Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


308 Phase equilibrium and phase diagrams
boundary is inconsistent with Schreinemaker’s rule. It also allows us to work with only a
partial data set, as being able to calculate only a couple of the reactions places constraints
on the locations of the others.
The second issue is that the information contained in a phase diagram such as that in
Fig. 6.8b, or even in a quantitative version of it, is incomplete. The composition of the
liquid phase is not fixed, and its location in the chemographic diagrams is only schematic.
Liquid composition is fixed at each point on each univariant curve, because the chemical
potentials of each of the liquid components are fixed at each point on each curve, but
the liquid composition is not constant along each curve, nor is it equal from one curve to
another. In the divariant fields the liquid composition also depends on bulk composition.
For example, consider a liquid in the field bound by the reactions (Th) and (V). If the bulk
composition of the system is such that there is excess mirabilite, then the liquid is saturated
in mirabilite and its composition will be that of the saturated solution at any given P and
T (which are the two degrees of freedom). If, on the other hand, all mirabilite dissolves
then the liquid will be in equilibrium with a gas phase rather than with mirabilite, and its
sodium sulfate content will be less than that of a saturated solution at the same pressure
and temperature. This shortcoming is inherent to this type of phase diagram. Recall that in
this section we set out to construct phase diagrams among phases of fixed composition. By
including a liquid phase we have gone beyond that specification, even if the resulting phase
diagram is still thermodynamically valid.
The third, and most important, issue, is whether the choice of intensive variables in
Fig. 6.8 is the most appropriate one for this particular example. We must now return to our
decision to ignore the fact that the Earth’s atmosphere consists chiefly of components other
than H
2
O. By considering a system of two components and using pressure as an intensive
variable we are assuming that the gas phase is made up exclusively of H
2
O, so that the
pressure on the system is the same as the partial pressure of H
2
O (see Worked Example
5.5). This would be true in a hypothetical planet in which the atmosphere consisted of H
2
O
vapor only, but it is certainly not true of the present-day Earth. Using pressure as an intensive
variable to represent the phase relations of sodium sulfate phases is not incorrect, but it leads
to unphysical interpretations if the diagram is used to study natural evaporites. For instance,
consider a system whose bulk composition is between Mi and L, and assume that conditions
are initially within the divariant field bound by (L) and (Th). If conditions change such that
reaction (Th) is crossed then all the vapor will be consumed and the assemblage will be
liquid + mirabilite. This description may work in a closed vessel in a laboratory, but it does
not work on a planetary surface. A better alternative would be to use µ
H
2
O
as an intensive
variable. By doing so we can track the evolution of the system as a function of changes
in atmospheric humidity at constant atmospheric pressure, as there is a simple relationship
between the mol fraction of H
2
O in air and the chemical potential of H
2
O (e.g., equation
(5.82)). We return to this in Worked Example 6.6.
Worked Example 6.3 Hydrous melting of silicate rocks
Melting of hydrous silicate mineral assemblages is an important process in the origin of felsic
terrestrial magmas, and in the origin and evolution of the continental crust. Whether or not
such processes ever took place in Mars or Venus is as yet unknown, and figuring this out will
be important in piecing together the crustal evolution of our sister planets. The fundamental
thermodynamic relations of hydrous melting of silicate rocks are a close parallel to those
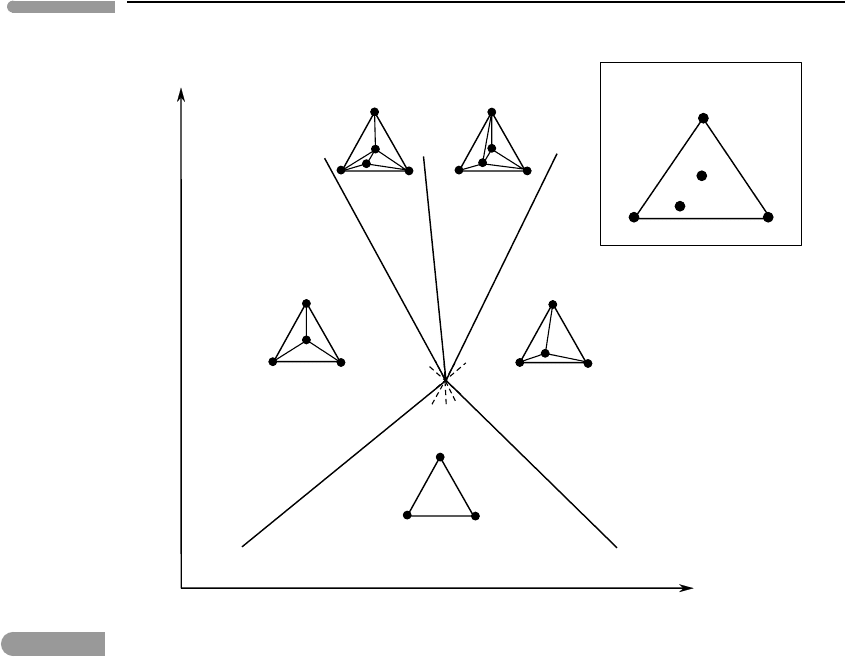
309 6.2 Analysis of phase equilibrium
P
(V)
(L)
(Co)
Ms
L
T
H
2
O KAlSi
3
O
8
Ms
L
Co
V
(Kf)
Kf
Ms
Co
Kf
V
Co
L
Kf
Kf
V
Ms
V
Co
L
Ms
L
Co
V
Al
2
O
3
Kf
(Ms)
Fig. 6.9 Phase diagram for melting of silicates in the system KAlSi
3
O
8
–Al
2
O
3
–H
2
O. The tie lines joining phases in the
chemographic diagrams define the possible divariant assemblages. For example, in the diavariant field bound by the
curves (V) and (Ms) there are three possible stable divariant assemblages: Co–L–V, Kf–L–V and Co–Kf–L. The only
phase stable in all assemblages is L, and the metastable extension of the (L) curve enters this field. This is one possible
statement of Schreinemakers’ rule. The other one (which I prefer) is that the metastable extension of (L) enters the
field in which L appears as a reactant on the two bounding curves, (V) and (Ms).
of evaporite–brine equilibria. A complete analysis of hydrous melting of rocks can only
be carried out in systems of at least four or five components, but a simple analog that
exhibits some of the key thermodynamic aspects can be based on the ternary system:Al
2
O
3
–
KAlSi
3
O
8
–H
2
O. Let us consider the five-phase invariant assemblage: muscovite–sanidine–
corundum–melt–vapor in this ternary system (Figure 6.9). In this simple example we will
assume that the vapor is pure H
2
O. The melt composition will be taken to correspond to a
hydrous and slightly peraluminous syenite (Fig. 6.9) – rocks that approximately match this
description are rare in nature, but not unknown.
A schematic P –T phase diagram for this system is shown in Fig. 6.9. Comparison with
Fig. 6.8 reveals some remarkable similarities and one important difference. Except for
the fact that there is one additional phase in each assemblage (because this is a ternary
system), reactions (Co), (L) and (V) are analogous to (Th), (L) and (V), respectively, in
the sodium sulfate–H
2
O system. Thus, the hydrous mineral assemblage (mirabilite, or
muscovite +sanidine) reacts with vapor to yield a liquid at (Th) and (Co), respectively. The
hydrous mineral by itself breaks down to an anhydrous solid assemblage (thenardite, or
sanidine + corundum) plus either vapor or liquid at the (L) and (V) curves in both systems.
For reasons that we will discuss in Chapter 10, in igneous systems we typically call the liquid
phase a melt, but, as these diagrams suggest, there are important thermodynamic similarities

310 Phase equilibrium and phase diagrams
between both systems. The signs of the Clapeyron slopes of these three reactions at low
pressure are the same in both systems, for the reasons that we discussed in Worked Example
6.2, but at high pressure, such as in the deep crust or upper mantle of the Earth, the Clapeyron
slopes of the (Co) and (V) curves may change signs, in response to the higher compressibility
of hydrous fluids relative to silicate melts, and of silicate melts relative to crystalline phases.
In the hydrous silicate system (c = 3) there is an additional univariant curve, (Kf), that
has no analog in the sodium sulfate–H
2
O system (c =2). This reaction is similar to (Co) in
the sense that in both of them muscovite reacts with vapor to produce liquid, except that an
anhydrous solid phase is produced together with the liquid along the (Kf) reaction, but not
along (Co). The one important difference between both systems is in the Clapeyron slope of
the reaction in which an anhydrous assemblage, thenardite or sanidine + corundum, reacts
with vapor to produce liquid. In the evaporite example we justified the positive slope of (Mi)
on the basis of the large value expected for the stoichiometric coefficient of vapor. We can
think of the liquid that forms at (Mi) as the result of condensation of vapor and dissolution
of the anhydrous solid in the resulting condensate. At the high temperature at which the
(Ms) reaction takes place, in contrast, the liquid forms by melting of the crystalline solids
and dissolution of vapor in the melt. The consequence is that the stochiometric coefficient
of vapor in the (Ms) reaction is characteristically quite small, and
r
S is dominated by
the entropy of melting of the silicates, which is of course positive. The volume change of
reaction, and therefore the Clapeyron slope, are negative.
In the discussion of igneous phase relations alternative names are used for some of
these reactions. Thus, (Co) is called the vapor-saturated solidus. This reaction maps the
minimum temperature at which melt can form. Because melt forms along this reaction
only if an aqueous vapor phase is present the melt at the solidus is saturated in H
2
O. Note
that for certain bulk compositions (e.g. inside the triangle defined by the phases vapor,
muscovite and corundum, see Fig. 6.9) the (Kf) reaction, rather than (Co), is the vapor-
saturated solidus. The liquid-absent curve is called the subsolidus dehydration reaction, as
the assemblage becomes anhydrous without melting. Finally, the (V) curve, where melt
forms in response to breakdown of the hydrous mineral without formation of a vapor phase,
is called the dehydration-melting or vapor-absent melting reaction.
Whether we call the liquid phase a liquid (or solution) or a melt depends on the com-
position of the coexisting solids. A melt is a liquid at equilibrium with a solid of its same
composition (Chapter 10). The composition of the liquid that forms in the silicate rock
example is close enough to the composition of the solid phases that it is properly called a
melt. In the evaporite example, on the other hand, the liquid composition is close to that
of a condensed gas and it contains dissolved ions, so we call it a solution. Liquid-forming
reactions such as (Co), (Th), (Mi) and (Ms), in which the only product of the reaction is a
liquid, are called congruent melting or congruent dissolution reactions. In contrast, along
equilibria such as (Kf) and (V) a solid phase crystallizes on the high-temperature side of the
reaction, together with formation of a liquid. Such reactions are called incongruent melting
or incongruent dissolution reactions.
6.2.4 Compositional degeneracy
A detailed discussion of the many possible chemographic relations in systems of two or
more components, and of the different topological varieties of phase diagrams that they
give rise to, exceeds the space available here (but see Zen, 1984). There is, however, one
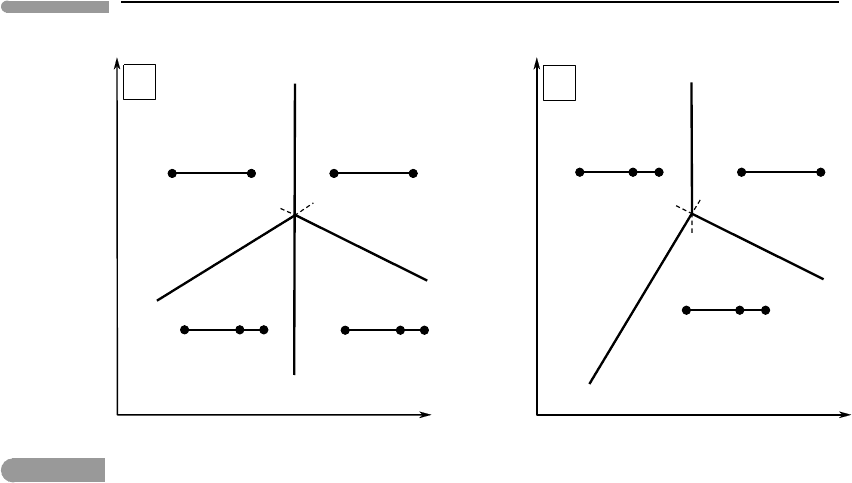
311 6.2 Analysis of phase equilibrium
Z
(A,D)
(B)
B
A
(C)
B
A
C
C
D
D
Y
DB
DBA
DC
Z
(A)
B
(C)
B
A
C
(B,D)
A
C
D
Y
(A,D)
B
C
DCA
DB
DB
C
D
DB
A
a
b
Fig. 6.10
Two types of compositional degeneracy in a binary system. If the degenerate reaction occurs at one of the ends of the
compositional range, as in (a), the univariant curve does not become metastable when it crosses the invariant point. If
the degeneracy is internal, as in (b), the univariant curve becomes metastable (see text).
instance that needs attention, as it occurs very commonly in natural systems. This is the case
in which a subset of phases can be described in terms of a smaller number of components
than the system as a whole. Phase transitions (including polymorphic transformations) are
an example of this situation, as they are described in terms of only one component (the
composition of the substance that undergoes the phase transition). Other examples are: a
set of three phases that plot along a straight line in a ternary chemographic diagram, so
that equilibria among these phases are described in terms of two components only; a set
of four co-planar phases in a four-component system, and so on. Such cases are said to be
compositionally degenerate.
Consider a binary system in which two phases have the composition of one of the system
components (Fig. 6.10a). The phase transition (B C in this example) is a univariant
equilibrium, which is represented by a curve, labeled (A, D), along which two phases are
missing. Two divariant fields, (A, D, C) and (A, D, B), radiate from this univariant curve,
and each of these divariant fields must switch from stable to metastable as it crosses the
univariant curve (A, D). The only way in which this can be accommodated is if the curve (A,
D) crosses the invariant point without becoming metastable, as shown in Fig. 6.10a. Another
way of seeing why (A, D) does not become metastable is by thinking of it as a phase boundary
in a one-component system in which phases A and D do not exist. The phase transition B
C takes place regardless of the presence of additional components that make phases A and
D possible. Alternatively, comparing Fig. 6.10a with Fig. 6.7, one may imagine that, as C
approaches B on the chemographic diagram, the slope of reaction (B) approaches that of
reaction (C) on the phase diagram. Coincidence in both diagrams occurs simultaneously, so
that when the reaction becomes degenerate each stable curve coincides with the metastable
extension of the other (this is the basis of the method developed by G. W. Morey to derive
Schreinemakers’ rule; see, for example, Williamson & Morey, 1918; Morey & Williamson,
1918). The important point here is to understand how Schreinemakers’ rule applies to this
degenerate case, which is easily seen in Fig. 6.10a. In my preferred versions of the rule,

312 Phase equilibrium and phase diagrams
each of the ends of the (A, D) curve, none of which is metastable, splits the only divariant
field in which one of the phases absent along the univariant curve appears as a reactant on
the two univariant curves bounding that field. Alternatively, each of the ends of the (A, D)
curve splits a divariant field in which a phase absent along the curve is stable for all bulk
compositions.
If the phase transition occurs for a composition that does not correspond to one of the
system components then the behavior is different, and is shown in Fig. 6.10b. In this case
the univariant curve that corresponds to the phase transition, (B, D) in the example, must
become metastable as it crosses the invariant point. This must be so because reactions (A)
and (C) correspond to the breakdown of the two phases with the same composition. Neither
of the phases is stable in the divariant field that extends between these two reactions,
and therefore the phase transition must be metastable in this field. Figure 6.10b shows
that the application of either version of Schreinemakers’ rule is immediately obvious. It
is important to see the subtle difference in Schreinemakers’ rule applied to both types of
degenerate systems shown in Fig. 6.10. In case (a) the phases absent along the degenerate
univariant reaction, A and D, appear on opposite sides of the curves that bound the divariant
fields, whereas in case (b) the two phases (B and D) appear on the same side.
Worked Example 6.4 A simple model for carbonatite melts
The fascination of petrologists with carbonatites is out of proportion to their scarcity in
the terrestrial igneous rock record. This is justified, however, as the processes that lead to
the formation of carbonate melts in a silicate planet are not as straightforward as those that
generate silicate melts. The simplestpossible model for carbonate melting can be constructed
in the binary system CaO–CO
2
(Fig. 6.11). If we assume that calcite melts congruently to
a liquid with the same composition then the melting reaction is degenerate, as shown in
the figure. When this reaction, labeled lime- and vapor-absent, crosses the invariant point it
becomes metastable – this is the case illustrated in Fig. 6.10b. The invariant point therefore
marks the minimum pressure at which calcite melts or, equivalently, the minimum pressure
at which calcite can crystallize from a carbonate melt. The pressure of the invariant point is
higher than 1 bar, as at atmospheric pressure calcite decarbonates to lime plus vapor along
the liquid-absent curve.
The parallels with the examples discussed in Worked Examples 6.2 and 6.3 should be
clear. We could call the (L) and (Lm, V) reactions the “subsolidus decarbonation” and
“decarbonation-melting” reactions, respectively, and we see that they are close analogs of
the corresponding reactions in the H
2
O-bearing examples. The vapor-saturated solidus is
missing in the carbonate system because we assumed a degenerate melting reaction. Phys-
ically, this means that we assumed that the carbonate liquid is not able to dissolve excess
CO
2
. If this were not the case then the liquid phase would be richer in CO
2
than calcite and
the melting reaction would not be degenerate. The (Lm,V) reaction would then split into
two different reactions, with the (Lm) reaction: Cc + V = L becoming an exact analog of
the (Co) or (Th) reactions in the previous examples. As with the (Mi) and (Ms) reactions,
the slope of the (Cc) reaction is the one that is most uncertain. I emphasize this in Fig. 6.11
by plotting it parallel to the T axis. We can be certain that liquid is the high-pressure
phase, but whether it is the high- or low-temperature phase depends on the entropy of molten
CaCO
3
relative to that of a stoichiometric mixture of CaO and CO
2
. Note that regardless
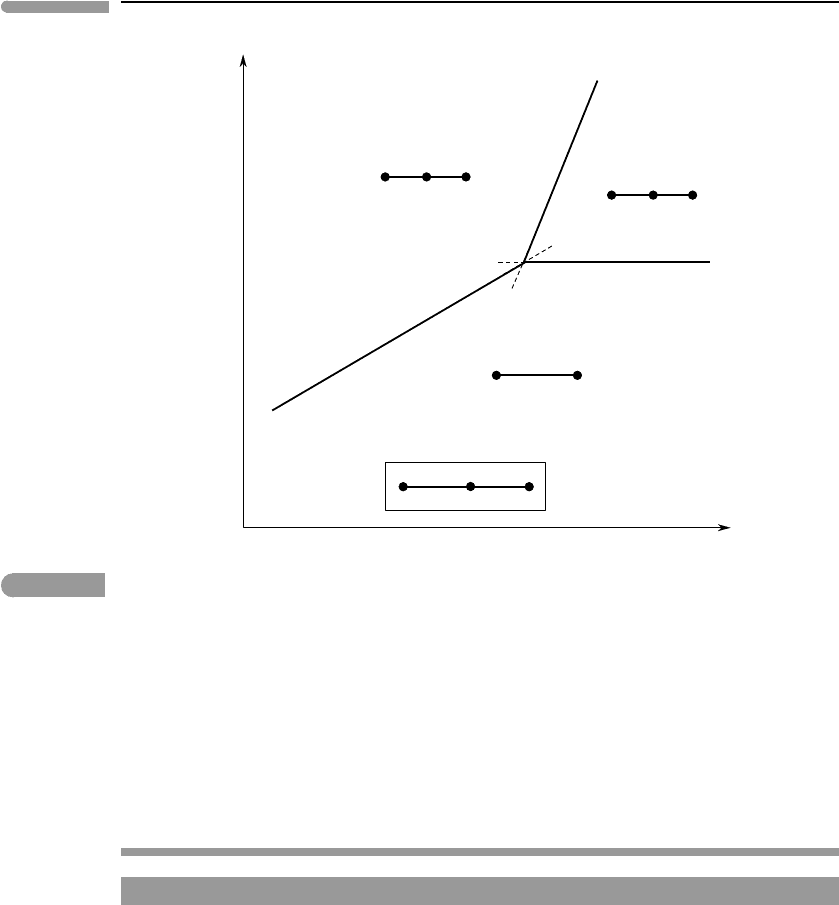
313 6.2 Analysis of phase equilibrium
P
(Lm,V)
(L)
(Cc)
T
LmVL
CaO
CO
2
LmV
Cc, L
LmV
LmVCc
Cc
L
L
Lm V
Lm
V
Cc
Fig. 6.11 Schematic phase diagram for melting of carbonates. Carbonatitic melts can only exist between the (Cc) and (Lm,V)
curves. Calcite cannot crystallize from a melt at a pressure lower than that of the invariant point. Compare Fig. 6.9.
of the slope of the (Cc) reaction the pressure of the invariant point is the minimum pressure
at which calcite can crystallize from a melt.
The phase diagram in Fig. 6.11 suggests that one way in which carbonatite melts can
form is by subduction of limestones. The “cold” conditions that prevail at subduction zones
may be able to keep limestones on the low temperature side of the (L) reaction until the
pressure of the invariant point is exceeded. Heating of subducted limestones at high pressure
would then produce carbonatite melts. Alternatively, carbonatite melts could be produced
by infiltration of CO
2
-rich fluids into clinopyroxene- or garnet-bearing peridotites.
Worked Example 6.5 Freezing of brines. Liquid water on the Martian surface?
Consider the system NaCl–H
2
O, and the four phases: ice–vapor–halite–liquid (Fig. 6.12).
The liquid phase in this system is brine, which we shall label L
2
, to distinguish it from
pure liquid H
2
O, which we label L
1
. The four phases I–V–Ha–L
2
exist at equilibrium at
an invariant point, O
2
in Fig. 6.12. Because neither ice nor vapor dissolve NaCl the phase
transition I V is a degenerate reaction, (L
2
, Ha), that in this case crosses the invariant
point without becoming metastable (this is the case depicted in Fig. 6.10a). The other two
univariant equilibria, (V) and (I), correspond to freezing and boiling of brine, respectively.
The negative slope of the (V) reaction arises from the fact that H
2
O expands when it freezes,
which is of course unusual.
Because sublimation of ice is a liquid-absent reaction, and the composition of both ice and
vapor is H
2
O, the (L
2
, Ha) reaction must be the same liquid-absent reaction that appears in
the one component system H
2
O, and which we can label (L
1
). This reaction meets the
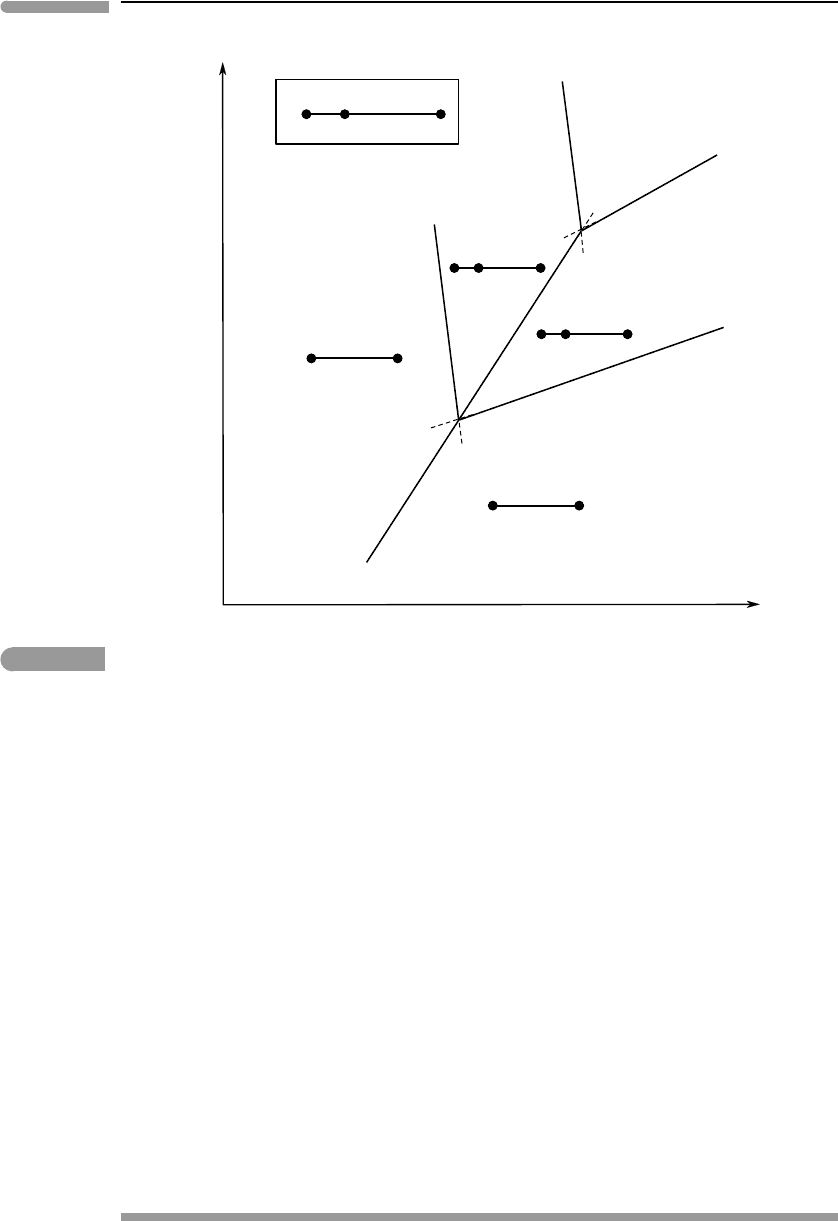
314 Phase equilibrium and phase diagrams
P
(L
2
,Ha)
(I)
T
NaCl
H
2
O
Ha
V,I,L
1
L
2
L
2
L
2
V
V
I
HaI
HaV
HaI
(V)
L
2
HaV
I
Ha
L
2
V
I
Ha
(I)
(V)
I
V
L
1
L
1
O
2
O
1
Fig. 6.12 Effect of a solute (e.g. halite) on the phase diagram of H
2
O. The triple point for pure H
2
O(O
1
) shifts to lower pressure
and temperature (O
2
). The freezing temperature drops and the boiling temperature rises.
freezing and boiling curves of pure water at the invariant point O
1
, which is the triple point
of H
2
O. Reaction (L
1
) must become metastable when it crosses O
1
, as no other behavior is
possible in a one-component system. The univariant curve for ice–vapor equilibrium joins
the two invariant points, even as its name changes (by convention) in the neighborhood of
each of the invariant points. Schreinemakers’ rule also tells us that O
1
must be located at
higher pressure than O
2
, because O
2
must lie on the stable part of the (L
1
) curve. Given
vapor’s higher volume and higher entropy relative to ice, the slope of the (L
2
, Ha) reaction
is positive. The two invariant points must therefore be located relative to one another as
shown in Fig. 6.12.
Schreinemakers’rule generates a qualitative result that we know well: addition of a solute
to water lowers its freezing point and raises its boiling point. The magnitude of the effect of
course depends on each particular solute, and thermodynamic data are needed in order to
calculate it (Chapter 11). But another prediction follows from this analysis, and this is that
the triple point of water shifts to lower pressure and temperature by addition of a solute.
Consider a planet (for instance, Mars), in which the partial pressure of H
2
O at the surface
is below O
1
(why we specify partial pressure will become clear in the next section). In the
absence of soluble salts liquid water is not stable at the planet’s surface, but if appropriate
solutes are available then the pressure of invariant point O
2
may be low enough to allow
brines to exist. The implications of these phase relations for the possible existence of liquid
H
2
O on the Martian surface are discussed in Chapter 11.
315 6.3 Phase diagrams in open systems
I used a binary system to discuss the concept of compositional degeneracy, but application
to systems with more components presents no difficulty, as further examples will show (see
end-of-chapter Exercises).
6.3 Phase diagrams in open systems
We can define a closed system as one in which the only intensive variables that can be con-
trolled independently and externally (or, equivalently, imposed on the system) are pressure
and temperature. An open system is one in which, in addition to pressure and temperature,
some chemical potentials can be controlled externally. Let the number of externally con-
trolled chemical potentials be v. Then the total number of independent intensive variables
in an open system is v + 2. Consider the evaporite system discussed in Worked Example 6.2.
If the gas phase is not pure H
2
O, and the H
2
O content of the atmosphere is variable, then
v =1 and there are three independent intensive variables: P , T and µ
H
2
O
. These additional
independently variable thermodynamic quantities allow extra flexibility in the construction
of phase diagrams for open systems.
6.3.1 Externally controlled chemical potentials
Recall that Schreinemakers’ rule is general, as we derived it on the basis of a set of arbitrary
and unspecified intensive variables. Pressure and temperature are not always the most
convenient combination of variables, particularly for open systems. Evaporites, for instance,
are most accurately described as open systems that exchange a chemical component (H
2
O)
with their environment (the atmosphere). In Worked Example 6.2 I suggested that the
chemical potential of H
2
O is a better intensive variable than pressure to study the behavior
of evaporites. This is so because, whereas pressure at the Earth’s surface is approximately
constant, atmospheric humidity is not. By using µ
H
2
O
as one of the intensive variables
we can track how changes in atmospheric humidity cause H
2
O to be transferred between
the atmosphere and the evaporite + brine assemblage, and predict changes in the evaporite
phase assemblage. Recall that equation (5.86) relates µ
H
2
O
to the partial pressure of H
2
O,
p
H
2
O
, and that p
H
2
O
is a convenient way of measuring atmospheric humidity.
Consider an equilibrium assemblage with f degrees of freedom, and subject to v externally
controlled (or imposed) chemical potentials. In such a system there are v + 2 independent
intensive variables (the v chemical potentials, pressure and temperature). If we fix the
values of any f of the v + 2 intensive variables then the assemblage appears to behave as
an invariant assemblage, as long as we keep the values of the f chosen intensive variables
fixed. Our goal is to map the f -variant assemblage onto a pseudo-invariant point in a two-
dimensional graph in which the coordinates are any two intensive variables, (Z, Y ), taken
from the v + 2 available independent variables. In order for this to be possible it must
be v = f . The variance of the assemblage is of course still f , so that it is stable over an
f -dimensional region of intensive-variable space. However, for each point in this region
there is a unique combination of the variables Z and Y for which the assemblage is stable.
This combination maps as a pseudo-invariant point on the Z–Y plane. Suppose further that
the pseudo-invariant assemblage consists of F = c +2 −f phases. Then, removing each
of the F phases one at a time generates F pseudo-univariant curves radiating from the
pseudo-invariant point, along each of which F −1 phases are stable. These curves separate
F pseudo-divariant fields, inside each of which F − 2 phases are stable. In general, a
316 Phase equilibrium and phase diagrams
“pseudo-q-variant assemblage” means that the assemblage actually has q + v degrees of
freedom, but the values of v intensive variables are held constant.
For example, suppose that we have a divariant assemblage (f = 2) in a four-component
open system (F = 4). In order for it to be possible to map this assemblage as a pseudo-
invariant point there must be two chemical potentials that are controlled externally (v = 2).
If we fix any two intensive variables, say pressure and temperature, then there is a unique
combination of the two chemical potentials for which the assemblage is stable. This com-
bination of values defines a pseudo-invariant point in an isobaric and isothermal phase
diagram in which the two coordinates are the two externally controlled chemical potentials.
Four phases are stable at the pseudo-invariant point, four pseudo-univariant three-phase
curves radiate from the point, and there are four pseudo-divariant two-phase fields between
the curves. Note that we can choose to fix any two intensive variables. In this example we
chose P and T, but if we had chosen, say, T and one of the chemical potentials then the
four-phase assemblage will be pseudo-invariant in a phase diagram in which the coordinates
are P and the other chemical potential. All of this is best seen in examples that will make
guest appearances throughout this and subsequent chapters.
Worked Example 6.6 Evaporites, part (ii)
We wish to recast the phase diagram for sodium sulfate evaporites shown in Fig. 6.8 as
a function of the intensive variables µ
H
2
O
and T (Fig. 6.13). Note that, whereas Fig. 6.8
assumes a closed system (constant bulk composition), in Fig. 6.13 we consider the system
mirabilite–thenardite–liquid to be an open system that exchanges H
2
O with its environment.
H
µ
2
0
(L)
(Mi)
(Th)
H
2
O
Mi
L
T
L
Th
H
2
O
Th
H
2
O
Mi
P = constant
Mirabilite
Thenardite
Liquid
T
1
T
2
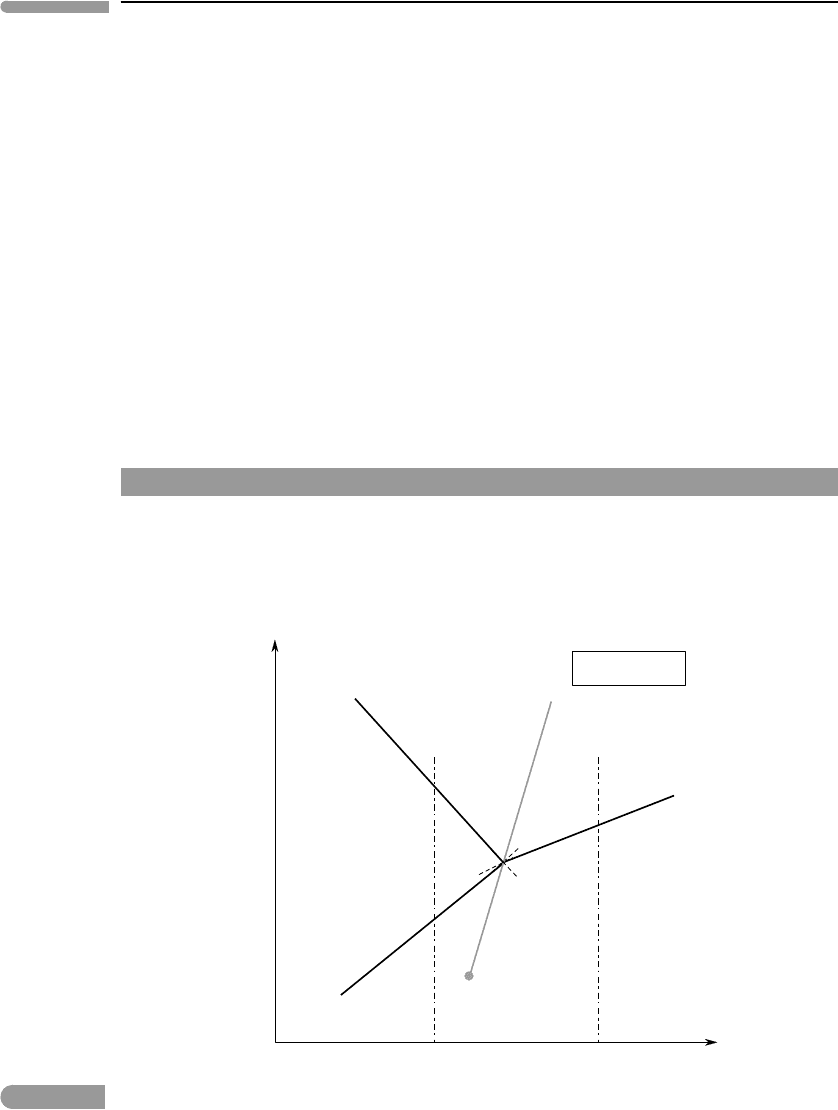
Fig. 6.13 Crystallization of sodium sulfate evaporites at constant pressure, as a function of temperature and µ
H
2
O
(e.g.
atmospheric humidity). The faded line shows the path of the psudo-invariant point with changing pressure (it is not a
phase boundary). The path of the pseudo-invariant point terminates at the dot, which corresponds to the
temperature of the invariant point for the binary system (Fig. 6.8). P–T along the path of the pseudo-invariant point
correspond to the (V) curve for the binary system.

317 6.3 Phase diagrams in open systems
In the former case the chemical potential of H
2
O is fixed by the assemblage that is stable
for each combination of pressure and temperature. In contrast, the chemical potential of
H
2
O in the open system depicted in Fig. 6.13 is an externally imposed variable, just as
pressure and temperature. In this case there is one externally controlled chemical potential
(v = 1), so that a univariant assemblage in the P–T diagram maps to a pseudo-invariant
assemblage if we fix one intensive variable. We choose to fix pressure, so that the three-
phase assemblage mirabilite +thenardite +liquid is stable at a pseudo-invariant point in the
isobaric µ
H
2
O
–T phase diagram. Three pseudo-univariant curves, along each of which two
phases coexist at equilibrium, radiate from the pseudo-invariant point. As usual, we label
each curve with the name of the absent phase. Vapor is no longer a phase in our analysis,
however. Rather, we are interested in how H
2
O is transferred between our system and its
environment (the atmosphere). We therefore substitute the name of this mobile species for
V along the pseudo-univariant curves (Fig. 6.13), which makes it clear that an increase
in µ
H
2
O
always causes H
2
O to move from the environment (atmosphere) to the system
(evaporite + brine), and vice versa. This is of course the same conclusion that we reached
in Section 5.3.2. A single phase is stable inside each of the pseudo-divariant fields. This
is the phase that is absent along the curve whose metastable extension enters that field.
Recall that by “pseudo-divariant field” we mean that there are 2 + v degrees of freedom:
the two coordinates in the phase diagram plus the variables that we chose to fix. In this case
v = 1, so that the three degrees of freedom required by the phase rule for a single phase
in a binary system are preserved. The same is of course true for the pseudo-univariant and
pseudo-invariant assemblages. The slopes of the phase boundaries are discussed in the next
section.
What about the vapor-absent reaction? The assemblage along this reaction is the same
one as in the pseudo-invariant point, so that it cannot exist along a curve on the isobaric
µ
H
2
O
– T phase diagram. The location of the pseudo-invariant point shifts with pressure,
along the (V) curve for the binary system in Fig. 6.8. It defines a path on the µ
H
2
O
– T
plane which is the locus of all points at which the pseudo-invariant assemblage mirabilite +
thenardite + liquid is stable. This is shown in Fig. 6.13 with a “ghost” line, to emphasize
that it is not a phase boundary but rather the path along which the pseudo-invariant point
slides with changing pressure. This path has a lower extremum, which corresponds to the
P –T conditions of the invariant point for the binary system (i.e. for the situation in which
the vapor phase is pure H
2
O, Fig. 6.8).
Note the similarities between the phase diagram in Fig. 6.13 and the phase diagram of
H
2
O (e.g. Fig. 6.12). We can think of the (Mi) curve as a “boiling” reaction, in the sense that
H
2
O in the liquid phase becomes vapor. Following this analogy the (Th) curve corresponds
to a freezing reaction, in which (most of) the H
2
O in the liquid phase “crystallizes” as
it is incorporated in the structure of mirabilite. The (L) curve behaves like a sublimation
reaction, in which H
2
O of crystallization of mirabilite becomes vapor.
Consider two evaporite basins in different locations, such that their characteristic tem-
peratures are below and above the temperature of the pseudo-invariant point, for instance
T
1
and T
2
in Fig. 6.13. Say that evaporation occurs at constant temperature and pressure, in
response to a decrease in atmospheric humidity (i.e. p
H
2
O
) and hence in µ
H
2
O
. Recall that,
at equilibrium, µ
H
2
O
is the same in the gas phase (atmosphere) as in the liquid phase (brine).
In the cooler climate the brine crystallizes mirabilite. If atmospheric humidity never drops
below the value at the (L) curve then the evaporite bed will consist of mirabilite only. On
the other hand, if dessication of the atmosphere continues then the (L) curve, where
