Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

278 Chemical equilibrium
0
X
b
b
-
0, b
RT ln
b
=
b, excess
G
excess
RT ln a
b
b, ideal
-
0, b
a
-
0, a
a, ideal
-
0, a
RT ln a
a
RT ln
a
=
a, excess
0
0
G
mixing
=
G
mixing, ideal
+G
excess
1
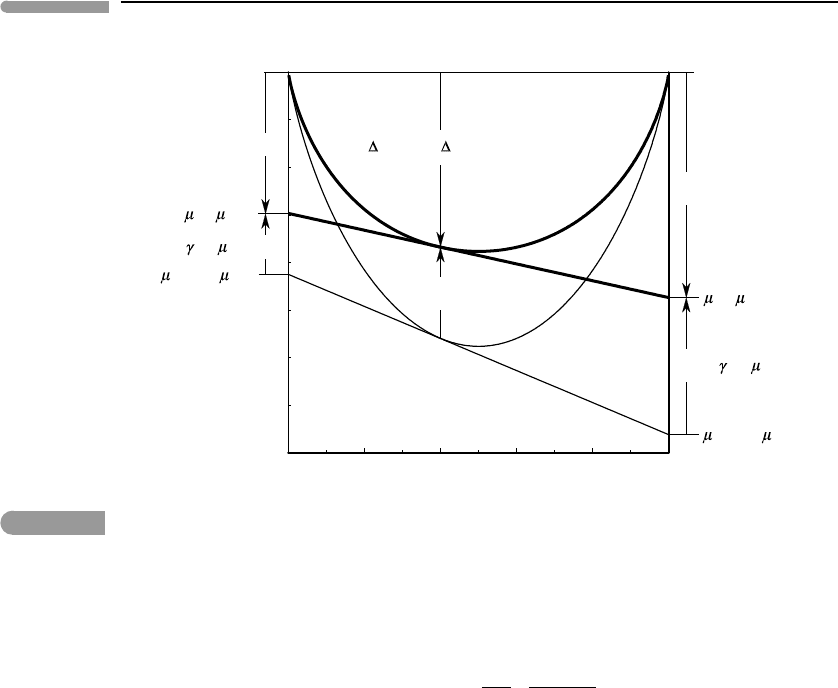
Fig. 5.14
Geometric interpretation of excess mixing properties. The thin curve and tangent line correspond to an ideal solution,
the thick ones to a (symmetric) non-ideal solution. Compare with Fig. 5.9.
We define a constant, K
1
H
, as follows:
K
1
H
=γ
1
X
1
→0
=exp
−
1
RT
∂G
excess
∂X
2
X
2
=1
, (5.135)
which, using (5.131), yields the following relation for an infinitesimally dilute component:
a
i
=K
i
H
X
i
, for X
i
→0. (5.136)
Equations (5.133) and (5.136) are known as Raoult’s and Henry’s laws, respectively, and
were found empirically by the eponymous scientists during the nineteenth century. Raoult’s
law is perhaps self-evident, but Henry’s law is not. It requires that ∂G
excess
/∂X
2
approach
a constant finite value as X
1
becomes infinitesimally small.
Raoult’s and Henry’s laws were originally derived for binary mixtures of liquid and gases,
but they are applicable to any type of solution. For complex multi-site solid solutions we
can re-state them as follows:
a
i
=a
i, ideal
, for X
i
→1 (5.137)
and:
a
i
=K
i
H
a
i, ideal
, for X
i
→0. (5.138)
Henry’s law is the thermodynamic basis of trace element geochemistry (Chapter 10) and is
also important in the thermodynamic treatment of electrolyte solutions (Chapter 11).

279 5.9 Non-ideal solutions
5.9.3 Polynomial expansion of excess mixing functions
The empirical approach to describing non-ideal solutions consists of finding a function of
T, P and composition that can reproduce observed values of G
excess
(i.e., the varying dis-
tance between the curves in Fig. 5.13) as closely as possible. This function must subsume
Raoult’s and Henry’s laws. Thus, G
excess
must vanish as the end-member compositions are
approached, and all partial derivatives ∂G
excess
/∂X
i
must be finite over the entire compo-
sitional range, and in particular at the end-members. In addition to these two properties,
the G
excess
function must be such that it is able to reproduce a range of possible non-ideal
behaviors, as depicted in Fig. 5.13. A polynomial in powers of mol fractions can, subject
to some constraints, be made to do all of these things. The most general way of construct-
ing such a polynomial is to begin with the following series, in which the w coefficients
are constant with respect to composition but are in general functions of temperature and
pressure:
G
excess
=
i
j
w
ij
X
i
X
j
+
i
j
k
w
ij k
X
i
X
j
X
k
+
i
j
k
l
w
ij kl
X
i
X
j
X
k
X
l
+···,
(5.139)
where i,j ,k,l, ... are solution components. Higher-order terms can be added as needed,
but it is often sufficient, or necessary owing to lack of data, to truncate the series after the
second term, in cubes of mol fractions.
A number of simplifications ensue. First, it is convenient to set coefficients with different
permutations of the same subindices equal to one another, i.e. w
ij
= w
ji
, w
ij k
= w
ikj
=
w
jik
=w
jki
=w
kij
=w
kj i
and so on. Second, in order for G
excess
to vanish for all pure end-
member components every coefficient in which all subindices are the same must vanish too,
i.e. w
ii
=w
iii
=w
iiii
=0, and so on if higher-order terms are included. Third, because of
the closure condition (
i
X
i
=1), there are dependencies among the non-zero coefficients,
which cuts down on the number of free parameters needed to fit G
excess
. This is easily seen
in a binary solution, but is true, although algebraically more complex, for solutions of any
number of components. Applying (5.139) to a binary solution, truncating the series at the
second term, collecting coefficients with different permutations of the same subindices and
omitting those coefficients that are zero we get:
G
excess
=2w
12
X
1
X
2
+3w
112
X
2
1
X
2
+3w
122
X
1
X
2
2
, (5.140)
which, noting that X
1
+X
2
=1, we write as:
G
excess
=X
1
X
2
[
3w
112
X
1
+3w
122
X
2
+2w
12
(
X
1
+X
2
)
]
(5.141)
or:
G
excess
=X
1
X
2
[
(
3w
112
+2w
12
)
X
1
+
(
3w
122
+2w
12
)
X
2
]
. (5.142)
In equation (5.142) there are only two independent coefficients, which we are free to rename
as follows: W
G
21
= 3w
112
+ 2w
12
and W
G
12
= 3w
122
+ 2w
12
. These parameters, written
with a capital W , are commonly called Margules parameters or interaction parameters.
Equation (5.142) simplifies to:
G
excess
=
W
G
21
X
1
+W
G
12
X
2
X
1
X
2
. (5.143)

280 Chemical equilibrium
The superscript G is added to the parameters to denote the fact that they measure excess
Gibbs free energy. It is important to realize that Margules parameters are functions of
temperature and pressure but not of composition.
We can verify that (5.143) is an acceptable algebraic representation of the excess Gibbs
free energy of a binary solution. First, G
excess
vanishes if the mol fraction of any of the two
end members becomes zero (Raoult’s law). Second, using infinitely dilute component 1 as
an example, we have:
∂G
excess
∂X
2
X
2
=1
=−W
G
12
, (5.144)
i.e. ∂G
excess
/∂X
i
at the limiting end-member composition has a finite non-zero value and
is related to the Henry law constant by:
K
1
H
=γ
1
X
1
→0
=exp
−
1
RT
∂G
excess
∂X
2
X
2
=1
=exp
W
G
12
RT
. (5.145)
There are no a priori conditions on the signs and relative magnitudes of the Margules param-
eters. An inflected G
mixing
curve (Fig. 5.13c) results if the two interaction parameters are
positive and large, whereas a G
excess
function that changes sign (Fig. 5.13d) arises if the two
parameters have different signs. An important simplification ensues if the two interaction
parameters are equal, in which case we have W
G
21
=W
G
12
and:
G
excess
=W
G
12
X
1
X
2
. (5.146)
Equation (5.146) corresponds to the symmetric behavior depicted in Figs. 5.13a and 5.13c,
whereas the general asymmetric case in Figs. 5.13b and 5.13d is reproduced by (5.143) with
W
G
21
= W
G
12
. A commonly used terminology refers to the symmetric solution described by
(5.146)asasimple mixture, and to the asymmetric solution given by (5.143)asasubregular
solution. To make things more confusing, a symmetric solution (=simple mixture) for which
S
excess
= V
excess
= 0 (i.e. for which G
excess
is independent of P and T) is called a regular
solution. I find this terminology, which is used primarily for historical reasons, unnecessarily
confusing, and will eschew it in favor of the more descriptive terms symmetric solution (e.g.
Fig. 5.13a and c) and asymmetric solution (e.g. Fig. 5.12b and d), with or without T and/or
P dependencies.
Because G
excess
is a linear function of interaction parameters, identities (5.123)to(5.125)
carry over to equivalent identities among interaction parameters, so that we have:
W
G
ij
=W
H
ij
−TW
S
ij
(5.147)
∂W
G
ij
∂T
P
=−W
S
ij
(5.148)
∂W
G
ij
∂P
T
=W
V
ij
. (5.149)
Note that the subscript n
i
is no longer needed in the partial derivatives, because the interac-
tion parameters as defined by (5.139) are not functions of composition. Excess enthalpies,
entropies and volumes are calculated with (5.143)or(5.146), using W
H
, W
S
,orW
V
,as
needed.

281 5.9 Non-ideal solutions
Excess chemical potentials and activity coefficients are obtained by substituting the
polynomial expansion for G
excess
(i.e. equations (5.143)or(5.146), depending on whether
the solution is asymmetric or not) in (5.126) and (5.129). For example, for an asymmetric
binary solution we have:
µ
1,excess
=
W
G
12
+2X
1
W
G
21
−W
G
12
X
2
2
. (5.150)
which for a symmetric solution simplifies to:
µ
1,excess
=W
G
12
X
2
2
. (5.151)
The attentive reader must have noticed that, beginning with equation (5.140), the dis-
cussion has focused exclusively on binary solutions. This is because the corresponding
polynomial expansions for solutions of three or more components quickly become much
more cumbersome, and are best dealt with by means of a symbolic algebra package such
as Maple (see end-of-chapter exercises).
5.9.4 Perils and tribulations of excess mixing functions
Whenever one uses non-ideal mixing models the fact must be kept in mind that the values
of excess thermodynamic properties are not independent of the values of standard state
properties. In order to understand what this means, and the perils that ensue, it is necessary
to sketch out how excess mixing properties are measured. One way of doing this is by
means of phase equilibrium experiments. The method is based on equilibrating a phase
of variable composition in an assemblage in which all the other phases are end-member
species that do not change composition. An example is the determination of Al–Mg excess
mixing properties in orthopyroxene using reaction (5.102). The experimental data that one
seeks are orthopyroxene compositions coexisting at equilibrium with end-member spinel
and forsterite. The equilibrium condition for this reaction is equation (5.103), which con-
tains three free parameters: P , T and X
Mg,M1
in orthopyroxene (e.g. equation (5.104)). If
we wish to determine the excess mixing properties of Al–Mg in orthopyroxene then we
can perform a series of phase equilibrium experiments at controlled pressures and temper-
atures, and measure the composition of orthopyroxene that crystallizes in each experiment.
Equation (5.103) assures us that at each P and T there is a unique equilibrium orthopyrox-
ene composition, since neither spinel nor olivine will depart from their Mg end-member
compositions. We will not go into the details of how the experiments are performed, what
are the uncertainties in experimental temperatures and pressures, how we can ascertain
whether equilibrium was attained in the experiments, or what are the likely uncertainties in
orthopyroxene compositions arising from analytical techniques (see for example Holloway
& Wood, 1988; Berman, 1988; Holland & Powell, 1998; Anderson, 2005). Rather, we will
put ourselves in the somewhat optimistic position that none of these is a concern, and see
that there is a more fundamental issue in play.
Allowing for the possibility that Al–Mg mixing in orthopyroxene may not be ideal, we
rewrite equation (5.103) as follows (see (5.128)):
r
G
0,(ii)
P ,T
+RT ln
X
Al,M1
X
Mg, M1
+µ
MgTs,excess
opx
−µ
En, excess
opx
=0. (5.152)
If we know the pressure and temperature of the experiment then we can calculate the standard
state Gibbs free energy of reaction (the first term in the equation), and we get the second term
(the ratio of ideal activities) from the measured orthopyroxene composition. One experiment

282 Chemical equilibrium
thus gives us the difference between the excess chemical potentials of Mg–Tschermakite and
enstatite. We will see in a second that with multiple experiments we can find the absolute
value of each of the excess chemical potentials, but before getting into that you must
understand that equation (5.152) is the crux of the issue: the values of the excess chemical
potentials are anchored to the values of the standard state thermodynamic properties. The
problem is that standard state properties for many species of interest in the planetary sciences
are not known with high accuracy, and there may be significant differences among values
given in different data bases. Excess mixing properties measured by phase equilibria are
relative values. They are determined relative to standard state properties from a specific
data base, and they can ONLY be used together with standard state data from that same data
base. Of course, equation (5.152) shows that the values of excess mixing properties are also
anchored to our choice of ideal activity model, but accounting for this is less of a problem,
as it requires only that one be consistent when calculating ideal activities. In contrast,
combining excess properties with standard state properties different from those used in the
derivation of the excess properties renders the results questionable at best. Excess mixing
properties can also be derived from calorimetric measurements (see Navrotsky, 1986), and
although these can be absolute values, the same caveat applies: they should not be used in
conjunction with standard state properties derived by some other method.
Extracting Margules parameters from (5.152) is straightforward but requires that we per-
form multiple experiments over a range of pressures and temperatures, so as to obtain a range
of orthopyroxene compositions. Assuming that Mg–Al non-ideal mixing is asymmetric, we
use (5.150) to re-write (5.152) as follows:
r
G
0,(ii)
P ,T
+RT ln
X
Al,M1
X
Mg,M1
+W
G
AlMg
X
2
Mg,M1
−2X
Al,M1
X
2
Mg,M1
−2X
Mg,M1
X
2
Al,M1
−W
G
MgAl
X
2
Al,M1
−2X
Al,M1
X
2
Mg,M1
−2X
Mg,M1
X
2
Al,M1
=0.
(5.153)
This is one equation in two unknowns (the two Margules parameters). In principle, and
assuming that there is no temperature nor pressure dependency of the W
G
parameters
(i.e. W
S
= W
V
= 0, see equations (5.148) and (5.149)), with two experiments at different
conditions, in which the values of
r
G
0
, X
Mg
and X
Al
are different, we could solve for the
two parameters. In practice many experiments are required so as to have an overdetermined
system of equations that allows us to analyze experimental errors, and detect possible P and
T dependencies of the Margules parameters (i.e. discriminate W
G
into W
H
, W
S
and W
V
,
see equation (5.147)). Assuming a symmetric solution simplifies 5.153 considerably, to:
r
G
0,(ii)
P ,T
+RT ln
X
Al,M1
X
Mg,M1
+W
G
X
2
Mg,M1
−X
2
Al,M1
=0. (5.154)
One commonly uses experimental data to test for symmetry vs. asymmetry, for example
by determining whether the difference between the two parameters fitted to an asymmetric
model is statistically significant.
The following example is designed to show that, no matter what model one chooses to
use to represent the behavior of a real solution, unless consistency between the various
sources of thermodynamic data is observed, the results can be spectacularly incorrect.
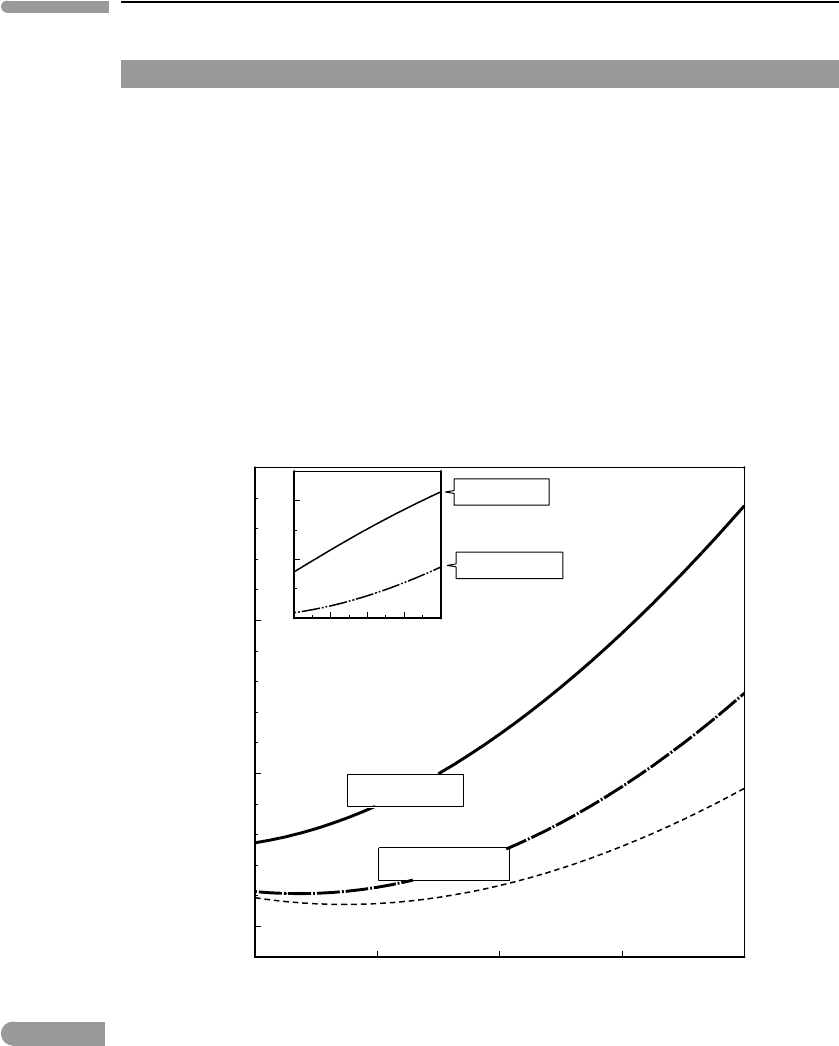
283 5.9 Non-ideal solutions
Worked Example 5.7 The spinel–garnet transition in planetary mantles, part (iv)
The spinel–garnet transition can be used as an example of the perils of combining mixing
and standard state properties that are not consistent with one another. The phase boundary
andAl contents in orthopyroxene in Fig. 5.10 were calculated using standard state properties
from Holland and Powell (1998) and ideal Al–Mg mixing in orthopyroxene. Relative to
their standard state properties, Holland and Powell find that W
G
AlMg
= 0. In other words,
Al and Mg appear to mix ideally in orthopyroxene. In truth what probably happens is that
the available experimental data are too sparse and do not make it possible to accurately
discriminate between standard state and excess mixing properties for this particular binary
join. The safest course of action in such case is to set the excess chemical potential equal to
zero, and let the standard state properties of Mg–Tschermakite “absorb” any excess mixing
properties – this is, I believe, what Holland and Powell have done. Whether or not Al–Mg
mixing in orthopyroxene is ideal, and it almost certainly is not, if we are going to use Holland
and Powell’s standard state properties to calculate the phase boundary then we must use
ideal mixing. The curves in Fig. 5.10 are therefore the correct ones.
800 1000 1200 1400 160
0
15
20
25
30
Temperature (°C)
Pressure (kbar)
forsterite
+ pyrope
spinel
+ 2
aluminous
enstatite
forsterite
+
pyrope
spinel
+
2
enstatite
800 1000 1200 1400 1600
0
0.1
0.2
T(
o
C)
X
Al
X
Al
in enstatite
forsterite
+
pyrope
spinel
+
2
aluminous
enstatite
CONSISTENT
INCONSISTENT
CONSISTENT
INCONSISTENT
Fig. 5.15 The effect of calculating a phase boundary using excess mixing properties that are inconsistent with the standard
state properties. Curves labeled “CONSISTENT” were calculated using standard state properties from Holland and
Powell (1998), that require that Al–Mg mixing in orthopyroxene be considered ideal. Curves labeled “INCONSISTENT”
were calculated with the same standard state properties, and Al–Mg excess mixing properties from Klemme and
O’Neill (2000b). This does not mean that Klemme and O’Neill’s mixing properties are incorrect, only that they are
anchored to a different set of standard state properties. The purpose of the diagram is to show that using standard
state and mixing properties that are inconsistent with one another can engender very significant errors.
284 Chemical equilibrium
Perusing the literature, however, one finds other studies that report significant non-ideality
for Al–Mg mixing in orthopyroxene. One such paper is the one by Klemme and O’Neill
(2000b), who find a symmetric non-ideal behavior with W
G
=20 kJ mol
−1
, but relative to
a different set of standard state values (which unfortunately they do not make explicit). Let
us ignore this “but” and recalculate the phase diagram using Holland and Powell’s standard
state data and Klemme and O’Neill’s non-ideal mixing model. The calculation is very
easily implemented in Maple (Software Box 5.5) and the results are shown in Fig. 5.15.
The calculated phase boundary shifts by ∼5 kbar, and the calculated Al mol fraction in
orthopyroxene drops by about 0.1 to 0.15. These are non-negligible displacements, and the
new curves (labeled “INCONSISTENT” in the figure) are incorrect. Note that this does
not mean that Klemme and O’Neill’s non-ideal mixing model is incorrect. Their model is
a different way of allocating experimental measurements among standard state and excess
properties, as equation (5.152) should make clear. This exercise is designed to demonstrate
the fallacy of combining excess properties and standard state properties that are not mutually
consistent. I emphasize: I made this error on purpose, with purely didactic goals.
Software Box 5.5 Incorporation of non-ideal Al–Mg mixing in orthopyroxene to the calculation
of spinel–garnet equilibrium
The Maple worksheet sp_grt_MAS.mw contains a procedure named
spgrAlMASni that adds non-ideal mixing terms to equations (5.101) and (5.104).
Assuming symmetric non-ideal Al–Mg interactions in orthopyroxene, we find from
equation (5.151):
µ
En, excess
opx
=W
G
AlMg
X
2
Al
(5.155)
and:
µ
MgTs,excess
opx
=W
G
AlMg
X
2
Mg
. (5.156)
Incorporating these excess chemical potentials in (5.101) and (5.104), and recalling that
X
Al,M1
+X
Mg,M1
=1, we get:
r
G
0,(i)
P ,T
−2RT ln X
Mg,M1
−2W
G
AlMg
1 −X
Mg,M1
2
=0 (5.157)
and:
r
G
0,(ii)
P ,T
+RT ln
1 −X
Mg,M1
X
Mg,M1
+W
G
AlMg
X
2
Mg,M1
−
1 −X
Mg,M1
2
=0.
(5.158)
As in the ideal solution case (Software Box 5.4) we have two equations that, if we
fix temperature, we can solve for the two unknowns, P and X
Mg,M1
in orthopyroxene.
Procedure spgrAlMASni is thus otherwise identical to spgrAlMAS.
In the example discussed in Worked Example 5.7 I have purposely used values of
standard state and excess mixing properties that are inconsistent with one another. Of
course, the Maple procedure can be used with any combination of thermodynamic prop-
erties, by modifying the standard state values stored in the spreadsheet RefStateData
and/or the value of the excess mixing parameter stored in the variable WAlMg.

285 Exercises for Chapter 5
Exercises for Chapter 5
5.1 Calculate the Clapeyron slope for the reaction spinel + 2 enstatite
→
←
forsterite +
pyrope at 298 K and 1 bar, and compare your result to the slope of the reaction in
Figure 5.5.
5.2 Using the reaction spinel + 2 enstatite
→
←
forsterite + pyrope, verify that the assem-
blage with higher entropy is the one that is on the high temperature side of the phase
boundary, and the assemblage with higher density is on the high pressure side of the
phase boundary.
5.3 Calculate the phase boundary for the transition between plagioclase lherzolite and
spinel lherzolite for the end-member Ca–Mg system, assuming that both diopside
and enstatite remain as pure end-member phases, and ignoring the order–disorder
transition in anorthite (more on this in Chapter 7). You need to write a balanced
reaction among anorthite, forsterite, diopside, enstatite and spinel, and program this
reaction in the Maple worksheet th_template_3.mw (see Software Box 5.2).
Use standard state properties from Holland and Powell (1998). You will refine this
calculation in Exercises 5.15 through 5.17.
5.4 Calculate the activity of diamond relative to the standard state graphite, and the activity
of graphite relative to the standard state diamond, at 298 K and 1 bar. What is the
physical meaning of a>1? Of a<1?
5.5 Show that the conclusion that chemical species are transferred down chemical poten-
tial gradients (i.e. equation (5.26)) is valid in systems with any arbitrary number of
phases and components.
5.6 Prove equations (5.95), starting from (5.70).
5.7 Prove equations (5.97), starting from (5.70).
5.8 Prove equations (5.100), starting from (5.70).
5.9 Write equations for the ideal activities of eastonite (KMg
2
AlAl
2
Si
2
O
10
(OH)
2
) and
muscovite (KAl
2
AlSi
3
O
10
(OH)
2
) in a trioctahedral mica.
5.10 Prove equation (5.91), i.e.:
G
mixing
=H
mixing
−TS
mixing
.
5.11 Prove that V
mixing
for an ideal solution is zero.
5.12 Use Maple’s plotting capabilities to explore the conditions under which a non-ideal
symmetric solution develops an inflected G
mixing
curve, as in Fig. 5.13c. (Hint: vary
the relative values of temperature and the interaction parameter.)
5.13 Use Maple’s plotting capabilities to show that a G
excess
function for an asymmetric
solution that changes sign (as in Fig.5.13d) arises only if the two interaction parameters
have different signs.
5.14 Prove equation (5.150).
5.15 A first step in refining the anorthite–spinel phase boundary in lherzolites is to include
the Mg–Tschermak’s component in orthopyroxene (using reaction (5.102)) and the
Ca–Tschermak’s component in clinopyroxene: CaAlAlSiO
6
. Assume that Ca fills the
M2 site incpx, and thatAl and Mg mix in the M1 site of cpx, as in opx.You need to come
up with an additional balanced chemical reaction that includes the Ca–Tschermak’s
species in cpx. There are several possibilities, but the simplest one is a reaction among
the two Tschermak’s components, diopside and enstatite. You will end up with three
linearly independent equations ((5.102), the one that you derived in Exercise 5.3, and

286 Chemical equilibrium
your new reaction) in four unknowns: P, T,(X
Al,M1
)
opx
and (X
Al,M1
)
cpx
. The system
has one degree of freedom, and can be solved by fixing one variable, for example
temperature. Use the discussion in Software Box 5.4, and the worksheet described
there, to help you program the solution of this system of equations in Maple, assuming
ideal mixing in both pyroxenes. Plot the new plagioclase–spinel phase boundary and
compare it with the one you generated in Exercise 5.3. Use standard state properties
from Holland and Powell (1998).
5.16 In order to further refine the plagioclase–spinel phase boundary you should include
excess mixing properties in pyroxenes. According to Holland and Powell, Al–Mg
mixing in the M1 site of both pyroxenes is symmetric, with (W
AlMg
)
opx
= 0 and
(W
AlMg
)
cpx
=7 kJ mol
−1
. With the discussion in Software Box 5.5 as a guide, include
the corresponding excess chemical potentials, recalculate the plagioclase–spinel phase
boundary and compare it with the ones you generated in Exercises 5.3 and 5.15.
5.17 In reality, Ca and Mg also mix in the M2 site of both clinopyroxene and orthopyroxene.
You can then add the following two reactions to your system of equations:
(CaMgSi
2
O
6
)
cpx
→
←
(CaMgSi
2
O
6
)
opx
(Mg
2
Si
2
O
6
)
cpx
→
←
(Mg
2
Si
2
O
6
)
opx
and end up with a system of five equations and six unknowns: P , T ,(X
Al,M1
)
opx
,
(X
Al,M1
)
cpx
,(X
Mg,M2
)
opx
, and (X
Mg,M2
)
cpx
. The system still has one degree of free-
dom, and can thus be solved by fixing one variable, e.g., temperature. According to
Holland and Powell, Ca–Mg mixing in the M2 site of pyroxenes is symmetric, with
(W
CaMg
)
opx
=(W
CaMg
)
cpx
= 30 kJ mol
−1
. Recalculate the plagioclase–spinel phase
boundary and compare it with the ones you generated in Exercises 5.3, 5.15 and 5.16.
6
Phase equilibrium and phase diagrams
A phase diagram is a graph that shows the distribution of stable phase assemblages as a
function of the values of the intensive variables used to describe the thermodynamic system.
If pressure and temperature are the variables of interest then the stable assemblage is the
one with the lowest Gibbs free energy, although other thermodynamic potentials may be
used if the variables of interest are different, for example, Helmholtz free energy if one
is interested in temperature and volume (more on this in Chapter 9). Phase diagrams are
powerful analytical tools in many branches of planetary sciences, as they provide a way
to quickly visualize how phase assemblages change in response to changes in pressure,
temperature, chemical potentials, or any other combination of intensive variables. There
are many different types of phase diagrams and it is not the purpose of this book to offer a
comprehensive review of all of them. Rather, in this chapter I will focus on the fundamental
rules that phase diagramsmust abide by inorder to be thermodynamicallyvalid.We therefore
begin with a discussion of the thermodynamic underpinnings of phase diagrams.
6.1 The foundations of phase equilibrium
6.1.1 The Gibbs–Duhem equation
Any extensive thermodynamic property can be written as a sum of products of partial molar
properties times mol numbers (equation (5.27)). Let us re-write equation (5.27) specifying
the identity of the phase that it applies to with the index α (α can be a solution or a pure
phase, in which case identity (5.27) is trivial):
Z
α
=
i
z
i
α
n
α
i
. (6.1)
Taking derivatives:
dZ
α
=
i
z
i
α
dn
α
i
+
i
n
α
i
dz
i
α
. (6.2)
Now, since Z is a state variable it is a function of temperature and pressure, i.e. the following
function exists:
Z
α
=Z
α
P , T , n
α
i
. (6.3)
287
