Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


268 Chemical equilibrium
taking R=Mg gives us the Mg-Tschermak’s end-member component: MgAlAlSiO
6
. The
two Al cations are kept separate in the formula to emphasize that they occupy different
crystallographic sites. In order to calculate the ideal activity of Mg-Tschermak’s we begin
by noting that the two octahedral sites in pyroxenes are distinct. One of them, labeled M1, is
smaller than the other one, M2. In a “perfect” orthopyroxene M2 sites are occupied by Mg
and Fe only. If such orthopyroxene dissolves Al then half of the Al cations enter the M1 site,
where they substitute for Mg or Fe cations, and the other half enter a neighboring tetrahedral
(T) site, where they substitute for Si. Because we assume that this coupled substitution
preserves local electrical neutrality the couples AlAl and MgSi behave as mixing units. In
a binary Mg–Al orthopyroxene (i.e., no Fe, Ca, etc.) the ideal activities of enstatite and
Mg-Tschermak’s in orthopyroxene are then given by equation (5.98):
a
MgTs,ideal
opx
=X
Al,M1
=X
Al,T
=
1
2
n
Al
a
En, ideal
opx
=X
Mg,M1
=1 −X
Al,M1
=1 −
1
2
n
Al
(5.99)
where n
Al
is the total number of Al cations per orthopyroxene formula unit: A
2
B
2
O
6
.
In the case of an Fe–Mg–Al orthopyroxene one possibility is that the coupled M1–T
substitution is independent of Fe–Mg substitution, and that Fe and Mg do not order
between M1 and M2 sites. The latter condition implies that (X
Mg,M1
)/(X
Fe,M1
) =
(X
Mg,M2
)/(X
Fe,M2
) = n
Mg
/n
Fe
, i.e. the ratio of Mg/Fe occupancy of M1 and M2 sites
equals the ratio between the number of Mg and Fe cations per orthopyroxene formula unit.
The following activity–composition expressions then follow from (5.96) and (5.98):
a
MgTs,ideal
opx
=X
Al,M1
·X
Mg,M2
a
En, ideal
opx
=X
Mg,M1
·X
Mg,M2
a
Fs,ideal
opx
=X
Fe,M1
·X
Fe,M2
.
(5.100)
As an exercise, you should derive equations (5.100) formally starting from (5.70).
Worked Example 5.6 The spinel–garnet transition in planetary mantles, part (iii)
In Worked Example 5.1 we calculated a hypothetical location of the spinel–garnet tran-
sition by calculating the phase boundary for the Mg end-member reaction (5.15). This
phase boundary cannot be correct, as examination of natural peridotite samples shows that
orthopyroxene always dissolves some amount of Al. By (5.99), the activity of enstatite in
orthopyroxene must be less than 1, so that the equilibrium constant for reaction (5.15), given
by (5.56), does not vanish. Calling reaction (5.15) equilibrium (i), and using (5.99) for the
activity of enstatite, we re-write (5.57) as follows:
r
G
0,(i)
P ,T
−2RT ln X
Mg,M1
=0 (5.101)
since the activities of the other species remain unity. We can now write another reac-
tion, involving the Mg-Tschermak’s component in orthopyroxene, which we shall call
equilibrium (ii):
MgAl
2
O
4
+Mg
2
Si
2
O
6
MgAlAlSiO
6
+Mg
2
SiO
4
(5.102)
269 5.8 More complex ideal activity–composition relationships
the equilibrium condition for which is (see (5.99)):
r
G
0,(ii)
P ,T
+RT ln
X
Al,M1
X
Mg,M1
=0. (5.103)
Equations (5.101) and (5.103), together with the crystallographic constraint for a binary
Al–Mg orthopyroxene, X
Mg,M1
+X
Al,M1
= 1, constitute a system of three equations with
one degree of freedom, as there are four unknowns: P , T , X
Mg,M1
, X
Al,M1
. We can specify
one of the variables, which in this case will be T, and solve the system of equations for the
other three variables, so as to obtain the location of the spinel–garnet phase boundary and
the composition of orthopyroxene as a function of temperature and pressure.
We begin by eliminating one of the mol fractions, say X
Al,M1
, and re-write (5.103)as
follows:
r
G
0,(ii)
P ,T
+RT ln
1 −X
Mg,M1
X
Mg,M1
=0. (5.104)
We now have a system of two equations, (5.101) and (5.104), in three unknowns
(P , T ,X
Mg,M1
), which we solve by specifying temperature. The Maple implementation is
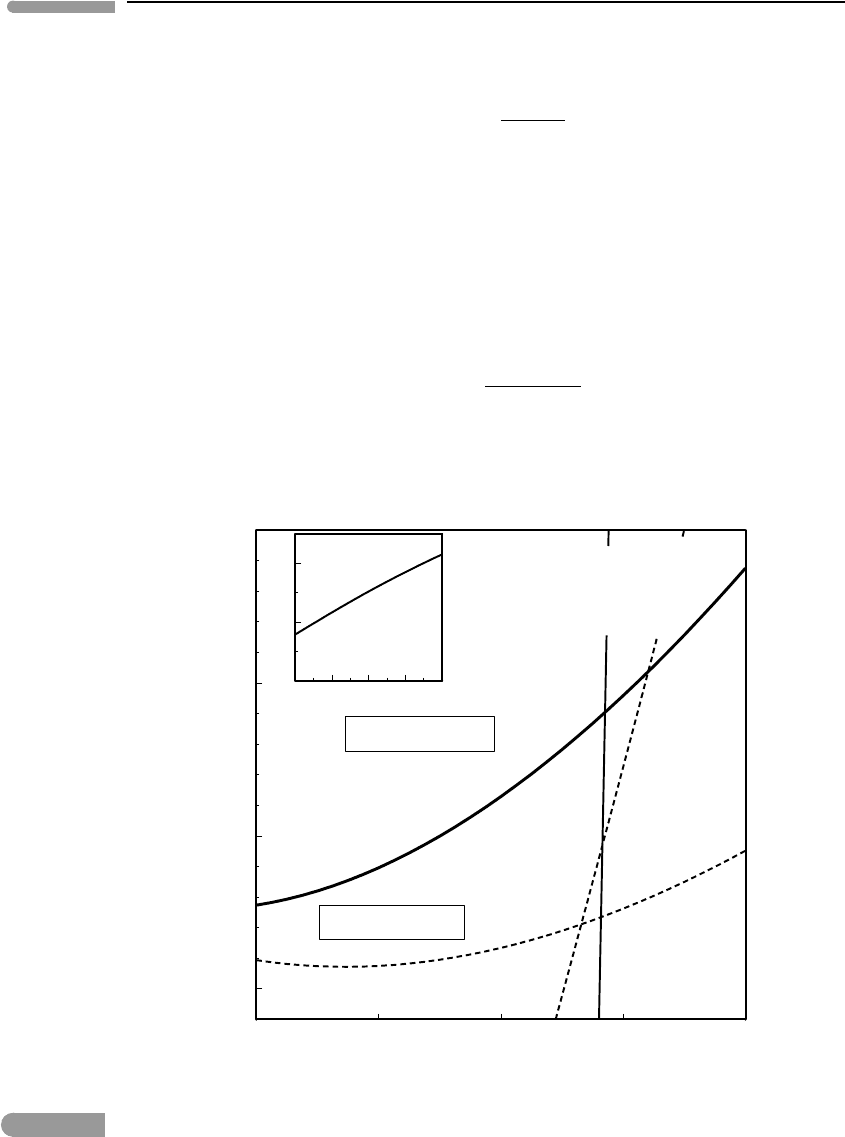
described in Software Box 5.4, and the results are shown in Fig. 5.10.
800 1000 1200 1400 160
0
15
20
25
30
Temperature (°C)
Pressure (kbar)
forsterite
+
pyrope
spinel + 2 aluminous enstatite
Garnet lherzolite
Spinel lherzolite
MOR adiabat
M
antle solidus
forsterite + pyrope
spinel + 2 enstatite
800 1000 1200 1400 1600
0
0.1
0.2
T(
o
C)
X
Al
X
Al
in enstatite
Fig. 5.10 Phase boundary between spinel lherzolite and garnet lherzolite calculated taking into account the Mg-Tschermak’s
component in orthopyroxene. The incorrect phase boundary from Fig. 5.5 (dashed line) is shown for comparison.
Predicted initial melting under mid ocean ridges shifts from the garnet to the spinel stability field. Inset shows
calculated mol fraction of Al in orthopyroxene as a function of temperature (pressure is not constant in this graph).
270 Chemical equilibrium
Software Box 5.4 Calculation of spinel–garnet equilibrium, assuming ideal Al–Mg mixing in
orthopyroxene
The Maple worksheet sp_grt_MAS.mw contains a procedure named spgrAlMAS
that solves the two simultaneous equations, (5.101) and (5.104), for the two unknowns,
P and X
Mg,M1
in orthopyroxene. The two equations are labeled R1 and R2 in the Maple
procedure, which calls dGPT (see Software Box 5.2) to calculate the values of the stan-
dard state Gibbs free energy change of equilibria (5.15) and (5.102). Maple’s numerical
solver, fsolve, is quite powerful and is able to find solutions to systems of non-linear
equations with relative ease, as this example shows and as we shall have plenty of
opportunity to test in later chapters.
Because spinel–garnet equilibrium is relatively “flat” in P –T space the procedure
solves for pressure and orthopyroxene composition at a given temperature, for tempera-
tures over a range that is specified in the procedure call. The name of the file for output
is also specified in the procedure call. Output is: T −P −X
Al,M1
. Thermodynamic data
are stored in tab-delimited format in a file named spgrt. The procedure can be modi-
fied to calculate other equilibria, by changing and/or adding tables defining the required
reactions (e.g. reaction1, reaction2, etc.).
The calculated phase boundary is located at pressures 2–10 kbar higher than those
obtained assuming that there is no Al in opx (Fig. 5.5). Solubility of Al in orthopyrox-
ene increases with temperature (inset in Fig. 5.10), which agrees with the fact that the
right-hand side of reaction (5.102) has the higher entropy (exercise left for the reader). The
pressure shift arises because dissolution of Al causes the activity of enstatite to decrease
(equation (5.99)), so that its chemical potential decreases as well (equation (5.45)). Looking
Constant temperature
spinel + 2 enstatite
spinel + 2 aluminous enstatit
e
forsterite + pyrope
Pressure
Gibbs free energy
spinel lherzolite
garnet lherzolite
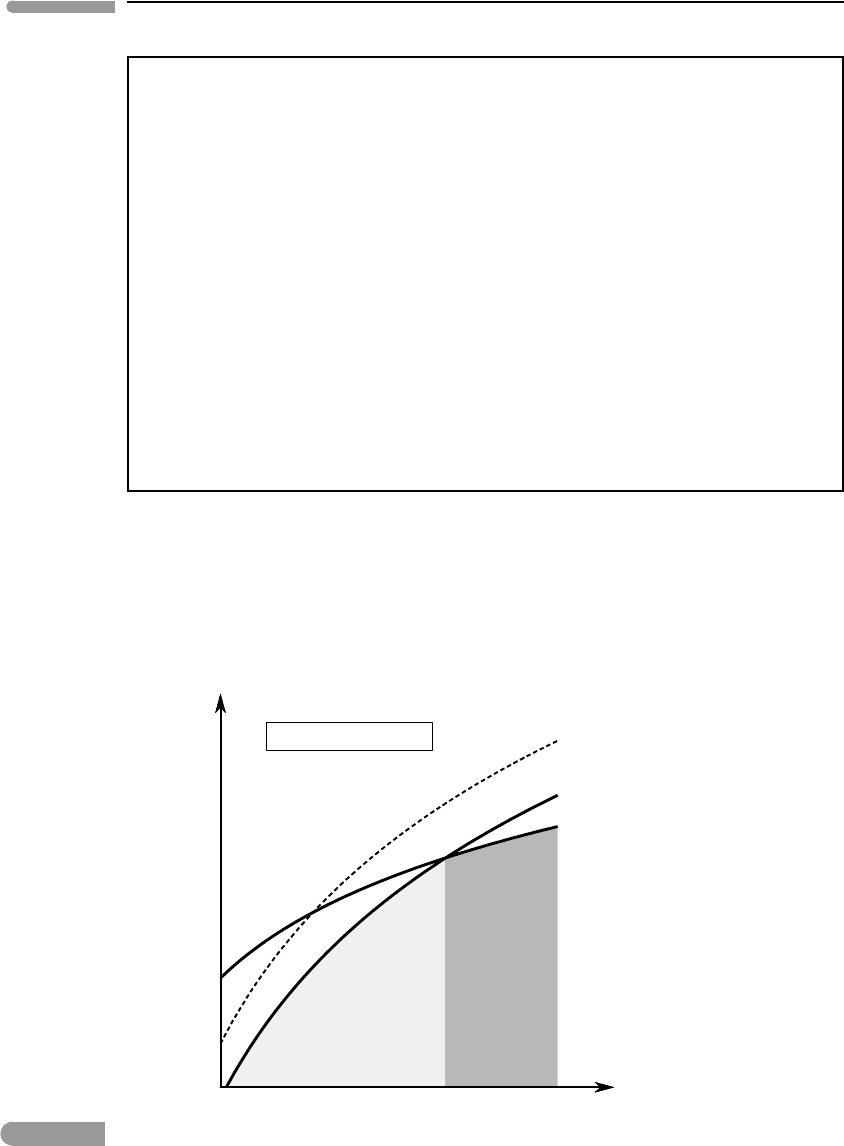
Fig. 5.11 Isothermal section across the Gibbs free energy surfaces for the assemblages in Fig. 5.10, showing that dissolution of
Al in orthopyroxene lowers the Gibbs free energy of the assemblage orthopyroxene +spinel, and must therefore shift
the equilibrium to higher pressure.
271 5.8 More complex ideal activity–composition relationships
at a schematic G–P diagram (Fig. 5.11), we see that lowering the Gibbs free energy of the
low-pressure assemblage in a reaction forces the equilibrium position to higher pressure.
This is a necessary consequence of the geometry of Gibbs free energy surfaces (Fig. 4.13).
The phase boundary in Fig. 5.10 is a better approximation than that in Fig. 5.5 to the con-
ditions under which the spinel–garnet transition takes place in planetary mantles, because
it accounts for the non-negligible solubility of Al in orthopyroxene. In contrast to what
we would have predicted if we had ignored the Al content of orthopyroxene, the more
complete phase diagram predicts that garnet becomes stable under mid-ocean ridges at
pressures of ∼24 kbar, and that MORB magmas form in the stability field of spinel lher-
zolites (Chapter 10). Our phase boundary in Fig. 5.10 is, however, still deficient on two
accounts. First, there are at least three other elements – Ca, Cr and Fe – that can be expected
to have non-trivial effects on the location of the spinel–garnet equilibrium. Second, our cal-
culations have been based on the assumption that crystalline solid solutions behave ideally,
but we have not examined the validity of this assumption.
5.8.2 Crystalline solutions with non-vanishing configurational entropy
in the end-member species
Because they can exist in several different states of cation ordering, plagioclase feldspars
are an excellent example of the effect of variable degrees of configurational entropy on
ideal activity. They also serve to illustrate further the use of equation (5.70). Using the
nomenclature from Worked Example 4.3, we recall that we can distinguish two T1 sites
and two T2 sites among the four T (tetrahedral) sites in one formula unit of feldspar. We
will consider mixing along the albite–anorthite join using three different assumptions about
cation ordering in the tetrahedral sites, as follows.
Model (i) assumes perfect local charge balance, such that Al–Si substitution only
occurs on T sites adjoining an octahedral site in which Ca–Na substitution occurs
simultaneously.
Model (ii) assumes that Al and Si mix randomly over the two T1 sites, so that the
two T2 sites are occupied by Si only. Mixing in tetrahedral and octahedral sites is
independent, so that there is no local charge balance.
Model (iii) assumes that Al and Si mix randomly over the four T sites, so that, again,
octahedral and tetrahedral mixing are independent and local charge balance is not
preserved.
The number of atoms of Na, Ca and Al, n
Na
, n
Ca
and n
Al
, per formula unit of plagioclase
and the mol fractions of Na and Ca, are related by the following equations:
n
Na
+n
Ca
=1
n
Al
=1 +X
Ca
=2 −X
Na
.
(5.105)
The local charge balance model, case (i), corresponds to the simple example of coupled
substitution discussed in Section (5.8.1). For this model we therefore have:
a
Ab,ideal(i)
plg
=X
Na
a
An, ideal(i)
plg
=X
Ca
.
(5.106)
In order to calculate the activities for model (ii) we must go back to equation (5.70) and
write out the number of microstates explicitly. Because octahedral and tetrahedral mixing

272 Chemical equilibrium
are now independent of one another the total number of microstates equals the product of
the numbers of octahedral and tetrahedral microstates:
O
m
Ab
=O
m
An
=
(n
Na
+n
Ca
)!
n
Na
!n
Ca
!
·
(n
Al
+n
Si,T1
)!
n
Al
!n
Si,T1
!
. (5.107)
The number of Si atoms in the T1 sites is given by n
Si,T1
=2 −n
Al
. Adding one molecule
of albite we find:
O
m
Ab+1
=
(n
Na
+n
Ca
+1)!
(n
Na
+1)!n
Ca
!
·
(n
Al
+n
Si,T1
+2)!
(n
Al
+1)!(n
Si,T1
+1)!
(5.108)
and similarly for one molecule of anorthite:
O
m
An+1
=
(n
Na
+n
Ca
+1)!
n
Na
!(n
Ca
+1)!
·
(n
Al
+n
Si,T1
+2)!
(n
Al
+2)!n
Si,T1
!
. (5.109)
Substituting these equations in (5.70) and simplifying:
a
Ab,ideal(ii)
plg
=C
Ab(ii)
X
Na
X
Al,T1
X
Si,T1
(5.110)
and:
a
An, ideal(ii)
plg
=C
An(ii)
X
Ca
X
Al,T1
2
. (5.111)
The mol fractions of Al and Si in the T1 sites are given by:
X
Al,T1
=
n
Al
2
=
2 −X
Na
2
=
1 +X
Ca
2
X
Si,T1
=
n
Si,T1
2
=
X
Na
2
=
1 −X
Ca
2
.
(5.112)
We now note that for pure albite it is X
Al,T1
= X
Si,T1
=
1
2
, whereas for pure anorthite
X
Al,T1
=1. Substituting these values in (5.110) and (5.111), respectively, and recalling that
we require activities to be unity at the standard state (pure end-member species), we find
C
Ab(ii)
= 4 and C
An(ii)
= 1. With these values for the constants and mol fractions as in
(5.112) we obtain:
a
Ab,ideal(ii)
plg
=
(
X
Na
)
2
(
2 −X
Na
)
a
An, ideal(ii)
plg
=
1
4
X
Ca
(
1 +X
Ca
)
2
.
(5.113)
We follow the same procedure in order to calculate ideal activities for model (iii).
Equation (5.107) needs to be modified slightly, in order to reflect the fact that we must
now count all four silicon atoms, with n
Si
=4 −n
Al
:
O
m
Ab
=O
m
An
=
(n
Na
+n
Ca
)!
n
Na
!n
Ca
!
·
(n
Al
+n
Si
)!
n
Al
!n
Si
!
. (5.114)
Adding one molecule of each end-member:
O
m
Ab+1
=
(n
Na
+n
Ca
+1)!
(n
Na
+1)!n
Ca
!
·
(n
Al
+n
Si
+4)!
(n
Al
+1)!(n
Si
+3)!
(5.115)
and:
O
m
An+1
=
(n
Na
+n
Ca
+1)!
n
Na
!(n
Ca
+1)!
·
(n
Al
+n
Si
+4)!
(n
Al
+2)!(n
Si
+2)!
, (5.116)
273 5.8 More complex ideal activity–composition relationships
which, using (5.70) and simplifying, leads to:
a
Ab,ideal(iii)
plg
=C
Ab(iii)
X
Na
X
Al,T
X
Si,T
3
(5.117)
and:
a
An, ideal(iii)
plg
=C
An(iii)
X
Ca
X
Al,T
2
X
Si,T
2
. (5.118)
The mol fractions of Al and Si over the four T sites are:
X
Al,T
=
n
Al
4
=
2 −X
Na
4
=
1 +X
Ca
4
X
Si,T
=
n
Si
4
=
2 +X
Na
4
=
3 −X
Ca
4
.
(5.119)
Unit activities at the standard state require that C
Ab(iii)
=256/27 and C
An(iii)
=16, so that
we finally arrive at:
a
Ab,ideal(iii)
plg
=
1
27
X
Na
(
2 −X
Na
)(
2 +X
Na
)
3
a
An, ideal(iii)
plg
=
1
16
X
Ca
(
1 +X
Ca
)
2
(
3 −X
Ca
)
2
.
(5.120)
It is now clear that C
i
=1 if different cations mix on the same site in an end-member species,
as in this instance their mol fraction in the end-member species is not unity, but the activity
for the chosen standard state must be unity. From (5.63) we see that C
i
= 1 implies that
the entropy of mixing and configurational entropy of the solution are not the same, and we
can now understand why. The end-member plagioclase species may contain configurational
entropy that arises from Si–Al mixing in the tetrahedral sites (see also Worked Example 4.3),
and this entropy is “carried over” into the solution, so that it must be subtracted from the
total configurational entropy of the solution in order to obtain the net increase in entropy
generated by mixing.
The three sets of equations, (5.106), (5.113) and (5.120), yield significantly different
ideal activities for plagioclase of a given composition, but none of them is more “correct”
than any other. Choosing among the three models requires that one knows the structural
state of the particular plagioclase of interest. This is seldom trivial, and is complicated by
the fact that the two end-members may show different Al–Si ordering in their standard
states, as anorthite always has full long-range ordering (except perhaps near its melting
point) whereas the ordering state of end-member albite changes with temperature (see,
for example, Putnis, 1992). We will not discuss the details of configurational entropy in
plagioclase any further, but it is important to gain some insight into the energetic effects of
the different ideal activity models and their likely geological significance. This is shown
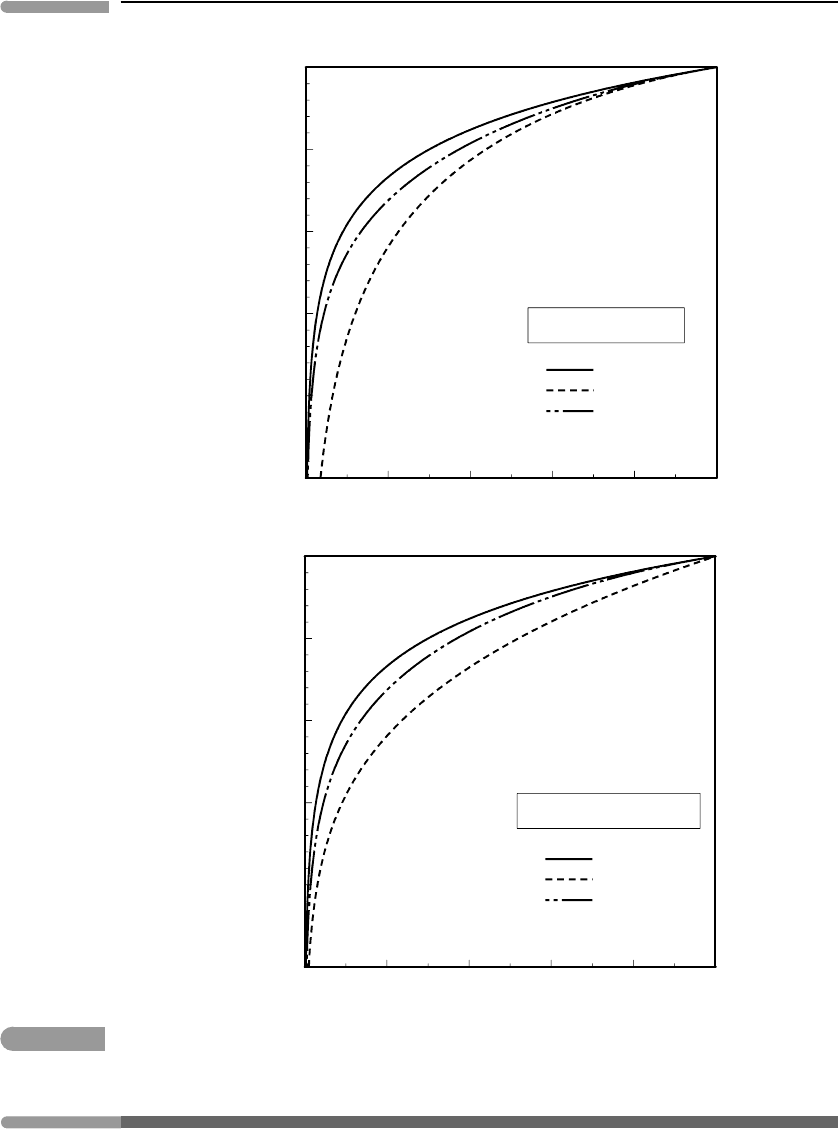
in Fig. 5.12, where we plot values of RT ln a
i, ideal
using T =1000 K as an example. The
calculated chemical potentials shift by the same amount as the energy difference between
models (equation (5.45)), and the effect is far from trivial. For example, a typical anorthite
content in plagioclase in medium to high-grade metamorphic rocks is An
20−40
. Figure 5.12
shows that the calculated chemical potential for anorthite would vary by 5–10 kJ mol
−1
,
depending on the ideal activity model used. Plagioclase in mantle rocks and in mafic igneous
rocks may contain ∼10–20% albite, and in this case the shift in calculated chemical potential
could be 20 kJ mol
−1
or more, as the temperature in such rocks is likely to be greater than
the 1000 K assumed in the figure. Energy differences of this magnitude would translate to
displacements in calculated equilibrium positions of hundreds of degrees or of kilobars to
perhaps tens of kilobars (see Section 5.2.1).
274 Chemical equilibrium
0 0.2 0.4 0.6 0.8 1
–50
–40
–30
–20
–10
0
TRnla
,bAlaedi
k(J lom
1–
)
Model (i)
Model (ii)
Model (iii)
0 0.2 0.4 0.6 0.8 1
–50
–40
–30
–20
–10
0
X
Ca
X
Na
TRnla
,nAlaedi
k(J lom
1–
)
Model (i)
Model (ii)
Model (iii)
Albite, T = 1000K
Anorthite, T = 1000K
Fig. 5.12
Comparison of three ideal activity models for plagioclase, at a constant temperature of 1000 K.
5.9 Non-ideal solutions
We defined an ideal solution as one for which H
mixing
= 0. Ideal solutions have another
important property, which is that V
mixing
in them vanishes too, as is easy to prove starting

275 5.9 Non-ideal solutions
from (5.37) and (5.40). Ideal solutions are an algebraic construct based upon Boltzmann’s
postulate, but is it reasonable to expect that real substances will follow the ideal behavior
that is defined by the condition H
mixing
= V
mixing
= 0? There are physical reasons to
believe that in general this will not be the case. For example, molecules in some fluids
are polar (e.g. H
2
O), whereas in others they are not (CO
2
). When the two fluids mix
CO
2
molecules screen electrostatic interactions among H
2
O molecules, and this should be
observed macroscopically as a non-zero V
mixing
. Different ions mixing in a crystalline
lattice generally differ in size, even if slightly, and therefore their surface charge densities
will differ too. Ionic substitution should then be accompanied by absorption or liberation
of energy (H
mixing
= 0), and perhaps by a change in volume relative to the equivalent
macroscopic aggregate (V
mixing
= 0). More subtly, mixing of different ions may have
effects on microscopic ordering that are not accounted for by the ideal mixing model, so
that the actual configurational entropy of the solution may be different from the value given
by (5.63). For example, ions may arrange themselves as if they are forming compounds (at a
microscopic level) and this will affect the configurational entropy of a crystal. Or molecules
may react to some extent in a mixed gas phase.
There are different ways of treating the behavior of real solutions. Here I focus on what
is perhaps the most widely used, and certainly the simplest, approach to real solutions. This
consists of assuming a reasonable microscopic mixing model from which one calculates
ideal activities, and then approximating the departure of the real (= observed) behavior
of the solution from this ideal model by fitting an empirical or semi-empirical function
with a variable number of free parameters. This is not the most elegant approach, as the
function has no strong physical justification, but there are a number of arguments that can
be made in its defense. Above all, it is simple and makes it possible to construct at least
a rough description of the behavior of real solutions on the basis of limited experimental
observations. Calibration of non-ideal solution models that have a better physical basis
often require experimental observations that for many phases and chemical species of
planetary interest do not exist. Their application to complex multicomponent phases under
very high temperatures and pressures may become computationally unwieldy, yet they
carry uncertainties that may make their results indistinguishable from those of simpler
empirical models, especially when compounded with possibly large uncertainties about
physical conditions in planetary interiors.
5.9.1 Excess mixing functions
Equation (5.91)(G
mixing
=H
mixing
−TS
mixing
) is valid for any solution, ideal or non-
ideal, as no assumptions about the nature of the solution were made in its derivation. If a
solution is non-ideal then in general it must be:
G
mixing
=G
ideal
mixing
. (5.121)
The inequality may arise from a combination of enthalpy and entropy contributions to
Gibbs free energy, but there may not be an a priori way of discriminating between them.
We therefore convert (5.121) into an identity by adding a Gibbs free energy contribution to
the Gibbs free energy of ideal mixing. This contribution is called excess Gibbs free energy,
G
excess
, and is defined by the following equation:
G
excess
≡G
mixing
−G
ideal
mixing
. (5.122)
276 Chemical equilibrium
0
∆G
mixing
∆G
mixing
∆G
mixing
∆G
mixing
0
0
X
i
X
i
X
i
X
i
0
(a)
(c)
1
1
1
1
(b)
(d)
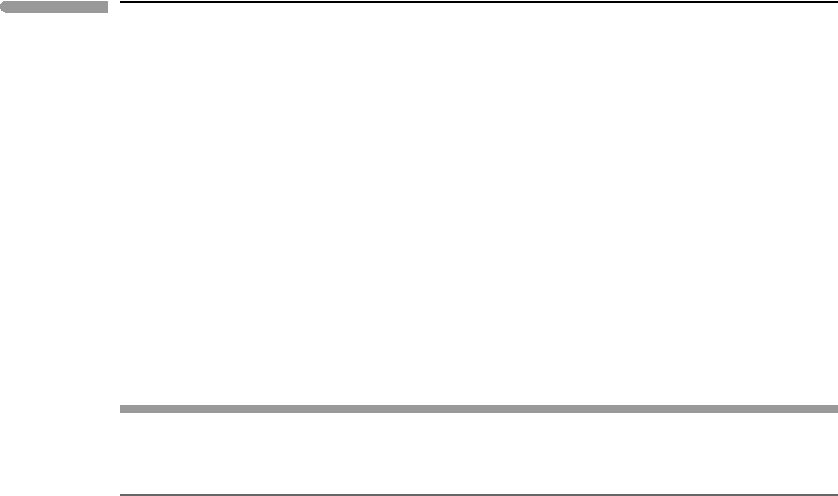
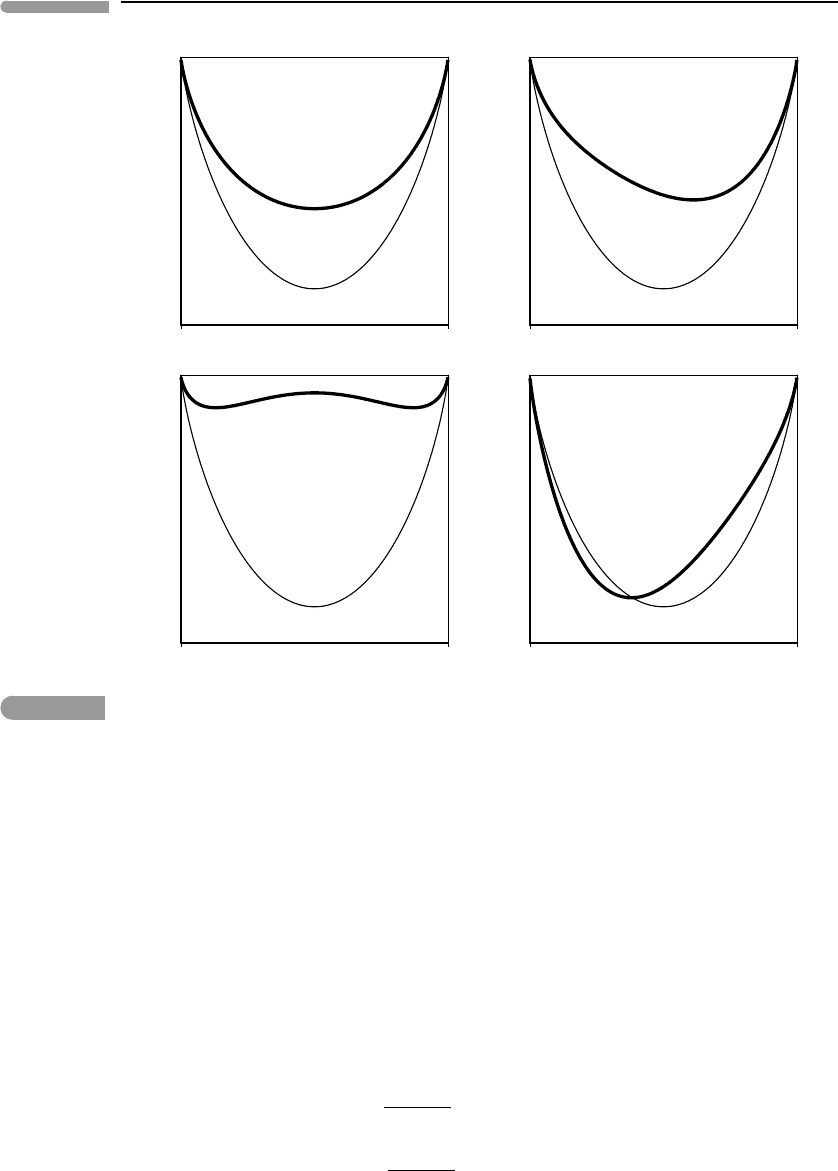
Fig. 5.13
Possible non-ideal behaviors of a binary one-site solution, shown by the thick curves. The thin curve is the same in all
four graphs and shows ideal one-site mixing. The examples shown do not exhaust all possibilities. For example, the
inflected behavior shown in (c) is symmetric, but it can also be asymmetric, as in (b). Negative deviation from ideality,
as in the left side of (d), can also be symmetric (at least in principle).
Figure 5.13 exemplifies possible behaviors of real solutions. The thick curves in the figure
represent the Gibbs free energies of four possible real solutions. The thin curves are the
Gibbs free energies of the same solutions, assumed to be ideal. The excess Gibbs free energy
is the distance between the curves. We see that G
excess
may be symmetric (Fig. 5.13a) or
asymmetric (Fig. 5.13b) relative to composition, it may be such that the curvature of the
G
mixing
function changes with composition (Fig. 5.13c), or such that the sign of G
excess
changes with composition (Fig. 5.13d), or some combination of these behaviors. Because it
is a Gibbs free energy, G
excess
is related to excess enthaply, entropy and volume functions:
G
excess
=H
excess
−T S
excess
(5.123)
∂G
excess
∂T
P ,n
i
=−S
excess
(5.124)
∂G
excess
∂P
T ,n
i
=V
excess
. (5.125)
277 5.9 Non-ideal solutions
We use (5.2.12) to define a partial molar property, called excess chemical potential, µ
excess
,
as follows:
µ
i, excess
≡G
excess
+
j=k
j=2
δ
ij
−X
j
∂G
excess
∂X
j
(5.126)
so that, by equation (5.29), G
excess
=
i
X
i
µ
i
. From (5.126), (5.122) and (5.93) we get:
G
mixing
=
i
X
i
RT ln a
i, ideal
+
i
X
i
µ
i, excess
(5.127)
and, by using (5.45):
µ
i
−µ
0,i
=RT ln a
i
=RT ln a
i, ideal
+µ
i, excess
. (5.128)
In order to construct a compact equation for the activity of a component in a non-ideal
solution we define a new non-dimensional parameter, called the activity coefficient and
symbolized by γ , as follows:
γ
i
≡exp
µ
i, excess
RT
(5.129)
so that:
a
i
=γ
i
·a
i, ideal
. (5.130)
Equations (5.122) through (5.130) are the foundations of the mathematical treatment of
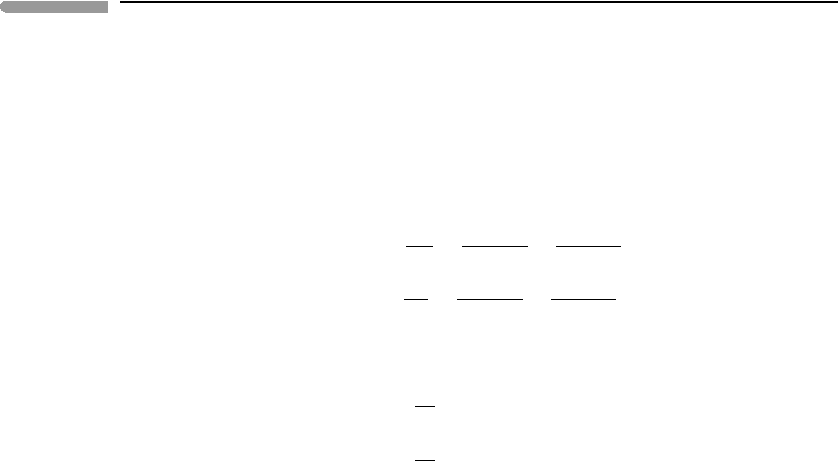
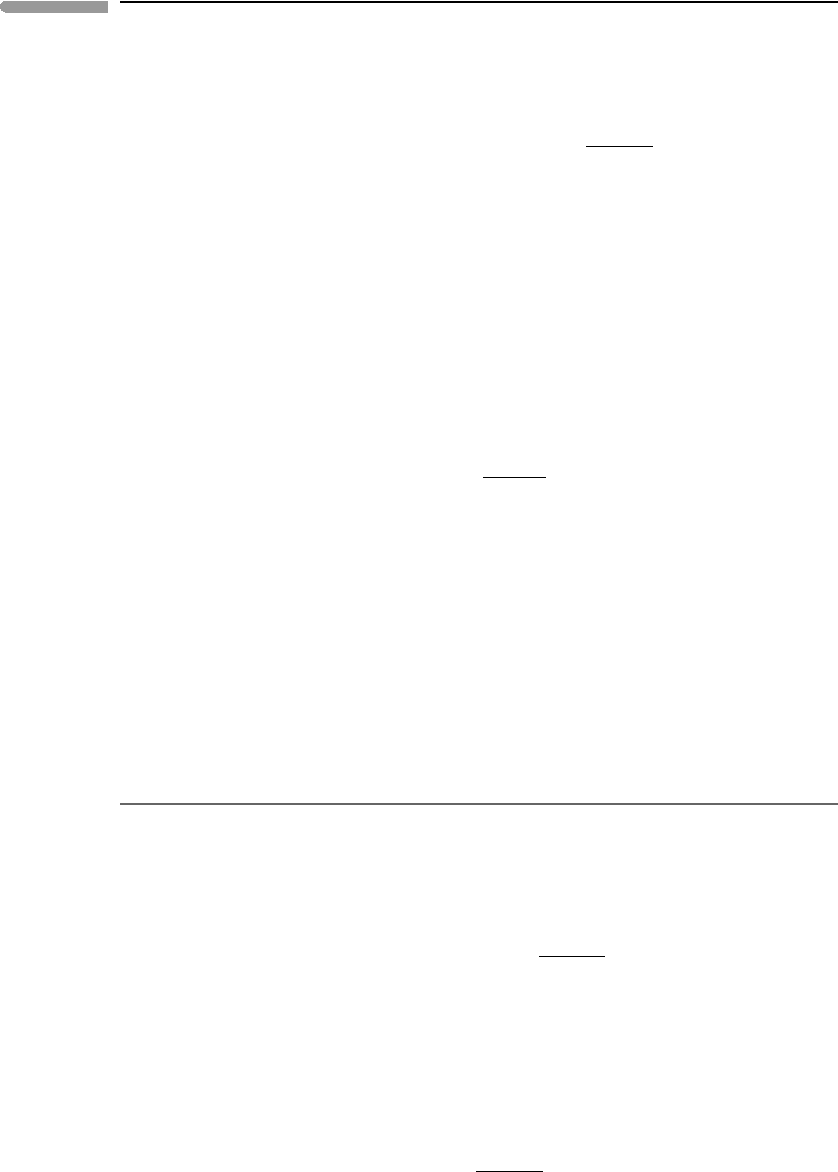
non-ideal solutions. They have a simple geometric interpretation which helps to visualize
the behavior of real solutions (Fig. 5.14). The excess chemical potential of a species is the
distance between the intersects of the tangents to the ideal and real Gibbs free energy of
mixing curves with the G axis for that species (compare Fig. 5.9). This distance vanishes
as the mol fraction of the species of interest approaches unity, and approaches a constant
finite value as the species becomes infinitely dilute (Fig. 5.14).
5.9.2 Raoult’s law and Henry’s law
If we consider a simple one-site binary solution then (5.130) becomes:
a
i
=γ
i
X
i
(5.131)
and, calling the two components 1 and 2, we get from (5.126):
µ
1,excess
=G
excess
−X
2
∂G
excess
∂X
2
. (5.132)
For X
1
→ 1 we have X
2
→ 0 and also G
excess
→ 0 (see Fig. 5.14), so that, as long as
∂G
excess
/∂X
2
stays bound, µ
1,excess
→0. We then have, for a nearly pure component:
a
i
=X
i
, for X
i
→1. (5.133)
On the other hand, for X
1
→ 0, X
2
→ 1 but it is still G
excess
→ 0. Assuming again that
∂G
excess
/∂X
2
stays bound, we have:
µ
1,excess
X
1
→0
=−
∂G
excess
∂X
2
X
2
=1
. (5.134)
