Das Sh.P. Statistical Physics of Liquids at Freezing and Beyond
Подождите немного. Документ загружается.


3.3 The density-functional approach 141
The corresponding choice for the set of order parameters represents a saddle point for
[n] in the function space of density. The ordinary differential equations obtained for
the order parameters are given by
∂
∂γ
i
D[γ ]+
αβ
j
∂
∂γ
j
#
αβ
ij
[γ ]{∇
α
γ
i
(x)∇
β
γ
j
(x)}
− #
αβ
ij
[γ ]∇
α
∇
β
γ
j
(x) −
#
∇
α
#
αβ
ij
[γ ]
$
∇
β
γ
j
(x)
= 0 (3.3.15)
for the index i =1,...,m running over the set. The above equations represent the
spatial variation of the local amplitudes γ
i
across the crystal–liquid interface in the
critical nucleus.
From what we have described above, the first step in the construction of the density-
functional model of the nucleus in the melt therefore involves constructing (a) the density
function parametrized in terms of a set of local order parameters that are chosen so as to
represent a spherical nucleus in the melt and (b) the proper grand-canonical potential
as a functional of the inhomogeneous density function. In the simplest approximation the
first term in (3.3.13) is computed in terms of a low-order functional Taylor-series expan-
sion of the Ramakrishnan and Yussouff (1979) model described in Chapter 2. However,
this would imply an ad-hoc truncation of the density expansion for the crystalline state in
which the density fluctuations are not small. Alternatively,
u
is computed using the more
sophisticated weighted-density-functional methods described in Chapter 2. We consider
both cases here.
3.3.2 The critical nucleus
In the simplest approximation
u
is computed (Harrowell and Oxtoby, 1984) in terms of
the expansion (3.3.10) around the uniform liquid state and keeping terms of up to second
order in the density fluctuations around the uniform liquid state. The functional is
to be optimized with respect to a suitably chosen density function, which represents the
crystal nucleus. We consider for the density n
0
(r) representing the spherical nucleus the
functional form (3.3.3) with the local amplitudes γ(r) ≡{η(r), A
i
(r)} being functions
solely of the radial distance r. This implies spherical symmetry for the order parameters
but not for the density function itself. Using the parametrization (3.3.3), we obtain for
the density fluctuation δn
0
(x;γ) = n
0
(x;γ)− n
l
of the inhomogeneous state around the
uniform liquid state density n
l
δn
0
(x
2
;γ(x
1
)) = n
l
η(x
1
) +
i
A
i
(x
1
)e
iK
i
· x
2
. (3.3.16)
142 Crystal nucleation
On substituting (3.3.17) into (3.3.10), the grand potential function
u
is obtained as
u
= n
l
dx
&
˜η(x) +
i
A
i
(x)e
iK
i
· x
'
ln
⎡
⎣
˜η(x) +
j
A
j
(x)e
iK
j
· x
⎤
⎦
− n
l
dx
&
η(x) +
i
A
i
(x)e
iK
i
· x
'
−
n
2
l
2
dx
dx
c(x, x
;γ)
×
&
η(x) +
i
A
i
(x)e
iK
i
· x
'
⎧
⎨
⎩
η(x) +
j
A
j
(x)e
iK
j
· x
⎫
⎬
⎭
, (3.3.17)
where ˜η =1 + η. Next, with the present choice of the density function, we obtain the
following values for the partial derivatives:
∂n
0
(r)
∂η
= n
l
, and
∂n
0
(r)
∂A
i
= n
l
e
iK
i
· r
. (3.3.18)
Using the results (3.3.18) in the expression (3.3.12) the kernel #
αβ
ij
is obtained in a purely
diagonal form,
#
αβ
ij
=
⎧
⎨
⎩
c
0
4
δ
αβ
, for γ
i
,γ
j
≡ η,
c
n
4
δ
ij
δ
αβ
, for γ
i
≡ A
i
,γ
j
≡ A
j
.
(3.3.19)
We represent here c(k) as the spatial Fourier transform of the direct correlation c(r), which
is assumed to be translationally invariant for the uniform liquid state. We have used here
the notation c
n
= n
l
c(K
n
) for the liquid density n
l
and the double-primed quantity, i.e.,
c
n
, is the corresponding second derivative of c
n
with respect to the wave vector K
n
.Itis
useful to note the following relations in this respect:
n
l
dr c(r)rr =−n
l
I
d
2
c(K )
dK
2
K =0
=−c
0
I, (3.3.20)
n
l
dr c(r)e
iK
n
· r
rr =−n
l
ˆ
K
n
ˆ
K
n
d
2
c(K )
dK
2
K
n
=−
ˆ
K
n
ˆ
K
n
c
n
, (3.3.21)
where
ˆ
K
n
is the unit vector along K
n
. The subscript 0 in c
0
denotes the same quantity
for zero wave vector. Note that with the present choice (3.3.3) for the density function the
kernel #
αβ
ij
is not only diagonal but also determined solely in terms of the static correlation
of the uniform liquid state.
The total difference of the grand potentials between the inhomogeneous and homoge-
neous states is obtained by evaluating the RHS of (3.3.13) in this case as
β= β
u
+
n
l
4
dr
c
0
|∇η(r)|
2
+
i
c
i
{
ˆ
K
i
· ∇A
i
(r)}
2
. (3.3.22)
3.3 The density-functional approach 143
Using the above expression for in (3.3.15), a set of ordinary differential equations for
the order parameters {η, A
i
} is obtained. Differentiating (3.3.22) with respect to η and A
i
,
respectively, gives
V
−1
dx
1
ln
1 + η +
m
A
m
e
iK
m
· x
1
= c
0
η −βU
0
, (3.3.23)
V
−1
dx
1
e
iK
i
· x
1
ln
η +
m
A
m
e
iK
m
· x
1
= c
i
A
i
− βU
i
. (3.3.24)
Note that the LHS of each of the above equations follows from the corresponding results
for the uniform system with local order parameters {η, A
i
}. We have used for simplification
in the RHS of (3.3.23) the following definitions for U
0
and U
i
:
βU
0
(x) =
c
0
2
∇
2
η(x), βU
i
(x) =
c
i
2
(
ˆ
K
i
· ∇)
2
A
i
(x). (3.3.25)
U
0
and U
i
are the Fourier components of an external field U (x) that maintains the density
profile in a uniform system with the space-dependent order-parameter fields {η(x), A
i
},
βU (x) = βU
0
+
i
βU
i
e
iK
i
· x
(3.3.26)
=
c
0
2
∇
2
η(x) +
i
e
iK
i
· x
c
i
2
(
ˆ
K
i
· ∇)
2
A
i
(x). (3.3.27)
For the special case of the spherical nucleus considered here the order parameters η and
A
i
are merely radial functions and the defining relations for U
0
and U
i
in (3.3.25) are
expressed with the following differential equations:
βU
0
(r) =
c
0
2
d
2
dr
2
+
2
r
d
dr
η(r ), (3.3.28)
βU
i
(r) =
c
i
6
d
2
dr
2
+
2
r
d
dr
A
i
(r). (3.3.29)
In (3.3.28) we have averaged over the angular dependence present in (3.3.26) due to
the directional operator (
ˆ
K
i
· ∇)
2
. These two equations are coupled to the two equations
(3.3.23) and (3.3.24) obtained above. In order to solve for the optimum density distribution
in the critical nucleus corresponding to the saddle point in the space of the density function,
the last two equations are expressed as a single relation,
ln
&
1 + η +
m
A
m
e
iK
m
· x
1
'
= c
0
η −βU
0
+
i
e
iK
i
· x
1
{c
i
A
i
− βU
i
}. (3.3.30)
Equivalently, we have,
1 + η +
m
A
m
e
iK
m
· x
1
= e
c
0
η−βU
0
exp
i
{c
i
A
i
− βU
i
}e
iK
i
· x
1
. (3.3.31)
144 Crystal nucleation
Hence the order parameters η and A
i
are obtained in terms of the self-consistent equations
1 + η =
e
c
0
η−βU
0
V
dx
1
exp
i
{c
i
A
i
− βU
i
}e
iK
i
· x
1
(3.3.32)
and
A
m
=
e
c
0
η−βU
0
V
dx
1
e
iK
m
· x
1
exp
i
{c
i
A
i
− βU
i
}e
iK
i
· x
1
. (3.3.33)
The coupled set of equations (3.3.26), (3.3.27), (3.3.32), and (3.3.33) constitutes the den-
sity distribution for the critical nucleus. To solve it the required input is the structure factor
for the uniform liquid.
The excess free energy per particle of the critical nucleus is then obtained from (3.3.1)
by evaluating for the optimum density distribution. We show in Appendix A3.2 that
is obtained as
n
l
k
B
T
= 4π
∞
0
r
2
D
0
[η, A
i
]−
c
0
4
dη
dr
2
−
i
c
i
12
dA
i
dr
2
dr.
(3.3.34)
D
0
[η(r ), A
i
(r)] represents the local contribution to the excess free energy. The latter is
obtained from the corresponding functional for a uniform system characterized by {η, A
i
}.
ThelasttwotermsontheRHSof(3.3.34) represent the nonlocal contribution due to the
variation of the order-parameter field.
In the first application of the density-functional method to the nucleation problem
Harrowell and Oxtoby (1984) solved the model equations for a simplified case by set-
ting c
0
= 0 and considered only a single RLV, K
m
, corresponding to the peak of the liquid
structure factor. It follows from eqns. (3.3.28) and (3.3.29), respectively, that βU
0
= 0 and
βU
1
(r) =
c
m
6
d
2
dr
2
+
2
r
d
dr
A
1
(r). (3.3.35)
η is now an algebraic function of A
1
as follows from (3.3.32). D
0
exhibits two minima
as a function of the order parameter A
1
, corresponding to the crystalline and uniform
liquid states, respectively. The liquid minimum exists down to the lowest supercooling
temperature, indicating the absence of a spinodal point in the present model. From the r
dependences on the order parameters η and A
1
for the critical nucleus corresponding to
various temperatures below the freezing point it follows that the crystal–liquid interface
is a few atomic diameters thick and that this is essentially independent of supercooling.
With lowering of the temperature the order parameter A
1
remains essentially constant near
r =0, being equal to the corresponding value for the solid state. This indicates that the
center of the nucleus has properties characteristic of the bulk solid. On the other hand,
the order parameter η in the same region steadily decreases with supercooling. Thus for
the critical nucleus the structure and average density, characterized by A
1
and η, respec-
tively, do not change at the same point in the interface. From the liquid side the crystalline
3.3 The density-functional approach 145
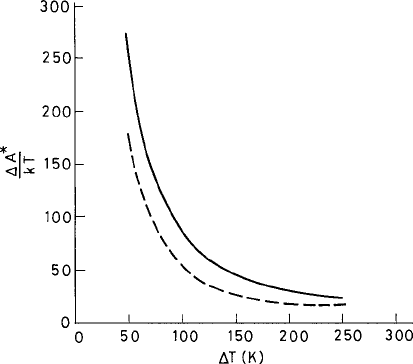
Fig. 3.6 The free-energy barrier A
∗
(in units of k
B
T ) to nucleation vs. the extent of undercooling
T , from the DFT (solid) and CNT (dashed) with the capillary approximation. From Harrowell and
Oxtoby (1984).
c
American Institute of Physics.
structure appears first in the interface region and the density changes more inside the
nucleus. It is energetically less favorable to compress a disordered liquid and once the
order appears it is easier to compress. The free energy of the critical nucleus is computed
from the formula (3.3.34) in the present calculation. The result is shown in Fig. 3.6.The
corresponding result according to the CNT is estimated from the formula (3.1.17).Here
G
v
(noted as A in Fig. 3.6) was computed using the DFT model. For the surface free
energy γ
s
the DFT results for the planar interface were used. Figure 3.6 shows that the
dependence of the free energy on the supercooling as predicted in the CNT is qualitatively
the same as that in the present DFT model, though the former somewhat underestimates
the free energy of the critical nucleus. In the CNT the radius of the corresponding critical
nucleus is estimated from (3.1.18). The DFT approach by construction does not assume
any sharp interface. However, a radius for the critical nucleus can be defined (Harrowell
and Oxtoby, 1984) as the distance at which A
1
falls to half of its value at the center of the
nucleus. Such an estimate indicates that the CNT somewhat underestimates the radius of
the critical nucleus in comparison with that predicted from the DFT.
3.3.3 The weighted-density-functional approach
The DFT model for the critical nucleus using a simple Ramakrishnan–Yussouff-type per-
turbation expansion for the free energy was subsequently improved using more advanced
techniques like the modified weighted-density-functional approximation (MWDA) by Shen
and Oxtoby (1996a, 1996b). We have already discussed the MWDA calculation of the
bulk free energy in the previous chapter. Here we will consider its specific application for

146 Crystal nucleation
identifying the critical nucleus. This involves the identification of a set of order-parameter
fields {γ
i
(x)} characterizing the density function for the nucleus forming in the melt.
Let us first identify a suitable set of parameter fields in defining the density function. The
inhomogeneous density is expressed in terms of the usual RLV expansion in the following
form:
n
0
(x) =¯n
0
+ n
s
m
μ
m
e
iK
m
· x
, (3.3.36)
where ¯n
0
denotes the average density, n
s
is the average density of the solid and the μ
m
denote the Fourier coefficients corresponding to the RLV K
m
. Note that the amplitude
μ
m
is dependent only on the magnitude of K
m
. The average density ¯n
0
changes from the
density of the liquid (n
l
) to that of the solid (n
s
). The above expansion (3.3.36) in the RLVs
can also be identified with a simple parametrization of the inhomogeneous density as a
sum of the Gaussian density profiles centered at the lattice sites R
i
,
n
0
(x) =
#
2a
3
0
¯n
0
$(
α
π
)
3/2
{R
i
}
e
−α(x−R
i
)
2
, (3.3.37)
where the inverse of α gives the width of the density profile. a
0
is the lattice constant
with the conventional unit cell being in the shape of a cube of side 2a
0
and having four
particles in each cell for the f.c.c. lattice structure. 2a
3
0
is the volume of the primitive
unit cell such that the factor 2a
3
0
¯n
0
on the RHS of (3.3.37) represents the dependence
of the density function on the average density ¯n
0
. The two parametrizations (3.3.36) and
(3.3.37) are equivalent to each other (see the discussion accompanying eqns. (2.2.15) and
(2.2.16) in Chapter 2) with the choice μ
m
= exp
!
−K
2
m
/(4α)
"
for the Fourier amplitudes.
Therefore defining the density function in terms of Gaussian density profiles is equivalent
to parametrizing all the RLV amplitudes μ
m
(m > 1) in (3.3.36) in terms of the amplitude
μ
1
corresponding to the smallest nonzero K
1
,
μ
m
= (μ
1
)
(K
m
/K
1
)
2
. (3.3.38)
Therefore the two order parameters ¯n
0
and μ
1
completely define the inhomogeneous den-
sity function. The grand potential D for the bulk system is a functional of the inhomoge-
neous density function characterized by a pair of values for the parameters {¯n
0
,μ
1
}.For
a Lennard-Jones system free energy is obtained as a sum of two different contributions.
The Lennard-Jones interaction potential (see eqn. (1.2.117) for its definition) is divided
into a short-range repulsive part and a long-range attractive part. The contribution to the
free energy from the repulsive part of the interaction is computed in terms of an equiva-
lent hard-sphere system using the MWDA as described in Chapter 2. (See eqns. (2.2.13)
and (2.2.20).) The contribution to the free energy from the attractive part of the interac-
tion is obtained in a mean-field approximation involving the pair correlation function for
the uniform liquid state. For example, Shen and Oxtoby (1996a) obtained for a Lennard-
Jones system at T = 0.6 the stable bulk solid phase for ¯n
0
= 1.04 with the parameter
3.3 The density-functional approach 147
μ
1
= 0.85 and the metastable bulk-liquid minimum for ¯n
0
= 0.89 and μ
1
= 0. All densi-
ties and temperatures for the Lennard-Jones system were expressed in dimensionless units
(see Chapter 1).
Now let us consider the technique being applied to the critical nucleus. The spherically
shaped critical nucleus nucleating from the melt is described in terms of the two order
parameters {¯n
0
,μ
1
}, which are treated as functions of the radial distance r from the cen-
ter. This form is similar to (2.4.32) used in the previous chapter for studying interfaces
using the weighted-density-functional model. The spatial dependence of the order param-
eters fields {¯n
0
,μ
1
} is controlled by the corresponding Euler–Lagrange equations (3.3.14)
obtained from the minimization of the grand potential. The optimum order-parameter
functions therefore follow from the solution of the simple differential equations given by
(3.3.15) as discussed above. The boundary conditions in this case are chosen in accord
with the requirement that at the center of the nucleus (r =0) and at large distance (r →∞)
the order parameters are equal to their characteristic values for the stable (crystalline)
and metastable (liquid) phases, respectively. The order-parameter profiles for the critical
nucleus obtained in the present case (Shen and Oxtoby, 1996a) are qualitatively sim-
ilar to those obtained in the model described above, with the Ramakrishnan–Yussouff
(Ramakrishnan and Yussouff, 1979) functional for the free energy. The results for the
optimum density profile for the critical nucleus obtained with the MWDA are shown in
Fig. 3.7. The density profile (¯n
0
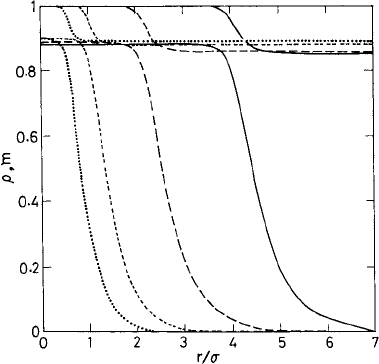
) decays more rapidly across the interface than the structural
order parameter (μ
1
). However, the results obtained from the Ramakrishnan–Yussouf (RY)
free-energy functional and the MWDA approach follow opposite trends when compared
Fig. 3.7 The density (ρ) and structural order parameter (m), denoted by n
s
and μ
1
, respectively,
in the text, for the crystal nucleus at temperatures T =75 K (solid), T =70 K (long-dashed line),
T =60 K (short-dashed line), and T =50 K (dotted line). Reproduced from Shen and Oxtoby
(1996b).
c
American Institute of Physics.
148 Crystal nucleation
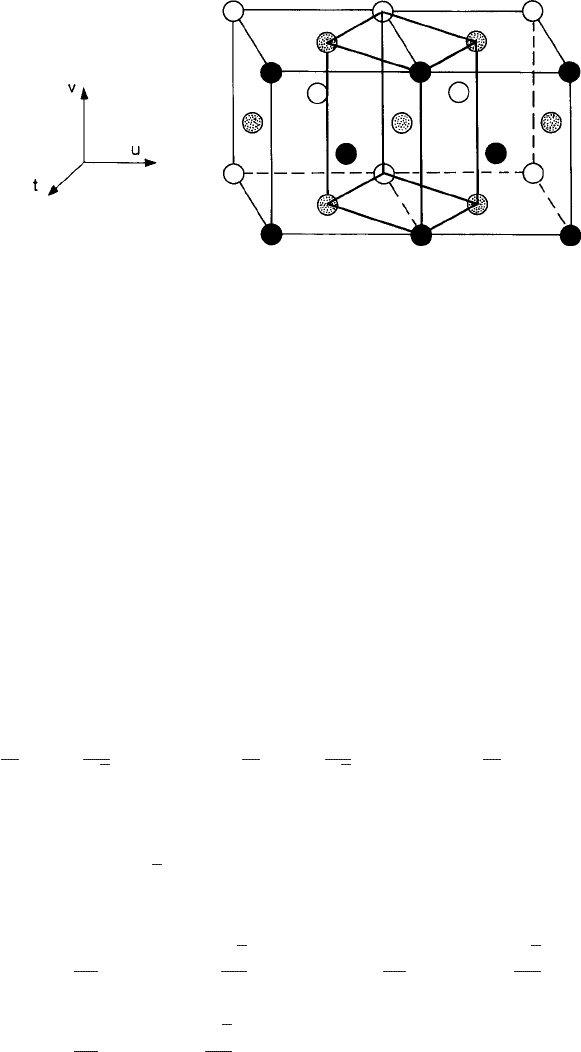
Fig. 3.8 An illustration of Bain’s distortion. Two f.c.c. cells (each of size a) outlined with thin black
lines are shown. v, t,andu form an orthogonal triad of vectors. Along the t axis, the atoms are shown
with open circles on the t = 0 plane, dot-filled circles on the t = a/2 plane, and solid circles on the
t = a plane. With Bain’s distortion along the v axis, the cube outlined with thick dark lines becomes
b.c.c. From Shen and Oxtoby (1996a).
c
American Physical Society.
with the corresponding CNT predictions. The free energy of the critical nucleus and its
size obtained with the CNT are less than the corresponding results from the DFT formu-
lated with the RY expansion. On the other hand, the CNT results are greater than the
corresponding quantities obtained in the MWDA model.
The nature of the critical nucleus is further analyzed by using the MWDA model (Shen
and Oxtoby, 1996b) to study the free-energy landscape for the system corresponding to
different possible crystalline structures. In this extension of the theory the parameter space
for the inhomogeneous density function includes a new order parameter, χ, which refers to
the symmetry of the crystal lattice. χ changes in a continuous manner monitoring Bain’s
distortion (Bain, 1924)(seeFig. 3.8) in the lattice structure, which is defined in terms of
the following set of real-space vectors:
a
1
=
a
0
2
ˆκ
1
+
χ
√
2
ˆκ
2
, a
2
=
a
0
2
ˆκ
3
+
χ
√
2
ˆκ
2
, a
3
=
a
0
2
( ˆκ
1
+ˆκ
3
), (3.3.39)
where ˆκ
1
, ˆκ
2
, and ˆκ
3
represent an orthogonal triad of basic vectors (indicated in Fig. 3.8
as v, t, and u, respectively). The lattice symmetry corresponds to b.c.c. and f.c.c. for χ
taking the values 1 and
√
2, respectively. The RLVs corresponding to (3.3.39) are obtained
as linear combinations of the following set of basis vectors:
b
1
=
2π
a
0
−ˆκ
3
+ˆκ
1
+
√
2
χ
ˆκ
2
, b
2
=
2π
a
0
ˆκ
3
−ˆκ
1
+
√
2
χ
ˆκ
2
,
b
3
=
2π
a
0
ˆκ
3
+ˆκ
1
−
√
2
χ
ˆκ
2
. (3.3.40)
3.3 The density-functional approach 149
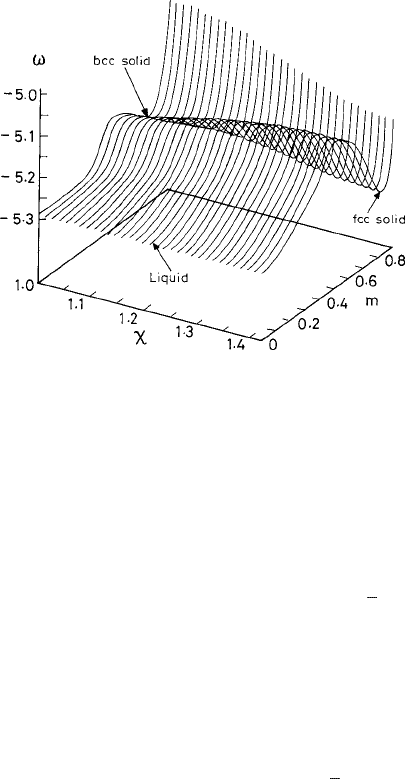
Fig. 3.9 The landscape of the local grand-canonical free-energy density (ω) at coexistence tempera-
ture T =83.1Kand ¯n
0
= 0.89. The symbol m denotes the parameter μ
1
used in the text. The three
minima shown correspond to (a) the stable liquid with m =0, (b) the stable f.c.c. solid with χ =1.414
and m =0.818, and (c) the metastable b.c.c. solid with χ =1.0andm =0.750. The metastable b.c.c.
solid creates a saddle point near χ =1.0. From Shen and Oxtoby (1996a).
c
American Physical
Society.
At fixed values of the density ¯n
0
and temperature T , the landscape for the local grand-
canonical free-energy density F in the parameter space of χ and μ
1
exhibits (Shen and
Oxtoby, 1996b) a minimum for a stable f.c.c. structure
χ =
√
2
as well as one for a
metastable b.c.c. structure (χ =1). This landscape is displayed in Fig. 3.9 for ¯n
0
=0.89
and coexistence temperature T =83.1 K. In this extended description for studying the
critical nucleus, the order parameters which characterize the nucleus are given by the
set {¯n
0
,χ,μ
1
}. As the liquid is undercooled below the coexistence temperature, study of
the MWDA model obtains the profile for χ(r ) corresponding to the critical nucleus. The
spatial dependence of χ represents a substantial b.c.c. character (χ =1) of the nucleus
near the interface changing over to f.c.c. structure
χ =
√
2
towards the center. With
increased supercooling the b.c.c. character spreads further inside, until at temperature 50 K
the nucleus is essentially all b.c.c. We note at this point that the computer studies of nucle-
ation of the crystal from a melt (ten Wolde et al., 1996) discussed next also suggest that in
the critical nucleus the interfacial region displays strong signatures of the b.c.c. symmetry
while near the center there is f.c.c. symmetry. This changes the surface free energy of the
nucleus. For a small-sized nucleus even the nature of the crystal symmetry near the center
is changed and is more b.c.c. like. Such a metastable b.c.c. phase has also been seen (Löser
et al., 1994) in experiments on rapidly cooled liquid metals whose stable crystalline phase
is f.c.c. An earlier theoretical study by Klein and Leyvraz (1986) using a field-theoretic
model of the phase transition also suggested the possibility of a metastable b.c.c. phase in
the first-order phase transition.
150 Crystal nucleation
3.4 Computer-simulation studies
The phenomenon of nucleation of a crystal from the melt has been studied using real exper-
iments as well as computer simulations. There are some obvious problems in making an
accurate study of the evolution of the nucleation process experimentally. It is difficult to
study in an experiment the formation of the critical nucleus since the crystallite spends
only a microscopic time in this stage of its evolution as the top of the potential barrier
is approached. Computer simulation, which is a useful tool for studying the microscopic
description of the fluid, has also been used for studying the nucleation process. In the
present section we discuss some of the recent developments in the study of the nucleation
process using such numerical methods. The straightforward approach of simulation for
this problem is to supercool the liquid below the freezing point and watch the formation
of the crystal nucleus. The free-energy barrier to the nucleation process falls as the inverse
square of the supercooling (see eqn. (3.1.17) for the nucleation barrier in the CNT) so that
at large supercooling the barrier is low. Mandell et al. (1976) simulated a small Lennard-
Jones system of 128 particles to study the nucleation process by monitoring the structure
factor. Honeycutt and Andersen (1984) investigated the effect of system size on nucleation.
Assuming the nucleation rate to be proportional to the volume, the observed nucleation rate
in the simulation is expected to grow with the number of particles. The observed nucleation
rate, however, decreased with the number of particles N, possibly indicating that the phe-
nomenon being observed was not homogeneous nucleation. The simulations were strongly
influenced by the periodic boundary conditions used in standard molecular-dynamic meth-
ods. Subsequent work by Swope and Andersen (1990) considered nucleation with one
million Lennard-Jones particles. The study shows that, although b.c.c. and f.c.c. crystalline
phases are formed in the early stage of nucleation, only the f.c.c. nuclei grow beyond the
post-critical stage. In all these cases the nucleation is studied at deep supercooling (more
than 40% below melting).
The free-energy barrier to critical-nucleus formation is not small enough unless the sys-
tem is deeply supercooled and as a consequence the rate of nucleation is too low to be
observed. For example, at 20% supercooling in a typical simulation of 10
6
or so particles
one has to wait up to 10
30
simulation steps to observe one nucleation event (ten Wolde
et al., 1996). Thus without deep supercooling the “brute-force” method for studying crys-
tallization with computer simulations fails. At least 50% supercooling is needed in order
to observe crystal formation over the time scale of a simulation. Studying the nucleation
of the crystal from a melt using computer simulations at low undercooling (close to the
freezing point) when the free-energy barrier is large requires the development of special
techniques. In this regard it is useful to identify suitable reaction coordinates characteriz-
ing the crystallinity of the system. The free energy for the nucleus is obtained from the
probability of occurrence of the corresponding value of the reaction coordinate, and this
probability is computed through efficient sampling of the configuration space. By locating
the maximum in the free-energy barrier against the reaction coordinate the critical nucleus
is identified.
