Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


248 Chemical equilibrium
potential of the species KAlSi
3
O
8
must be the same in the crystal as in the melt, and in
order for water ice to be stable on the Martian surface the chemical potential of H
2
O must
be the same in surface ice as in the Martian atmosphere. This seemingly trivial corollary of
(5.22) is a fundamental concept that we will rely on many times.
Suppose now that the system composed of the two phases A and B is not at equilibrium.
Then a spontaneous transfer of component σ between the phases at constant temperature
and pressure, say from A to B, can take place only if it leads to a decrease in the Gibbs free
energy of the system. Expanding (5.21) we have:
dG =
µ
σ
A
−µ
σ
B
dn
σ
A
< 0. (5.25)
But if matter is transferred from A to B then it must be dn
A
σ
< 0, which implies that:
µ
σ
A
>µ
σ
B
. (5.26)
In other words, chemical species are transferred down chemical potential gradients. This is
what we should have expected given that µ and n are conjugate variables (Section 4.8.4) and
also justifies the name chemical potential for the thermodynamic variable µ. Equilibrium
is reached once mass transfer among phases eliminates chemical potential gradients. The
proof that equation (5.26) generalizes to systems with any number of phases and chemical
species is left as an exercise.
5.4 Partial molar properties
Finding the function that relates the chemical potential of a phase component to its standard
state Gibbs free energy and to the composition of the solution requires several steps. We
begin by noting that we can express any extensive thermodynamic property of a solution,
Z
sol
, as a sum of products of intensive quantities z
i
for each of the i components of the
solution, times the amount (an extensive quantity, for example the number of mols, n
i
)of
each component, as follows:
Z
sol
=
i
z
i
n
i
. (5.27)
We make no claims as to the functional form of the z
i
s, and in particular, as to whether
or not the various z
i
s are a function of each other. Equation (5.27) is simply an algebraic
identity. We do require, however, that the amount of each component, n
i
, be independently
variable, so these must be phase components (for example, we can add forsterite to olivine
without changing the amount of fayalite). Z
sol
being a thermodynamic variable, it is also
a function of temperature and pressure. It then follows from (5.27) that the quantities z
i
,
called partial molar quantities, are defined by the following derivative:
z
i
≡
∂Z
sol
∂n
i
P ,T ,n
j≡i
. (5.28)
It is important to understand what this equation means: partial molar z of component i,an
intensive variable symbolized z
i
, equals the rate of change of Z of the solution (an extensive
variable) relative to a change in the amount of component i (also extensive), while keeping

249 5.4 Partial molar properties
pressure, temperature and the amounts of all other components constant. Dividing (5.27)by
the total number of mols in the system we obtain molar Z of the solution, Z
sol
, as follows:
Z
sol
=
i
z
i
n
i
i
n
i
=
i
z
i
X
i
, (5.29)
where X
i
is the mol fraction of component i.
Obviously, the thermodynamic property Z also takes a definite value for each of the
solution components when they are in their standard states. For a component i we symbolize
this by Z
0,i
, reserving the subscript slot to state the pressure and temperature of the standard
state, if needed (e.g. Section 1.13.1). On dimensional grounds it is evident that z
i
and Z
0,i
refer to the same type of thermodynamic property (e.g. they must both have dimensions of
volume per mol, energy per mol or entropy per mol). Note, however, a very important point:
we have made no assumptions about the relationship between z
i
and Z
0,i
. In particular,
the definitions of the two variables are not the same. Whereas the standard state property,
Z
0,i
, is defined independently of the properties of a solution (for example, for a pure
chemical species), the definition of the corresponding partial molar property z
i
is based on
the behavior of a particular solution that contains i. There is no expectation that the two
variables take on the same value, so that we can write the following equation:
Z
sol
=
i
z
i
X
i
=
i
Z
0,i
X
i
+Z
mixing
. (5.30)
We take (5.30) as the definition of Z
mixing
, noting that this quantity is defined for the
solution (not for the components). For a pure chemical species (equivalently, a phase of
fixed composition), there is only one component, for which X
i
= 1, so that in this case z
i
is always equal to Z
0,i
, for any thermodynamic variable, and Z
mixing
is obviously zero.
There is an additional relationship among partial molar properties and the molar prop-
erty of the solution that plays a central role in the study of solutions. For a solution of
k components, the partial molar property of the ith components is given by:
z
i
=Z
sol
+
j=k
j=2
δ
ij
−X
j
∂Z
sol
∂X
j
, (5.31)
where the symbol δ
ij
, called the Kronecker delta, takes the value 1 if i =j , and 0 otherwise.
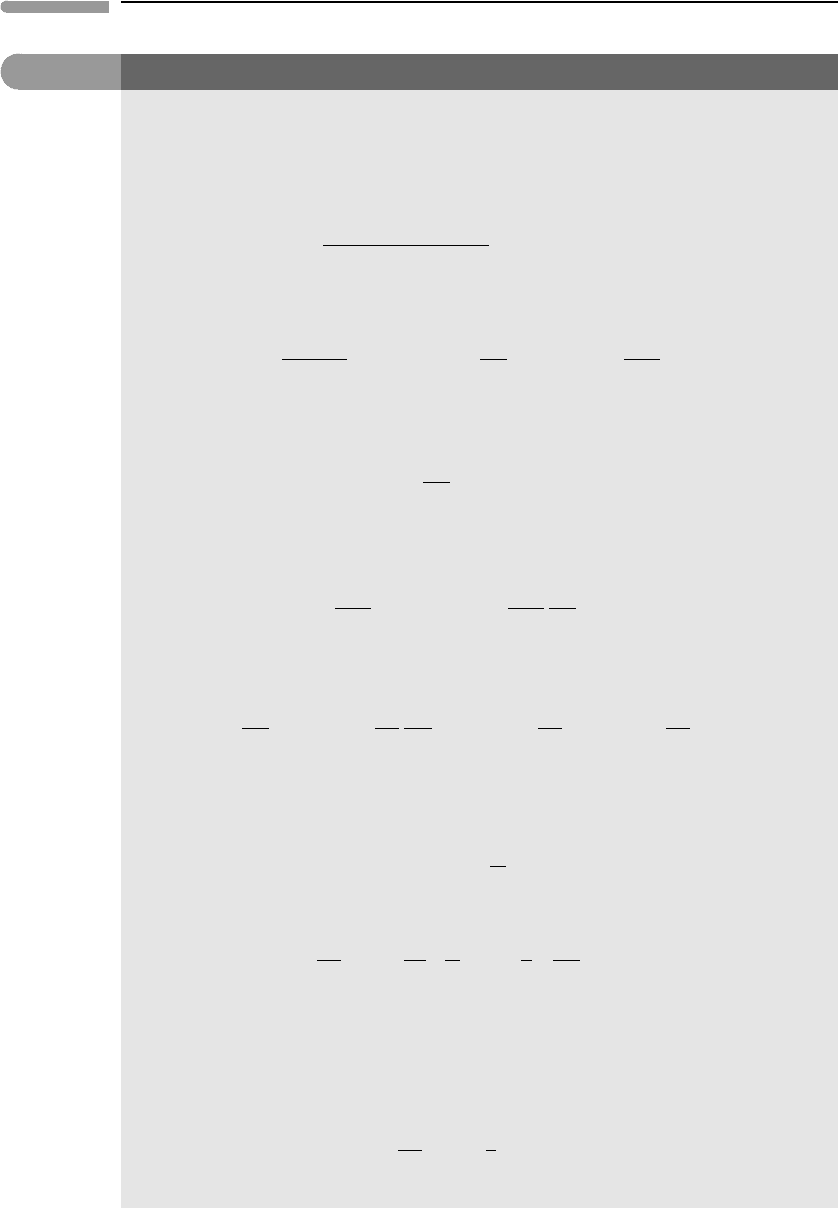
The proof and geometric interpretation of (5.31) are given in Box 5.2.
Box 5.2
Proof of equation 5.31
Let Z be any extensive thermodynamic variable. We can write Z for a solution of k components as a function
of the form:
Z
sol
=Z
sol
(
P, T, n
1
, ...,n
k
)
(5.2.1)
or, equivalently, as a function of the total number of mols, n =
i
n
i
and the mol fractions of any k −1
components (the kth is not linearly independent):
Z
sol
=Z
sol
(
P,T,n,X
2
, ...,X
k
)
. (5.2.2)
250 Chemical equilibrium
Box 5.2
Continued
This is a simple change of coordinates: note that the functions (5.2.1) and (5.2.2) have the same number of
variables, and that, as long as we know n, it is always possible to convert the n
i
stoX
i
s and vice versa. Molar
Z of the solution, Z
sol
, is obtained by dividing (5.2.2)byn, and, because n is the only extensive independent
variable in (5.2.2), this results in:
Z
sol
=
Z
sol
(
P, T, n, X
2
, ...,X
k
)
n
=Z
sol
(
P, T,1,X
2
, ...,X
k
)
. (5.2.3)
We now use (5.2.3) to re-write the definition of the partial molar property z
i
(equation (5.28)) as follows:
z
i
=
∂
nZ
sol
∂n
i
P,T,n
j≡i
=Z
sol
∂n
∂n
i
P,T,n
j≡i
+n
∂Z
sol
∂n
i
P,T,n
j≡i
. (5.2.4)
Because all n
j=i
are kept constant, it is:
∂n
∂n
i
P,T,n
j≡i
=1. (5.2.5)
Using (5.2.3) we write the partial derivative in the second term of the right-hand side of (5.2.4) as follows:
∂Z
sol
∂n
i
P,T,n
j≡i
=
j=k
j=2
∂Z
sol
∂X
j
∂X
j
∂n
i
P,T,n
j≡i
(5.2.6)
and, using (5.2.5):
∂X
j
∂n
i
P,T,n
j≡i
=
∂X
j
∂n
∂n
∂n
i
P,T,n
j≡i
=
∂X
j
∂n
P,T,n
j≡i
=
∂X
j
∂n
P,T
. (5.2.7)
In the last identity we have dropped the constraint n
j=i
because we are now differentiating relative to the
total number of mols. Each mol fraction is given by:
X
j
=
n
j
n
(5.2.8)
so:
∂X
j
∂n
P,T
=
∂
∂n
n
j
n
P,T
=
1
n
∂n
j
∂n
−X
j
P,T
. (5.2.9)
Component i can be any arbitrary component, either component 1 or any one of the k −1 components
between j = 2 and j = k. We need to account for all possibilities. For n
j=i
it is ∂n
j
/∂n = 0, whereas for
n
j=i
we have ∂n
j
/∂n =1. We can then write (5.2.9) using the Kronecker delta: δ
ij
=1ifi = j, δ
ij
=0if
i = j, as follows:
∂X
j
∂n
P,T
=
1
n
δ
ij
−X
j
. (5.2.10)
251 5.4 Partial molar properties
Box 5.2
Continued
Substituting into (5.2.6):
∂Z
sol
∂n
i
P,T,n
j≡i
=
1
n
j=k
j=2
δ
ij
−X
j
∂Z
sol
∂X
j
. (5.2.11)
Substituting (5.2.11)in(5.2.4) and using (5.2.5):
z
i
=Z
sol
+
j=k
j=2
δ
ij
−X
j
∂Z
sol
∂X
j
, (5.2.12)
which is (5.31).
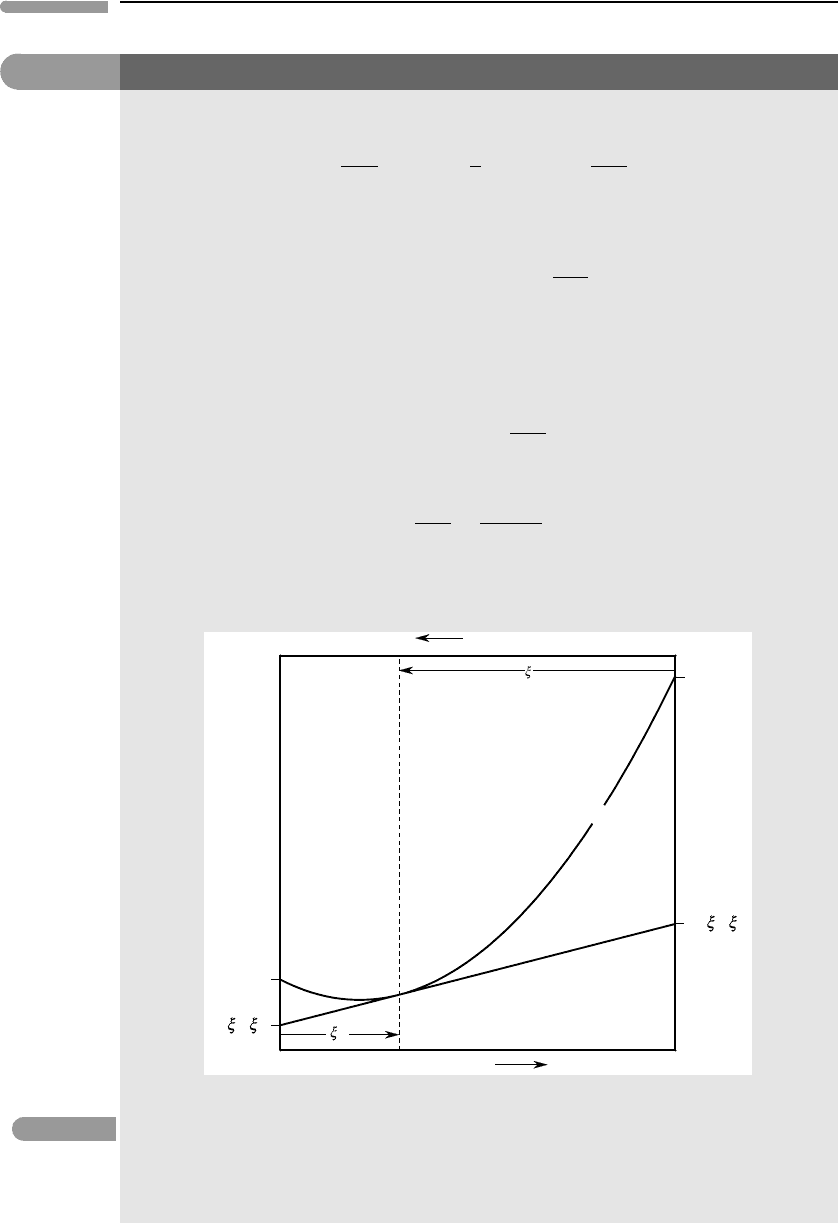
This equation comes up repeatedly in the study of solutions, and it is helpful to visualize its geometry.
This is best done by considering a system of two components, a and b, in which case (5.2.12) becomes:
z
a
=Z
sol
−X
b
∂Z
sol
∂X
b
. (5.2.13)
Rearranging as follows:
∂Z
sol
∂X
b
=
Z
sol
−z
a
X
b
(5.2.14)
it is easy to see (Fig. 5.7) that the partial molar properties z
a
and z
b
for a given solution composition, say at
mole fractions ξ
a
, and ξ
b
=1 −ξ
a
, are the intercepts of the tangent line to Z
sol
at that composition with
01
X
b
01
X
a
Z
0, b
Z
sol
z
b
(
a
,
b
)
Z
0, a
z
a
(
a
,
b
)
a
b
Fig. 5.7 Geometric interpretation of the relationships between the molar property of a solution, Z
sol
, standard state molar
properties of end-member species, Z
0,a
and Z
0,b
, and partial molar properties of the species in solution, z
a
and z
b
.
Partial molar properties, shown in the figure for a specific solution composition given by ξ
a
and ξ
b
=1 −ξ
a
, are
functions of composition.

252 Chemical equilibrium
Box 5.2
Continued
the Z axis at X
a
= 1 and X
b
= 1, respectively. This is true for any extensive thermodynamic function that
can be written as (5.27), and regardless of the functional form of Z
sol
. The geometric point of view (Fig. 5.7)
is particularly helpful in emphasizing that, whereas Z
0,i
is a constant at a given P and T , z
i
varies with the
composition of the solution at constant P and T (except for a property for which Z
sol
is a linear function).
The geometry is the same for multicomponent solutions, except that the tangent line is replaced by a
tangent n −1 plane embedded in an n-dimensional space, where n is the number of components in the
solution.
Equations (5.27) through (5.31) are algebraic identities that can be applied to any exten-
sive thermodynamic state variable, for instance, enthalpy, entropy, volume and Gibbs free
energy. With one exception, the partial molar property is symbolized with the same let-
ter used for the standard state molar property but written in lowercase (e.g. partial molar
entropy, s) rather than uppercase (e.g. standard state molar entropy, S
0
). The exception
is Gibbs free energy. Comparison of (5.28) with (4.134) shows that the partial molar
Gibbs free energy is the chemical potential, µ. It is customary to symbolize the standard
state molar Gibbs free energy of a pure species by µ
0
, and to call it the standard state
chemical potential. This is physically appealing given that the interpretation of chemical
potential as a driving force for transfer of matter (equation (5.26)) is also true for pure
chemical species. In this book we will use both µ
0,i
and G
0,i
, depending on the context,
but you must keep in mind that the identity µ
0,i
= G
0,i
is always true. Summarizing,
we have:
G
sol
=
i
µ
i
X
i
=
i
µ
0,i
X
i
+G
mixing
(5.32)
S
sol
=
i
s
i
X
i
=
i
S
0,i
X
i
+S
mixing
(5.33)
H
sol
=
i
h
i
X
i
=
i
H
0,i
X
i
+H
mixing
(5.34)
V
sol
=
i
v
i
X
i
=
i
V
0,i
X
i
+V
mixing
. (5.35)
The pressure and temperature derivatives of chemical potential follow the same rules as
those of Gibbs free energy. Thus (omitting the constant variable subscripts for clarity, see
Box 1.3):
∂µ
i
∂P
=
∂
∂P
∂G
sol
∂n
i
=
∂
∂n
i
∂G
sol
∂P
=
∂V
sol
∂n
i
(5.36)
or:
∂µ
i
∂P
T
=v
i
. (5.37)

253 5.5 Generalized equilibrium condition
And by an identical procedure:
∂µ
i
∂T
P
=−s
i
. (5.38)
5.5 Generalized equilibrium condition. Activity and the
equilibrium constant
5.5.1 Definition of activity
We now have the background needed to derive the algebraic expression that describes
equilibrium among chemical species in any state, as a function of temperature, pressure,
composition and thermodynamic properties in the standard state. We begin by defining a
new thermodynamic variable called activity. Using equation (4.128) (last line) we write the
following equation for the molar properties of a solution:
G
sol
=H
sol
−TS
sol
. (5.39)
We can re-write this in terms of partial molar properties, by using (5.32), (5.33) and (5.34):
i
µ
i
X
i
=
i
h
i
X
i
−T
i
s
i
X
i
(5.40)
from which, given that the mol fractions can vary independently, we derive an identity valid
for each component i:
µ
i
=h
i
−Ts
i
. (5.41)
Equation (4.128) also yields the following relationship between standard state molar
properties:
µ
0,i
=H
0,i
−TS
0,i
. (5.42)
Subtracting (5.42) from (5.41) and rearranging:
µ
i
=µ
0,i
+
h
i
−H
0,i
−T
s
i
−S
0,i
. (5.43)
The terms in this equation have dimension of energy. We can therefore choose to write the
sum of the last two terms on the right-hand side as the product of the gas constant, R, times
temperature, T, times a non-dimensional variable. We define this non-dimensional variable,
called activity and symbolized by a, as follows:
ln a
i
≡
1
RT
h
i
−H
0,i
−
1
R
s
i
−S
0,i
(5.44)
from which we get:
µ
i
=µ
0,i
+RT ln a
i
. (5.45)

254 Chemical equilibrium
There are different ways of defining activity (see, for example, Guggenheim, 1967).
Equation (5.44) is not orthodox, but it is elegant. All definitions converge on equation
(5.45). It is very important to understand what this equation is saying. Activity is a non-
dimensional parameter that measures the chemical potential of a component in a solution
relative to the chemical potential of the same chemical species in a specified standard state.
Activity is a relative quantity that has meaning only if the standard state that it refers to is
given explicitly. This standard state could be, for example, the pure chemical species in the
same physical state as the solution (e.g. solid in a certain crystalline state, liquid, gas) and at
the pressure and temperature of interest. We could also specify a different standard state, and
if we do so then, as is readily apparent from (5.45), the value of the activity must also change,
because it now measures the same chemical potential relative to a different reference level.
Worked Example 5.2 Activity of components in silicate melts: an example of changing the
standard state
Consider a silicate melt saturated in quartz, for instance, a granitic melt. By “saturated” we
mean that crystalline quartz is at equilibrium with the melt, so that we have:
µ
0,SiO
2
quartz
=µ
SiO
2
quartz
=µ
SiO
2
melt
(5.46)
The identity µ
0,SiO
2
quartz
=µ
SiO
2
quartz
comes from (5.30), noting that for pure quartz it is X
SiO
2
=1.
We wish to specify the activity of SiO
2
in the melt, but in order to do so we must first define
the standard state relative to which the activity is to be measured. One possibility is to define
the standard state as pure crystalline quartz at the pressure and temperature of interest. As
with chemical potential, it is convenient to label activity with the identities of the chemical
species and the phase, thus: a
c
p
. If we call the activity of SiO
2
in the melt relative to a
standard state of pure crystalline quartz at the pressure and temperature of interest a
1
we
have, from (5.45):
µ
SiO
2
melt
=µ
0,SiO
2
quartz
+RT ln
a
SiO
2
melt
1
(5.47)
and, using (5.46):
a
SiO
2
melt
1
=1. (5.48)
Another possibility is to define the standard state as pure liquid SiO
2
at the pressure and
temperature of interest. This standard state, µ
0,SiO
2
liquid
, is related to the pure quartz standard
state by:
µ
0,SiO
2
liquid
=µ
0,SiO
2
quartz
+
m
G
0
P ,T
, (5.49)
where
m
G
0
P ,T
is the Gibbs free energy of melting of quartz at the pressure and temperature
of interest, i.e.
r
G for the reaction quartz → liquid SiO
2
at P and T. Using liquid SiO
2
as
the standard state and calling the activity relative to it a
2
we have:
µ
SiO
2
melt
=µ
0,SiO
2
liquid
+RT ln
a
SiO
2
melt
2
(5.50)
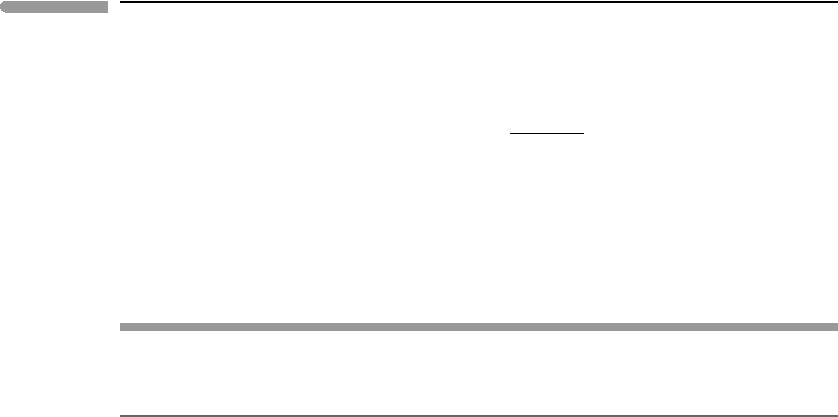
255 5.5 Generalized equilibrium condition
and, using (5.46) and (5.49):
a
SiO
2
melt
2
=exp
−
m
G
0
P ,T
RT
. (5.51)
Now,
m
G
0
P ,T
vanishes only if P and T are on the melting curve of pure quartz, in which
case the two standard state chemical potentials are equal (equation (5.49)), and a
1
= a
2
.
Otherwise, it is
m
G
0
P ,T
= 0 and a
1
= a
2
. Note very carefully that µ
SiO
2
melt
is the same in
(5.47) and (5.50). What has changed is the standard state, and therefore the activity scale
that measures this fixed chemical potential.
5.5.2 The equilibrium constant
Substituting (5.45)in(5.22), the condition of equilibrium among chemical species in an
arbitrary state and at temperature T can be written as follows:
i
ν
i
µ
0,i
+RT
i
ln
a
i
ν
i
=0. (5.52)
The first term of the sum in (5.52) is the standard state Gibbs free energy of reaction
(equation (5.7)):
i
ν
i
µ
0,i
=
i
ν
i
G
0,i
P ,T
=
r
G
0
P ,T
, (5.53)
but this is now not necessarily zero, as equation (5.8) is true only for species in their
standard state, and we have relaxed this restriction. We define another non-dimensional
variable, called the equilibrium constant of the chemical reaction, and symbolized by K,
as follows:
K =
i
a
i
ν
i
. (5.54)
Using (5.53) and (5.54)in(5.52) we get the following generalized equation of chemical
equilibrium:
r
G
0
P ,T
+RT ln K = 0. (5.55)
Finding the equilibrium position for a chemical reaction among species in any state entails
solving equation (5.55). This equation is a generalization of (5.8), and collapses to (5.8)if
all species are in their standard states, in which case, according to (5.45), it is a
i
= 1 for
all is, and, therefore ln K = 0. The following are two examples of equilibrium calculations
among solutions in heterogeneous and homogeneous systems.

256 Chemical equilibrium
Worked Example 5.3 The spinel–garnet transition in planetary mantles, part (ii)
In order to study phase changes in real planetary mantles we need to calculate the equilibrium
position of reaction (5.15) for the general case in which the Mg-bearing species are not pure
phases but components in mineral solid solutions. We define the standard state of each
Mg-bearing species as the pure crystalline solid (pyrope, forsterite, spinel or enstatite) at
the pressure and temperature of interest. Applying (5.54), we get the following expression
for the equilibrium constant of reaction (5.15):
K =
a
Mg
3
Al
2
Si
3
O
12
garnet
·a
Mg
2
SiO
4
olivine
a
MgAl
2
O
4
spinel
·
a
Mg
2
Si
2
O
6
opx
2
. (5.56)
We then write the equilibrium condition (5.55) like this:
r
G
0
P ,T
+RT ln
a
Mg
3
Al
2
Si
3
O
12
garnet
·a
Mg
2
SiO
4
olivine
a
MgAl
2
O
4
spinel
·
a
Mg
2
Si
2
O
6
opx
2
=0. (5.57)
Keeping in mind that
r
G
0
P,T
is a function of P and T, given by (5.13), we see that (5.57)
contains six unknowns: P ,T and the four activities, which, as we shall discuss beginning
in the next section, are functions of composition. Therefore, equation (5.57) by itself has
five degrees of freedom, which means that in order to solve it we must specify the values
of five of the variables. We can ask (i) which five, and (ii) how? The answer depends on
what we are trying to accomplish. Let us look at two distinct possibilities.
Suppose first that we have a sample of lherzolite that contains the four-phase assem-
blage olivine–orthopyroxene–garnet–spinel, and that we have good reasons (petrographic
or other) to be confident that the four phases crystallized at equilibrium. We can then calcu-
late the four activities from the respective mineral compositions (more on this in subsequent
sections), leaving us with two unknowns in (5.57), and thus one degree of freedom. We are
now in the same situation that we discussed in Worked Example 5.1: we can calculate a
curve in P –T space that maps all the possible P –T combinations at which minerals with the
observed compositions could have crystallized. The location of this curve will in general be
different from the one in Fig. 5.5 (unless, by chance, activities are such that K = 1) and its
physical meaning is also subtly different (more on this in Chapter 6). The curve nonetheless
constrains the possible crystallization conditions of our lherzolite. If we have an indepen-
dent way of fixing one of the two variables, for instance temperature, then equation (5.57)
can be solved for the pressure of crystallization (subject to the uncertainties discussed in
Section 5.2.1, plus others that arise from activity–composition relationships, as we shall
see). We may label this procedure inverting mineral compositions in order to determine the
values of intensive variables during crystallization. It is also called “geothermometry” or
“geobarometry”, depending on which is the target intensive variable.
Alternatively, we may wish to do forward modeling in order to predict mineral compo-
sitions as a function of pressure and temperature. In this case we specify arbitrary values
of P and T, leaving us with three degrees of freedom in equation (5.57). In order to solve
the problem we need to come up with three additional equations, or some other way of
constraining three of the compositional variables (Worked Example 5.6).

257 5.5 Generalized equilibrium condition
Worked Example 5.4 Speciation in a homogeneous gas phase. Carbon–oxygen equilibrium,
part (i)
Carbon–oxygen equilibrium plays important roles in many planetary processes, from deter-
mining the composition of volcanic gases (Chapter 9) to determining ice compositions in
the outer reaches of the solar nebula, and perhaps the compositions of early post-nebular
atmospheres in terrestrial planets (Chapter 14). One often wishes to know the speciation in
the gas phase, i.e. the relative amounts of chemical species present in the gas phase, which
in this case would be CO
2
, CO and O
2.
In almost every realistic speciation calculation in
planetary fluids a meaningful calculation requires that equilibria involving other system
components, chiefly hydrogen, nitrogen and sulfur, be included as well (more on this in
Chapters 9 and 14). The C–O example, however, allows us a simple first look into how the
calculations are carried out.
The following two equilibria describe the chemical reaction between carbon and oxygen:
Reaction 1: C+
1
2
O
2
←
→
CO,
Reaction 2: C+O
2
←
→
CO
2
.
(5.58)
Let us assume that equilibrium is established in the presence of excess graphite, i.e. there
is not enough oxygen to oxidize all of the carbon. The assemblage then consists of graphite
and a gas phase composed of CO, CO
2
and O
2
. It is convenient in this case to define pure
graphite at the temperature and pressure of interest as the standard state for C, as this results
in: a
C
graphite
=1. We can now write one version of (5.55) for each reaction, as follows:
K
1
=
a
CO
gas
a
O
2
gas
1/2
=exp
−
r
G
0,1
P ,T
RT
(5.59)
and:
K
2
=
a
CO
2
gas
a
O
2
gas
=exp
−
r
G
0,2
P ,T
RT
, (5.60)
where the 1 or 2 superscripts identify the reaction. If we specify pressure and temperature
then these are two equations in three unknowns (the three activities).The system of equations
appears to have one degree of freedom. We shall see, however, that we can recast the
activities as functions of the concentration, or mol fraction, of each of the three gas species
in the gas phase, X
CO
2
, X
CO
and X
O
2
. Solving for these concentrations is what we call
determining the speciation of the gas phase. If we assume that no other components are
present then we can write an additional equation which states that the gas contains only
CO
2
, CO and O
2
: X
CO
2
+X
CO
+X
O
2
=1. Together with (5.59) and (5.60) this constitutes a
system of equations with no degrees of freedom, that we can solve for the three mol fractions
(at given P and T). We have not defined the standard states for the three gas species: O
2
,CO
and CO
2
. Neither the activities nor the Gibbs free energies of reaction in equations (5.59)
and (5.60) have any meaning unless standard states are specified. The usual way of defining
standard states for gases is different from condensed phases, and will be discussed briefly
in Worked Example 5.5, where we will solve equations (5.59) and (5.60), and in detail in
Chapter 9.
