the necessary data can be obtained from Lodders and Fegley (1998), Table 1.20.
7
Critical phase transitions
The phase transitions that we discussed in Section 6.6 are all discontinuous phase transitions.
They are step-wise changes in the structure of matter, for instance, the destruction of the
crystalline structure during melting or sublimation, or the breakdown of molecular bonds
in a liquid during boiling. These are microscopic changes that are accompanied by a macro-
scopic exchange of heat with the environment, what we call the enthalpy of transition
(melting, vaporization, etc.), or also “latent heat”. There is another type of phase tran-
sition, which takes place without there being a discontinuity either in the microscopic
structure of a substance or in its macroscopic properties, and during which there is no
energy exchange with the environment. Such phase transitions are called continuous or crit-
ical phase transitions, and play important roles in many planetary processes. For example,
they underlie exsolution phenomena such as are observed in feldspars, pyroxenes, oxides
and meteoritic metal, hydrogen–helium unmixing in fluid planets and liquid immiscibility
phenomena in magmatic systems. They also explain order–disorder transformations in crys-
talline substances. Critical phase transitions also play an important role in the study of fluids
(Chapter 9).
7.1 An intuitive approach to critical phase transitions
Consider a binary solution between components A and B with unit site multiplicity. If we
use X for the mol fraction of component A then the mol fraction of B is 1 − X, and the
Gibbs free energy of ideal mixing is given by equation (5.93):
G
ideal
mixing
=RT
[
X ln X +
(
1 −X
)
ln
(
1 −X
)
]
. (7.1)
This function vanishes at X =0 and X =1, is negative everywhere in between, and becomes
more negative with increasing temperature. What this means is that, if two substances mix
ideally, then the solution (microscopic mixture) is always stable relative to a mechanical
(macroscopic) mixture of the pure substances, and becomes more stable (the tendency to
form the solution becomes stronger) with increasing temperature. The curve that represents
G
ideal
mixing
has a minimum and is concave up, as shown in Fig. 5.9. The second derivative
of (7.1) must therefore be everywhere positive, which you can verify by differentiating the
function. Let us now assume that the solution is not ideal. From (5.122):
G
mixing
=G
ideal
mixing
+G
excess
. (7.2)
We begin by considering a hypothetical non-ideal solution that can be described with a
symmetric excess Gibbs free energy function (equation (5.146)) that is neither a function of
temperature nor of pressure (i.e. W
S
= W
V
= 0). This is the simplest possible description
349
350 Critical phase transitions
of a non-ideal solution. G
excess
vanishes at X = 0 and X = 1 and is positive everywhere in
between and independent of temperature. Because G
excess
and G
ideal
mixing
have opposite signs
and G
excess
is a function of composition only whereas G
ideal
mixing
is a function of both compo-
sition and temperature, the relative magnitudes of the two functions, and hence of G
mixing
,
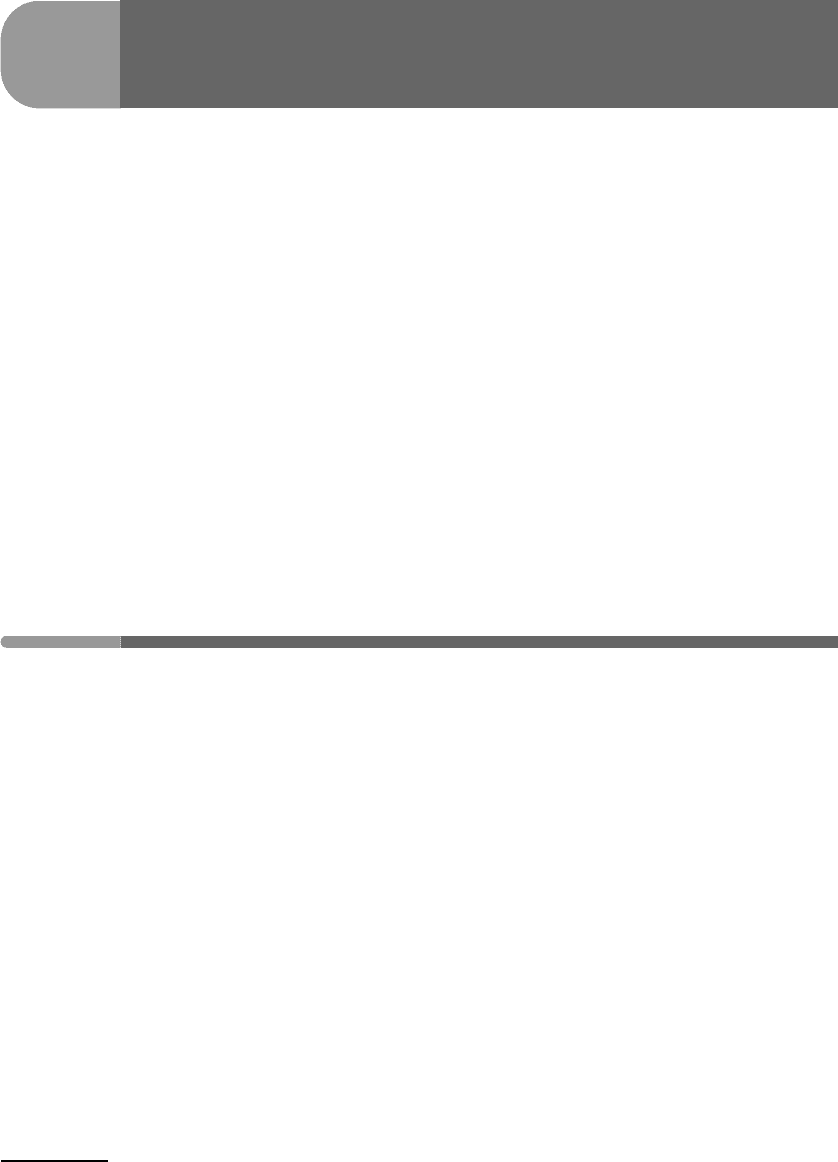
vary in interesting ways with temperature and composition. This is shown in Fig. 7.1.
At some high temperature T
1
the excess function is small enough relative to the ideal
function that G
mixing
has the properties of the ideal function, in particular, it is always
negative and concave up. This case corresponds to the top diagram on the left of Fig. 7.1.
0
0
X
A
∆G
mixing
∆G
mixing
∆G
mixing
0
0
X
A
0
0
X
A
0
X
A
T
T
1
T
c
T
2
phase phase
supercritical phase
T
1
T
c
T
2
X
A
( )
phase + phase
X
A
( )
X
A
( )X
A
( )
critical mixing point:
(X
c
,T
c
)
phase + phase
phase
phase
supercritical phase
A
( )
=
A
( )
B
( )
=
B
( )
phase = phase
= supercritical phase
ba
1
1
1
1
Fig. 7.1
Gibbs free energy of mixing curves curves (left) for a symmetric non-ideal solution at the critical mixing temperature,
T
c
, and at a higher and a lower temperature. The curve on the T–X diagram (right) is the solvus. See text.

351 7.1 An intuitive approach
Under these conditions any composition between pure A and pure B forms a stable solution,
just as in the case of ideal mixing. As temperature decreases, however, G
ideal
mixing
becomes
less negative but G
excess
remains unchanged (and positive). The properties of the two
functions are such that at some temperature T
2
<T
1
, G
mixing
takes the shape shown in the
bottom left diagram of Fig. 7.1 (see also Figure 5.13.c). At this temperature the curvature of
the G
mixing
function changes sign twice: there is a central interval with negative curvature
separated from two positively-curved intervals by two inflection points. Given a curve with
this shape it is possible to find two points, labeled a and b in the figure, which have a
common tangent. Note that in Fig. 7.1 these points happen to be minima, but this is only
because we are plotting Gibbs free energy of mixing, so that the vertical coordinate of
the two end-members is the same. A plot of Gibbs free energy of the solution would not
show these points as minima, but they would still have a common tangent. The common
tangent is what matters, for it means that there are two compositions in which each of the
components, A and B, have chemical potentials that are simultaneously the same (see also
Fig. 5.9). These compositions are labeled X
A
(β) and X
A
(α) in Figure 7.1. It is evident from
the figure that the Gibbs free energy of any solution that could form in between these two
compositions is greater than the Gibbs free energy of a macroscopic mixture of solutions
with compositions X
A
(β) and X
A
(α). Such a solution is therefore unstable relative to a
macroscopic mixture of phases with compositions X
A
(β) and X
A
(α).
Two distinct phases, which I have labeled phase α and phase β, are therefore stable at T
2
.
For any bulk composition in the interval X
A
(β)–X
A
(α) the stable assemblage consists of
two phases: phase β of composition X
A
(β) and phase α of composition X
A
(α). Two phases
in a binary system constitute a divariant assemblage, and since we have chosen arbitrary
values of pressure and temperature the compositions of the two phases are fixed. The stable
assemblage for bulk composition between X
A
(β) and X = 0 (pure B) consists of phase β
only. This can be seen from the fact (Figure 7.1) that any solution within this interval has
lower Gibbs free energy than a macroscopic mixture of pure B and X
A
(β). The one-phase
assemblage is trivariant, so that the composition of phase β if it exists at equilibrium by itself
is not fixed, but is rather given by the bulk composition of the system. Identical arguments
show that phase α alone is stable from X
A
(α)toX = 1 (pure A), and that its composition
within this interval equals the bulk composition of the system.
The two points X
A
(β) and X
A
(α) are compositions of phases that coexist at equilibrium.
They correspond to two points on a curve that maps compositions of coexisting phases as
a function of temperature, as shown on the right-hand side diagram of Fig. 7.1. This curve
is called a solvus and is the phase diagram for this system. Bulk compositions inside the
solvus consist of two phases at equilibrium, of compositions given by the two intersections
of the temperature coordinate with the solvus. Bulk compositions outside the solvus consist
of a single phase, of composition equal to the bulk composition. We will get to a formal
justification for the shape of the solvus below, but it should be intuitively apparent that the
solvus of the system that we are considering must become wider as temperature decreases
and the positive contribution of the excess term in (7.2) becomes greater in relative terms.
It is important to realize that the only thing that changed between T
1
and T
2
is temperature.
The nature of the phase has not changed, as reflected in the fact that the mathematical
description of mixing has not changed. The ideal and non-ideal contributions to Gibbs
free energy of mixing are described by the same equations and using the same values
for the interaction parameters throughout, and we have also implicitly assumed that the
standard state Gibbs free energies always refer to the same two end-members (otherwise
leveling G
mixing
at zero at the end-members for all temperatures would not be valid). The

352 Critical phase transitions
corollary follows that a solvus can exist only between condensed phases that have the same
or very similar microscopic structures, such that complete miscibility is possible at some
temperature. Examples include: two feldspars, two pyroxenes, two micas, two metals, two
liquids, etc. (but obviously not two gases, since gases are always fully miscible).
A temperature must exist at which the nature of the system changes from one in which
there is only one phase spanning the entire compositional range between A and B, such
as at T
1
, to one in which there is a compositional interval within which two phases are
stable, such as at T
2
. This temperature is called the critical temperature. The two branches
of the solvus converge on the same composition, X
c
, at the critical temperature, defining
the critical mixing point (X
c
, T
c
), see Fig. 7.1. This point is also called the consolute point,
but I prefer critical mixing point because it highlights the deep underlying analogies among
all critical phenomena, that we will explore further below and in Chapter 9.
But what exactly happens at the critical mixing point? If there are two distinct phases,
α and β, that can exist below the critical temperature, but only one above it, is the high-
temperature phase α or β, or something else altogether? The answers to these questions
begin with the middle diagram on the left-hand side of Fig. 7.1, which shows Gibbs free
energy of mixing at the critical temperature, T
c
. As we saw, for T < T
c
the Gibbs free energy
function has two inflection points, whereas for T > T
c
there are no inflection points. Recall
that at an inflection point the curvature of a function changes signs, and hence its second
derivative vanishes. We can move from T > T
c
to T < T
c
without any discontinuity, as
all that is entailed in doing so is changing the value of T, which is a continuous variable,
and monitoring how (7.2), which is a continuous function, responds. Somewhere along this
continuous path there must be a temperature – the critical temperature – at which the function
goes from having a second derivative that never vanishes to having a second derivative that
vanishes at two points. The only way in which this can happen without a discontinuity is
if at some temperature, which is the critical temperature, the second derivative vanishes
at one point only. Now, if the function vanishes at X = 0 and X = 1 and has a single
inflection point in between, then the sign of the curvature of the function cannot change at
this inflection point either. Mathematically this means that the third derivative of the Gibbs
free energy function vanishes as well. This special behavior is depicted in the middle left
diagram in Fig. 7.1. It corresponds to a curve that is very flat in the neighborhood of X
c
.
It is, if you wish, almost a straight line but not quite. Generally, the fourth derivative is
the lowest order derivative of the Gibbs free energy of mixing that does not vanish at the
critical point (Section 7.4).
Consider a phase of composition X
c
that forms at T > T
c
. We shall label any phase that is
stable at T>T
c
the supercritical phase. If the supercritical phase is cooled instantaneously
to a temperature T<T
c
it will have a finitely higher Gibbs free energy relative to a mixture
of phases α and β and this Gibbs free energy will act as a driving potential (see Section 5.3.2)
that will cause the single supercritical phase to unmix into a macroscopic mixture of the
subcritical phases α and β (albeit subject to kinetic constraints, Chapter 12). With an exper-
iment of this kind it would in principle be possible to distinguish between the supercritical
phase and the subcritical phases. However, if the final temperature of the experiment is made
progressively higher, all the while keeping it below T
c
, the driving potential for unmixing
becomes smaller (because the G
mixing
curve becomes flatter), and the compositions and
physical properties of the two subcritical phases become closer to one other, and also closer
to those of the supercritical phase. Finally, at T
c
it is no longer possible to distinguish
between the three phases. There is no discontinuous phase boundary between the supercrit-
ical phase and either of the two subcritical phases. Rather, at the critical point the system
353 7.1 An intuitive approach
changes continuously from a supercritical state in which only one phase is possible to a sub-
critical state in which two phases can exist at equilibrium. If the experiment is repeated with
a supercritical phase of composition other than X
c
then the phase will change continuously
and imperceptibly, as temperature is lowered, from the supercritical phase to either of the
two subcritical phases (depending on the bulk composition), until the temperature is reached
at which this particular bulk composition intersects the solvus and the other subcritical phase
will appear, in this case discontinuously. There is a direct analogy with the behavior of fluids:
the distinction between liquid and gas, and the discontinuous phase transition that separates
the two phases, vanishes continuously as the critical point is approached, and a single super-
critical fluid phase is stable above the critical temperature (Chapter 9). Application of the
phase rule at a critical point may be confusing, but it shouldn’t be (see Box 7.1).
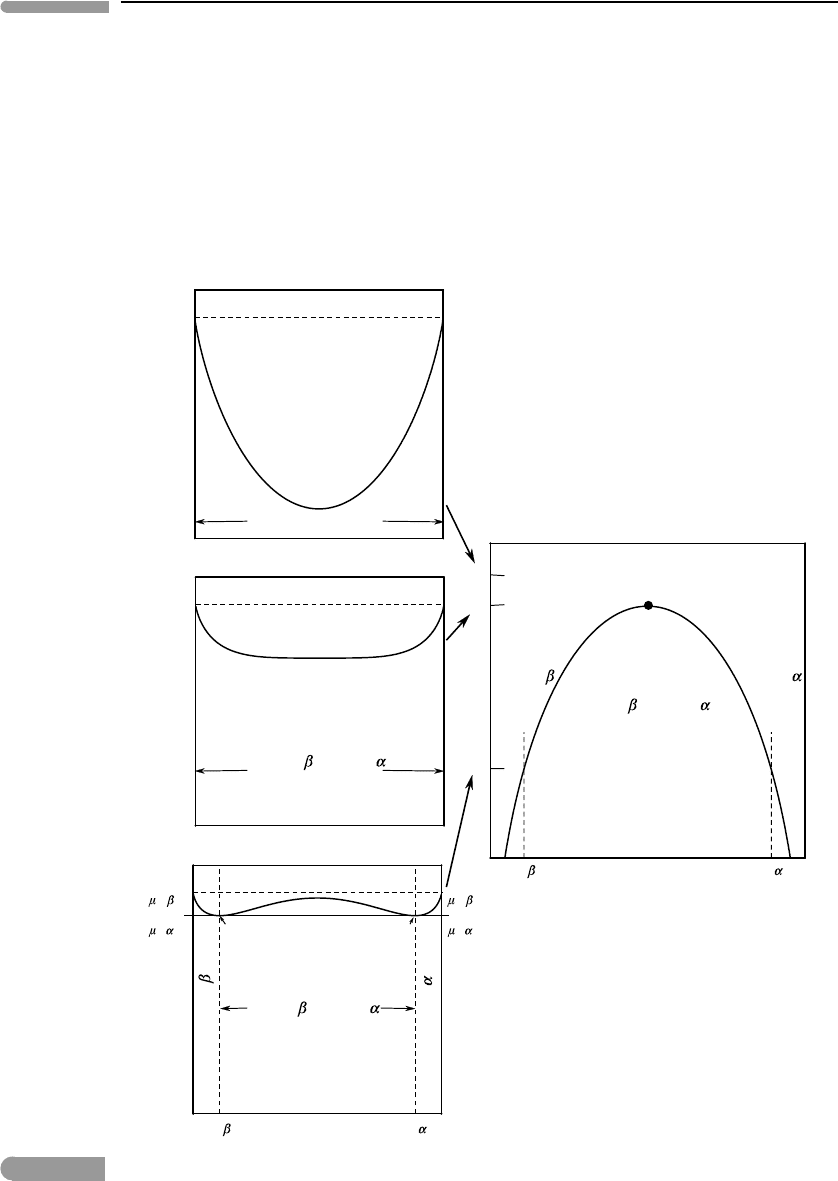
Box 7.1
The phase rule at a critical point
Consider a solvus in a binary system such as the one in the left-hand side panel of Fig. 7.2. The solvus is a
divariant phase boundary, as can be seen from an application of the phase rule. We have c =2, and along
the solvus the two phases, α and βs coexist at equilibrium, so F = 2 and f = 2. The solvus appears as
a one-dimensional curve, i.e. as a pseudo-univariant phase boundary, because we hold pressure constant,
thus “using up” one of the two degrees of freedom. At a different pressure the solvus will generally be
located at a different temperature. This is shown schematically by the two curves in the left-hand panel
of Fig. 7.2, where I have arbitrarily chosen the higher temperature solvus to correspond to a pressure, P
2
,
lower than that of the lower temperature solvus, P
1
. It could also be the other way around, but this diagram
is applicable, for example, to the system liquid molecular helium–liquid metallic hydrogen (see Fig. 2.16).
The solvus is also a first-order phase transition. Consider bulk composition X
z
, at temperature T
z
and
pressure P
2
. At these conditions the system consists of the two phases, α and β, of compositions given
by the intersection of the temperature coordinate with the two branches of the solvus. If we change the
01
T
P
P
2
<P
1
P
1
T
c
(P
2
)
T
c
(P
1
)
T
X
A
T
c
(P
2
)
T
c
(P
1
)
P
2
P
1
+
supercritical
phase
X
z
,T
z
Fig. 7.2 Two solvus curves for a hypothetical system (left) in which the critical mixing temperature varies inversely with
pressure. The P–T curve on the right-hand side diagram maps the location of the critical mixing point. It is not a
univariant phase boundary, because only one phase is stable along the curve. Following Ricci (1966) we can call it a
“singular curve” that separates supercritical conditions (to the right) from subcritical conditions (to the left).

354 Critical phase transitions
Box 7.1
Continued
temperature while holding the pressure constant one of the phases will form at the expense of the other,
and the two phases will change composition. If we raise the temperature then phase α will eventually
disappear when the vertical coordinate X
z
intersects the solvus. These changes are “first order” because there
is a difference in entropy and volume between the two phases, so that there is a non-vanishing enthalpy of
reaction associated with the phase changes.
More generally, this non-zero enthalpy change that characterizes first-order phase transitions is what
makes it possible for invariant (and univariant, divariant, and so on) assemblages to exist in the first place.
We can add or remove heat to an invariant system (e.g. an equilibrium assemblage of the three Al
2
SiO
5
polymorphs) and the system will stay at the P and T of the invariant point by forming some of the phases at
the expense of the others, until one or more of the phases disappears and the variance of the assemblage
increases. With two degrees of freedom rather than zero, this is what happens along the solvus.
Except at the critical point. Critical points have unique coordinates. Thus, in the two component system
shown in Fig. 7.2, the critical point at each pressure occurs at a unique temperature, which is thetemperature
at which coexistence of the two subcritical phases ends. We can show this by a curve in P–T space, as in the
right-hand panel of Fig. 7.2, that shows the combination of (T, P) coordinates at which the critical phase
transition takes place. The figure would appear to suggest that this curve is a univariant phase boundary
but
this is not correct
. By construction, the curve represents the P–T conditions at which phases α and β become
identical, and in effect identical to the supercritical phase. It is the locus of all critical points, two of which,
corresponding to the diagrams on the left, are shown in the figure.
Along the curve the system consists of a
one-phase assemblage
, so according to the phase rule it should be trivariant. Yet it shows as what looks like
a univariant phase boundary. Similarly, if one considers the critical point of a one-component fluid (Chapter
9), this is the point at which the liquid–vapor equilibrium curve ends, and only one phase is present at the
critical point. It looks like an invariant point, but according to the phase rule its variance is 2.
What is going on here? Simply, that the variance is indeed what the phase rule says it should be. We will
see why in a moment but first a note of caution. It is sometimes proposed that the way to fix this conundrum
is by modifying the phase rule at the critical point, by subtracting a number of degrees of freedom equal to
the number of phases that become indistinguishable at the critical point (two in both the example of the
solvus and that of the fluid critical point). The justification offered for this is having to account for “conditions
of criticality”, and although it may work arithmetically, it is thermodynamically incorrect. The critical point
in a one-component system
is not invariant, and the P–T locus of critical mixing points in a two-component
system (Fig. 7.2)
is not univariant.
The correct description was formulated by Ricci a long time ago (Ricci, 1966, pages 24–27). He pointed
out that a critical point along a univariant phase boundary (e.g. the end of the liquid–vapor coexistence
curve, more on this in Chapter 9)isa
singular point: it is simply the point where the boundary ends. It is
not an invariant point, because exchange of even an infinitesimal amount of energy will move the system away
from the critical point, which is distinctly different from what happens at a true invariant point.
Depending on
whether one adds (or subtracts) heat or work the system can move both in P and T, so the critical point is
indeed divariant. The fact that it is a “point”, with unique coordinates, does not make it invariant!
Consider a mixture at its critical mixing point. An infinitesimal exchange of heat, or of mechanical energy,
or of matter, will move the system away from the critical point in T, P and X, respectively. The critical
point is indeed trivariant. Following Ricci’s terminology, we can label the curve on the right-hand diagram
of Fig. 7.2 a
singular curve: it is the boundary of a two-dimensional divariant field, that extends to the
left of the curve in the figure. The coexistence of α and β ends at the singular curve because the phases

355 7.2 Location of the critical mixing point
Box 7.1
Continued
become identical. We can also state a general rule as follows: a critical phase transition occurs along an
n-dimensional boundary of an (n +1) dimensional region, over which an assemblage of subcritical phases
coexist. The phases become identical at the n-dimensional critical boundary, and only a single supercritical
phase exists on the other side of it.
So what makes phase equilibrium at the critical point different? Simply, the fact that there is no latent
heat associated with critical (continuous) phase transitions. Because entropy and volume are continuous
across a critical phase transition,
transition
H =0. It therefore becomes impossible to have, for example, an
invariant assemblage, as transfer of even an infinitesimal amount of energy will change the temperature
and/or pressure. The phase rule, and the variance that it predicts, are intact and there is no need to invent
“conditions of criticality”.
I must emphasize that although I used a symmetric Margules-type function to exemplify
the concept of critical mixing (because it generates simple diagrams, such as in Fig. 7.1),
the behavior is general. It arises from the fact that, if the relative magnitudes of the ideal and
excess contributions to Gibbs free energy of mixing change with temperature, then a transi-
tion from a Gibbs free energy function with no inflection points to one with two inflection
points must happen at some temperature. At this critical temperature both the second and
third derivatives of G
mixing
must vanish, giving rise to a continuous phase transition.
Unmixing of a homogeneous supercritical phase into two or more subcritical phases
is an important process in nature. Common examples include exsolution in high-pressure
mantle phases (such as pyroxenes from majoritic garnets), Widmanstätten textures in iron
meteorites, metal–sulfide–silicate liquid immiscibility, condensation of a supercritical fluid
into a liquid and coexisting vapor, and immiscibility between liquid helium and liquid
metallic hydrogen in the interiors of some giant planets (Chapter 2).
7.2 Location of the critical mixing point
Finding the critical mixing point of a solution is the first step in determining its solvus.
From the preceding discussion it follows that at the critical mixing point the following two
conditions must be satisfied:
∂
2
G
mixing
∂X
2
=
∂
2
G
ideal
mixing
∂X
2
+
∂
2
G
excess
∂X
2
=0 (7.3)
and:
∂
3
G
mixing
∂X
3
=
∂
3
G
ideal
mixing
∂X
3
+
∂
3
G
excess
∂X
3
=0 (7.4)
which, substituting (7.1), yield:
∂
2
G
excess
∂X
2
=−
RT
X
(
1 −X
)
(7.5)

356 Critical phase transitions
and:
∂
3
G
excess
∂X
3
=−
RT
(
2X −1
)
X
2
(
1 −X
)
2
. (7.6)
Equations (7.5) and (7.6) are general (for solutions of site multiplicity one). They contain two
free parameters, X and T, which means that they can be solved for a unique pair of values that
satisfy both equations simultaneously. These are the coordinates of the critical mixing point.
In order to solve the equations, however, it is necessary to substitute an explicit expression
for the excess Gibbs free energy function. As an example we will assume that the excess
Gibbs free energy can be represented by the asymmetric Margules function (5.143), with no
temperature dependency. Without loss of generality we can choose to label the components
such that W
G
12
≥W
G
21
, and make X =X
2
. We also define the variable K
W
, as follows:
K
W
=
W
G
21
W
G
12
(7.7)
and note that it must be K
W
≤ 1, with K
W
= 1 corresponding to the symmetric function
(5.146). Substituting these definitions in (5.143), taking the second and third derivatives of
the resulting function, and substituting in (7.5)and (7.6) we obtain, after some simplification:
−
RT
X
(
1 −X
)
=W
G
12
[
(
6X −4
)
K
W
−6X +2
]
(7.8)
and:
−
RT
(
2X −1
)
X
2
(
1 −X
)
2
=6W
G
12
(
K
W
−1
)
. (7.9)
Dividing (7.8)by(7.9) and simplifying some more we get:
2X −1
2X −3X
2
=
3
(
1 −K
W
)
2K
W
−1
(7.10)
which is a quadratic equation in X :
9
(
1 −K
W
)
X
2
+
(
10K
W
−8
)
X +
(
1 −2K
W
)
=0. (7.11)
The physical solution of this equation is X
c
, the composition of the critical mixing point.
Equation (7.11) shows that the composition of the critical mixture depends only on the
relative values of the interaction parameters, and not on their absolute values. In particular,
for a symmetric solution K
W
=1 and X
c
=0.5, which of course we could have inferred from
symmetry considerations. Let us now define a non-dimensional temperature, τ , as follows:
τ =
2RT
W
G
12
(7.12)
and call the value of τ at the critical temperature τ
c
. Substituting this definition in (7.8),
and with X
c
given by the physical solution of (7.11), we find that τ
c
is given by:
τ
c
=4X
c
(
1 −X
c
)
[
K
W
−
(
1 −K
W
)(
1 −3X
c
)
]
. (7.13)
This is a remarkable result: given that X
c
depends only on K
W
, the non-dimensional critical
temperature, τ
c
, is also a function of K
W
only. But, alas, this concise and rather elegant
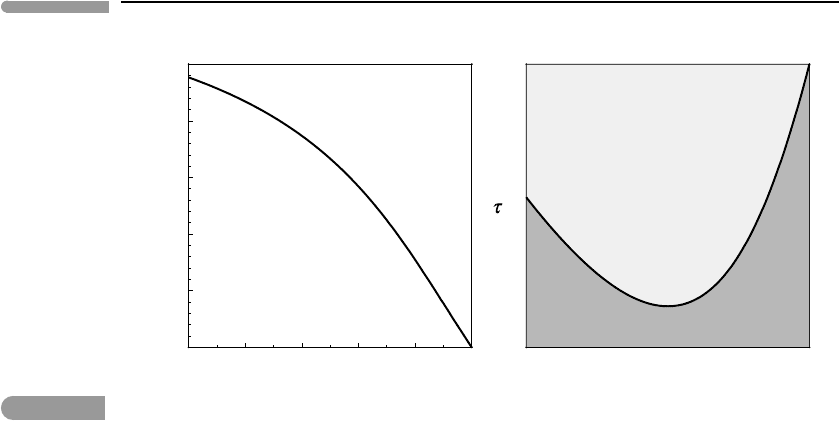
357 7.2 Location of the critical mixing point
0 0.2 0.4 0.6 0.8 1
0.5
0.6
0.7
K
W
X
c
0 0.2 0.4 0.6 0.8 1
0.9
0.95
1
K
W
c
supercritical mixing
(one phase)
subcritical mixing
(two phases)
Fig. 7.3
Calculated composition (X
c
) and non-dimensional temperature (τ
c
) at the critical mixing point, for (possibly
hypothetical) solutions with temperature-independent excess Gibbs free energy of mixing.
result is true only because we assumed that G
excess
is not a function of temperature, and is
given by the simple Margules function. Since the Margules approximation is no more than
an empirical fit, with little physical fundamentation, we should not expect equation (7.13)
to provide more than a rough approximation to the behavior of real solutions.
Even if equations (7.11) and (7.13) are no more than simplified models for the behavior
of real solutions, the results, which are summarized in Fig. 7.3, help make the significance
of the critical mixing temperature clear. The figure shows X
c
and τ
c
as functions of K
W
.
Note that K
W
is defined to be ≤ 1, and although it could in principle also be negative,
such behavior (which corresponds to Fig. 5.13d) is uncommon and is not included in the
figure. The figure shows that, starting from the value X
c
= 0.5 for a symmetric solution,
increasing asymmetry causes the composition of the critical mixture to shift in the direction
of the component that is associated with the larger interaction parameter (X
c
> 0.5), i.e.
the component that is responsible for generating the greatest amount of excess Gibbs free
energy of mixing. We will see in the next section that this asymmetry carries over into
the subcritical region. Symmetric solutions also have the largest value of non-dimensional
critical temperature, τ
c
= 1. Note that τ
c
decreases to a minimum of ∼0.89 for K
W
∼0.5,
but this range may have little practical significance in planetary processes. Figure 7.3
illustrates how equations (7.11) and (7.13) encapsulate a fundamental property of critical
mixing. Namely, all non ideal solutions (W
12
G
= 0) are in principle capable of exhibiting
critical mixing behavior. This means that all non-ideal solutions can undergo a continuous
phase transition between a supercritical one-phase region at τ>τ
c
, and a subcritical region
at τ<τ
c
that, in a binary system, is populated by two phases (Fig. 7.3, right panel). For
a given value of K
W
(asymmetry) the absolute value of the critical temperature is a linear
function of the interaction parameter W
G
12
, given by (7.12). This leads naturally to a couple
of important considerations, that arise from the fact that the stabilities of phases, both
supercritical and subcritical, unavoidably terminate at some discontinuous phase boundary.
If the excess Gibbs free energy of mixing is small then the critical mixing point may occur
at a temperature lower than that at which the supercritical phase breaks down to a lower-
entropy assemblage, i.e. the critical mixing point is metastable. The critical temperature may
also be sufficiently low that subcritical unmixing, even if thermodynamically favored, is
