Das Sh.P. Statistical Physics of Liquids at Freezing and Beyond
Подождите немного. Документ загружается.

3.1 Classical nucleation theory 121
G
i
∗
=
16πγ
3
s
3|G
v
|
2
. (3.1.17)
By equating the volume of i
∗
v
m
of the critical nucleus to the spherical volume of
(4/3)π R
∗
3
, we obtain for the radius of the critical nucleus
R
∗
=
2γ
s
|G
v
|
.
(3.1.18)
In this case the constants A and B in eqn. (3.1.13) are given by
A =
aγ
s
k
B
T
, (3.1.19)
B =
v
m
|G
v
|
k
B
T
. (3.1.20)
The maximum at n = n
∗
in the free-energy barrier and the corresponding radius R
∗
of the
cluster represent a limiting situation. Note that the above expression (3.1.17) for the free
energy computed from thermodynamic consideration of reversible processes represents
the minimum work needed to form the cluster of i monomers. Furthermore, it is also a
maximum with respect to the variation of the number of monomers i. The critical nucleus
is therefore in a state of unstable equilibrium. A cluster of radius less than R
∗
(having
fewer than n
∗
monomers) is subcritical and shrinks spontaneously. On the other hand,
clusters with radius greater than R
∗
(having more than n
∗
monomers) are supercritical and
increasingly likely to grow.
The classical nucleation theory (CNT) described above involves making approximations
that are questionable. First, it assumes that even a cluster of molecular size has bulk-like
properties at the center. Second, the clusters of monomers forming the nucleus are defined
with a sharp interface between the two phases. More specifically, the CNT uses a sur-
face free energy for the nuclei that is the same as that of the infinite planar interface,
whereas the actual interface for the small nucleus is in fact sharply curved. Thus, while the
basic ideas used in constructing the CNT capture the essential physics, there is a need for
improvement.
3.1.2 The nucleation rate
Since the supercooled liquid can be maintained in the metastable liquid state well below
the freezing point, considerations of the dynamics become important in understanding the
process of crystal formation from the melt. This dynamic process generally involves clus-
ters of various sizes of the new phase gaining or losing more molecules from the bulk
liquid. This process is idealized in terms of rate equations that are local in time, control-
ling the loss or gain of particles from the clusters of different sizes. At the simplest level,
namely the description of the rate process of growth (of small clusters), the role of mem-
ory is ignored. This essentially amounts to ignoring the correlation in the events which

122 Crystal nucleation
cause the growth or decay of the clusters. The temperature is assumed to remain constant
in the steady state as the cluster grows or decays. This implies that the clusters are in ther-
mal equilibrium while the numbers of particles belonging to them change. The latent heat
evolved in the process of phase change does not affect this condition. We also assume that
the clusters grow or decay by attaching or losing single units or monomers. In the fol-
lowing we consider two cases of nucleation of the crystal: (a) transition from a fluid to a
solid, i.e., crystallization from a low-concentration solution; and (b) crystallization from
a high-density melt, whereby the growth of the nucleus takes place through an activated
process.
Nucleation from low monomer concentration
Let the number of n-particle clusters of the crystalline phase present in the parent phase at
time t be denoted by N
n
(t).If
n
+
and
n
−
denote the rates of monomer addition and loss
from the n-particle cluster, respectively, the net number of clusters changing per unit time
from size n to size n + 1 at time t is given by
J
n
(t) =
n
+
N
n
(t) −
n+1
−
N
n+1
(t). (3.1.21)
Equilibrium is reached when N
n
(for all n) no longer changes with time. The equilibrium
distribution is given by J
n
(t) = 0, i.e.,
n
+
N
e
n
=
n+1
−
N
e
n+1
. (3.1.22)
On the other hand, a steady state is reached when the rates of change of the numbers
of differently sized clusters become constant, i.e., independent of time and size. This is
characterized by N
n
(t) ≡ N
s
n
, giving for the steady-state nucleation rate
J
s
≡ J
n
(t) =
n
+
N
s
n
−
n+1
−
N
s
n+1
. (3.1.23)
In the steady state the rate of change of the number of clusters of a particular size N
n
is
independent of time but not necessarily equal to zero. The latter is a particular case corre-
sponding to the equilibrium state. Using the relation (3.1.22), the steady-state nucleation
rate J
s
defined above satisfies the relation
J
s
=
i
+
N
s
i
− N
s
i+1
N
e
i
N
e
i+1
. (3.1.24)
Since the equilibrium density N
e
i
of the clusters of size i is given by eqn. (3.1.2), we obtain
from (3.1.24) the nucleation rate
J
s
=
i
+
N
s
i
− N
s
i+1
exp
3
G
i+1
− G
i
k
B
T
4
. (3.1.25)

3.1 Classical nucleation theory 123
Using the Taylor expansions
N
s
i+1
= N
s
i
+ i
dN
s
i
di
, (3.1.26)
G
i+1
= G
i
+ i
d
di
G
i
, (3.1.27)
and keeping terms up to lowest order, with i = 1, we obtain from (3.1.25) the following
differential equation for N
i
:
i
+
dN
i
di
+ β N
i
d
di
G
i
=−J, (3.1.28)
where we drop from now on the superscript s for the steady state for simplification and
use the notation β = 1/(k
B
T ). Now in general the nucleation rate is defined as
i
+
=
ai
2/3
λ
+
, where λ
+
is the dynamic factor denoting the number of monomers arriving at the
nucleus per unit time per unit area and ai
2/3
is the surface area of the nucleus containing i
monomers. Using this result for
i
+
, we obtain
dN
i
di
+ β N
i
d
di
G
i
=−
¯
Ji
−2/3
, (3.1.29)
where
¯
J = J/(aλ
+
). The formal solution of this equation is obtained by multiplying
(3.1.29) by the factor exp(β G
i
) and then integrating from an initial point i
0
up to i to
obtain
N
i
= e
−βG
i
−
¯
J
i
i
0
dw e
βG
w
w
−2/3
+ N
i
0
e
βG
i
0
, (3.1.30)
where we define the number of clusters N
i
= N
i
0
for i = i
0
. Now we can further simplify
the above expression for N
i
by choosing i
0
to be small enough relative to the critical
nucleus (i = i
∗
) that the number of critical nuclei can be expressed in terms of the number
of monomers N per unit volume as given in eqn. (3.1.2), i.e., N
i
0
= N exp(β G
i
0
). Hence
N
i
= e
−βG
i
−
¯
J
i
i
0
dw e
βG
w
w
−2/3
+ N
. (3.1.31)
Now, for the number of clusters N
i
with a very large number of particles we should
have lim
i→∞
N
i
= 0. On the other hand, since G
i
reaches a maximum at an inter-
mediate i = i
∗
and falls to a negative value for large values of i,wemustalsohave
lim
i→∞
exp(−βG
i
) =∞. Therefore, in the large-i limit of eqn. (3.1.30), we obtain the
result
lim
i→∞
−
¯
J
i
i
0
dw e
βG
w
w
−2/3
+ N
= 0. (3.1.32)
The nucleation rate J =
¯
Jaλ
+
is therefore given by
J =
Naλ
+
I
∗
, (3.1.33)
124 Crystal nucleation
where the integral I
∗
is obtained as
I
∗
=
∞
i
0
exp
[
βG
n
]
n
−2/3
dn. (3.1.34)
The free energy G
n
is maximum at n = n
∗
and the dominant contribution of the integral
I
∗
in the denominator of the RHS of eqn. (3.1.34) comes from values of n close to n
∗
.
We evaluate this integral by replacing G
n
with a Taylor expansion up to quadratic terms,
G
n
= G
n
∗
−
1
2
*
*
G
n
∗
*
*
(n − n
∗
)
2
, (3.1.35)
and take the limit over n from n = n
∗
to ∞ since the contribution from n = i
0
to n = i
∗
is
negligible. This gives
I
∗
= n
∗
−2/3
2πk
B
T
*
*
G
n
∗
*
*
1/2
exp
G
n
∗
k
B
T
. (3.1.36)
The steady-state nucleation rate J is obtained as
J = N
n
∗
+
*
*
G
n
∗
*
*
2πk
B
T
1/2
exp
−
G
n
∗
k
B
T
, (3.1.37)
where
n
∗
+
= (an
∗
2/3
)λ
+
represents the number of monomers that hit the nucleus of sur-
face area A
0
= an
∗
2/3
per unit time. The nucleation rate is thus expressed in the form of
the general expression
J = J
0
exp
!
−G
n
∗
/(k
B
T )
"
. (3.1.38)
The prefactor J
0
in the above expression for the nucleation rate is proportional to the num-
ber of monomers N per unit volume of the mother phase from which the nucleating cluster
is formed. J
0
also has the following two components.
(i) The dynamic factor
n
∗
+
represents the number of monomers attaching to the nucleus
per unit time. It is given by A
0
λ
+
, where A
0
is the area of the cluster and λ
+
denotes
the rate of arrival of monomers on the cluster per unit area.
(ii) The statistical prefactor Z , called the Zel’dovich factor (Zel’dovich, 1943),
Z =
*
*
G
n
∗
*
*
2πk
B
T
1/2
, (3.1.39)
is determined by the shape of the free-energy surface. Using the expression (3.1.13)
for G
n
∗
and taking the second derivative gives
*
*
G
n
∗
*
*
=|μ|/(3n
∗
). Hence the
Zel’dovich factor is expressed in terms of the chemical potential difference as
Z =
μ
6πk
B
Tn
∗
1/2
. (3.1.40)
3.1 Classical nucleation theory 125
The expression for the nucleation rate J is now obtained in the form
J = N
n
∗
+
μ
6πk
B
Tn
∗
1/2
exp
−
G
n
∗
k
B
T
.
(3.1.41)
Note that, in the above expression for the nucleation rate per unit volume per unit time,
N denotes the number of monomers in unit volume. In order to further simplify the
above expression, we need to estimate the dynamic prefactor present in the expression
for the nucleation rate.
In the case of transition from a dilute fluid to a solid, the dynamic prefactor is determined
by taking the ideal-gas approximation for the monomers hitting the surface of the nucleus.
The number of particles λ
+
falling on the surface per unit time is obtained from the simple
kinetic theory of ideal-gas particles in terms of the equilibrium pressure P
e
as
P
e
= λ
+
∞
0
dv φ(v)(mv)(2π)
π/2
0
d(cos θ)cosθ
= λ
+
;
(2πmk
B
T ), (3.1.42)
where φ(v) denotes Maxwell’s velocity distribution for the ideal gas. Using the ideal-gas
equation of state P
e
= N
e
k
B
T and the monomer concentration N
e
at pressure P
e
is reduced
from its value N at the pressure P of nucleation by a factor S, where S = P/P
e
. Thus we
obtain for
n
∗
+
for the nucleus of area A
0
n
∗
+
=
k
B
T
2πm
1/2
N
S
A
0
. (3.1.43)
For a spherically shaped critical nucleus the area is A
0
= 4π R
∗
2
with the radius R
∗
given
by (3.1.18).Using(3.1.15), (3.1.16), and (3.1.10), we obtain for the nucleation rate J the
result
J =
9
2γ
s
πm
N
2
v
m
S
exp
−
16πv
2
m
γ
3
s
3(k
B
T )
3
(ln S)
2
,
(3.1.44)
in which the chemical potential difference μ between the two phases is substituted in
terms of the supersaturation as μ = ln S/(k
B
T ).
Crystallization from the melt
For nucleation from the condensed phase, the dynamic prefactor in the nucleation rate is
approximated using the reaction-rate approach (Turnbull and Fisher, 1949). In this model
the process of addition of the monomer to the nucleus is assumed to take place through
transition into an intermediate activated state that represents an unstable maximum in the
free energy as shown schematically in Fig. 3.2. This maximum at A corresponds to the
nucleus in the activated state and is intermediate between the two minima corresponding
to the nuclei with i and i + 1 particles, respectively. These two minima correspond to

126 Crystal nucleation
Fig. 3.2 A schematic representation of the free energy of activation corresponding to the process of
adding a monomer to a nucleus.
the states of the nucleus before and after the monomer attachment. We assume that the free
energies of formation of the nuclei corresponding to i , A, and i +1 are respectively denoted
by G
i
, G
A
, and G
i+1
. The free-energy difference between the states corresponding
to A and i is given by
f
∗
i
≡ G
A
− G
i
= f
∗
+
1
2
(G
i+1
− G
i
)
= f
∗
+
i
2
dG
i
di
, (3.1.45)
where we use the definition f
∗
= G
A
−(G
i+1
+G
i
)/2. Similarly, the free-energy
difference between the nuclei corresponding to A and i + 1is
f
∗
i+1
≡ G
A
− G
i+1
= f
∗
−
1
2
(G
i+1
− G
i
)
= f
∗
−
i
2
dG
i
di
. (3.1.46)
The steady-state rate of the nucleation J in this case is obtained in a manner similar to that
used in formulating eqn. (3.1.23), as a sum of two opposite processes. These respectively
correspond to rate processes (at temperature T ) of crossing barriers f
∗
i+1
and f
∗
i
.The
nucleation rate is obtained as
J = ai
2/3
ν
0
!
exp
−βf
∗
i
N
i
− exp
−βf
∗
i+1
N
i+1
"
, (3.1.47)
where ν
0
= k
B
T /h is the primary frequency corresponding to the thermal energy k
B
T and
ai
2/3
is the surface area of the nucleus with i monomers. Using the Taylor expansions as
3.1 Classical nucleation theory 127
presented in eqn. (3.1.26) and keeping terms of up to lowest order in i, we obtain the
following differential equation for the variation of N
i
:
dN
i
di
+ β N
i
d
di
G
i
=−
¯
Ji
−2/3
, (3.1.48)
where
¯
J = J/{aν
0
exp[−f
∗
/(k
B
T )]exp[− f
∗
/(k
B
T )]}. Note that (3.1.48) is identical
to eqn. (3.1.29) in the earlier section except that now the rate of monomer attachment per
unit area λ
+
is replaced by ν
0
exp[−f
∗
/(k
B
T )]. Hence the nucleation rate J in this case
is obtained from (3.1.41) in the form
J = N ν
0
n
∗
2/3
μ
6πk
B
Tn
∗
1/2
exp
−
G
n
∗
+ f
∗
k
B
T
, (3.1.49)
where N denotes the number of monomers per unit volume. In the above expression for the
nucleation rate the exponent term involving the free-energy barrier G
n
∗
is further sim-
plified. Using (3.1.17), G
n
∗
is expressed in terms of μ ≡ v
m
G
v
, where the chemical
potential difference μ between the two phases is related to the molar enthalpy h and
the molar entropy s through the thermodynamic relation
μ = h − T s = s(T
m
− T ). (3.1.50)
We have used here the result that close to the melting temperature T
m
, h = T
m
s.Using
(3.1.50) in (3.1.17) and the definition μ = v
m
G
v
, we obtain for the free-energy barrier
G
i
∗
=
16πv
2
m
γ
3
s
3 s
2
(T
m
− T )
2
. (3.1.51)
The exponential factor in (3.1.49) simplifies further with the definition a =
36πv
2
m
1/3
and the expression (3.1.16) for i
∗
. We obtain the following result for the nucleation rate:
J = 2N v
m
(k
B
T γ
s
)
1/2
h
exp
−
f
∗
k
B
T
exp
−16πγ
3
s
v
2
m
3k
B
T s
2
(T
m
− T )
2
(3.1.52)
for the spherical nucleus. From eqn. (3.1.51) it follows that the free-energy barrier G
v
to
be surmounted for the critical nucleus formation falls sharply as the undercooling of the
liquid is increased, i.e., G
v
∝ T
−2
R
, where the extent of undercooling is defined as
T
m
− T
T
m
= 1 −
T
T
m
≡ 1 − T
R
≡ T
R
(3.1.53)
in terms of the scaled temperature T
R
= T /T
m
.AtT = T
m
the barrier G
v
is infinite,
making the nucleation rate zero. To initiate the formation of the crystal seed the temperature
of the liquid has to be well below the freezing point T
m
, i.e., T
R
of the liquid has to
be large. We now consider this dependence of the nucleation process on the extent of
undercooling.
128 Crystal nucleation
Dependence on undercooling
The temperature dependences of the two exponential factors in the above expression for the
nucleation rate J result in opposite trends. The first factor, exp[−f
∗
/(k
B
T )] decreases
with lowering of T . On the other hand, the second exponential factor increases as T
decreases, since the difference T = T
m
− T grows with lowering of the temperature.
In fact, the latter factor in the exponent produces the dominant effect and for an increase
of T by a few degrees the exponential factor in the nucleation rate J changes by orders
of magnitude. To see this, we rewrite eqn. (3.1.52) in the form
J = J
0
exp
−
G
i
∗
k
B
T
≡ J
0
exp
−
a
0
α
3
ζ
T
R
(T
R
)
2
(3.1.54)
using the definitions
α =
v
2/3
m
γ
s
h
,ζ=
h
k
B
T
m
. (3.1.55)
The constant a
0
= 16π/3 for a spherical droplet. In order to obtain this value of J
0
the
energy barrier f
∗
has to be estimated. J
0
is inversely related to the time τ
J
takenbya
monomer to cross this barrier at the interface and become attached to the nucleus. Let us
write J
0
in the form C
N
/τ
J
, where C
N
is a constant, and hence the nucleation rate J is
J =
C
N
τ
J
exp
−
G
i
∗
k
B
T
. (3.1.56)
τ
J
is inversely proportional to the diffusion constant D
s
and hence directly proportional
to the viscosity if we assume that the Stokes–Einstein law holds. A lower value of the
viscosity implies a shorter passage time and hence a lower barrier. We ignore the temper-
ature dependence of the viscosity of the undercooled liquid at first to keep J
0
within its
upper bound. The prefactor J
0
is estimated as 10
32
(Turnbull and Fisher, 1949), taking
the viscosity of the liquid as 10
−2
P. The effect of undercooling on the crystallization is
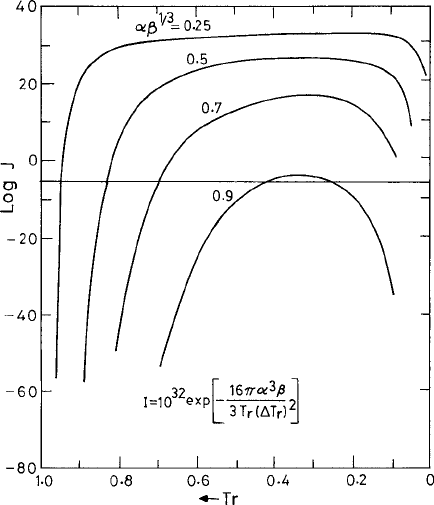
demonstrated through a plot of the nucleation rate with respect to the scaled temperature
T
R
.ThisisshowninFig. 3.3 (Turnbull, 1969). The curves are fixed by choosing constant
values of the parameter αζ
1/3
(≡
0
, say). Close to T
m
, i.e., at low undercooling (T
R
≈ 1),
the curve for J corresponding to a fixed
0
rises steeply. The curve reaches a maximum at
T
R
= 1/3 and falls off to zero as we approach T = 0. In order to be able to observe crys-
tallization experimentally, the rate J must exceed some minimum practical value, say one
nucleus per cubic meter per second (10
−6
cm
−3
s
−1
). This threshold value for J is shown
by a horizontal line in Fig. 3.3. We notice in this plot of Fig. 3.3 that at low undercooling or
close to T
m
, corresponding to most values of the parameter
0
, the nucleation rate J falls
much below the horizontal line and cannot be observed experimentally. In this case for
0
> 0.9 the crystallization practically never occurs through a homogeneous nucleation
process. On the other hand, crystallization is almost impossible to suppress for
0
< 0.25.
For intermediate values of
0
crystallization can remain suppressed only up to moderate
undercooling.
3.1 Classical nucleation theory 129
Fig. 3.3 The logarithm of the rate J
in cm
−3
s
−1
of homogeneous nucleation in the undercooled
liquid given by (3.1.54) with reduced temperature T
R
for various values of
0
≡ αζ
1/3
. The tem-
perature dependence of the viscosity η present in the prefactor J
0
in (3.1.54) is ignored here. From
Turnbull (1969).
c
Taylor & Francis Group, http://www.informaworld.com
The above analysis is based on two assumptions: first, that the Stokes–Einstein relation
holds; and second, that the temperature dependence of the viscosity can be ignored. Both
are violated in the liquid deeply supercooled below T
m
. A more realistic scenario for the
crystallization process in the supercooled liquid emerges when we take into account (a) the
sharp temperature dependence of the viscosity of the metastable liquid below the freezing
point and (b) the violation of the Stokes–Einstein law. This is considered in the next chapter
in our discussion of the glass transition.
3.1.3 Heterogeneous nucleation
In our discussion of the crystallization process we have so far considered the phenomena
to be taking place in the bulk of the liquid phase. This is generally termed homogeneous
nucleation. In reality, however, the process occurs in a heterogeneous manner around for-
eign particles or impurities distributed in the sample. The surface of the liquid droplet is
also often the source of heterogeneous nucleation. Turnbull (1950) extended the CNT for
homogeneous nucleation to the heterogeneous process by considering the liquid and crys-
talline phases in contact with a solid substrate. The outcome of this is a modification of
130 Crystal nucleation
the free-energy barrier to the nucleation as obtained in the CNT in eqn. (3.1.17) in the
following form:
G
i
∗
=
16πγ
3
s
3|G
v
|
2
f
HN
(θ). (3.1.57)
The factor f
HN
(θ) is the heterogeneous nucleation factor given by
f
HN
(θ) =
1
4
(2 + cos θ)(1 − cos θ)
2
, (3.1.58)
where θ is the contact angle between the substrate and the crystal droplet nucleating from
the liquid phase. This contact angle θ is determined in terms of the surface free energies of
the different phases,
cos θ =
γ
LS
− γ
CS
γ
s
, (3.1.59)
where γ
s
denotes the surface free energy of the liquid–crystal interface while γ
LS
and γ
CS
,
respectively, represent the surface free energies for the liquid–substrate interface and the
crystal–substrate interface. If the substrate on which the nucleation is occurring is identical
to the liquid phase we have (γ
LS
− γ
CS
) →−γ
s
and cos θ → 1, i.e., θ → π. In this limit
we have homogeneous nucleation occurring in the liquid and f
HN
(θ) = 1. The main impli-
cation of the heterogeneous nucleation is therefore represented in terms of a modification
of the nucleation barrier. There is also a corresponding modification in the pre-exponential
term J
0
for the nucleation rate as given in (3.4.7) later.
In a bulk heterogeneous process expectedly the factor modifying the nucleation rate
signifies the fraction of the impurity sites which has been used up. Gránásy and Iglól (1997)
assumed a modified nucleation rate in the following phenomenological form:
J ={ϑ J
0
}exp
− f
HN
G
∗
k
B
T
, (3.1.60)
where ϑ<1 is the fraction of molecules active in nucleation. f
HN
< 1 is the factor repre-
senting the reduction of the potential barrier due to the presence of heterogeneities, as has
already been discussed above. From eqn. (3.1.17) the exponential factor f
HN
[G
∗
/(k
B
T )]
in (3.1.60) is proportional to γ
3
T
/
TG
3
v
, where γ
T
is the surface free energy at temper-
ature T . In the CNT the quantity γ
T
is usually taken to be independent of temperature
and this is rectified in terms of the quantity χ(T ) = γ
T
/γ
s
, which is the ratio of the
temperature-dependent surface tension γ
s
to the bulk surface tension γ
s
between the two
phases. Therefore a plot of log[J/J
0
] against the quantity X ≡ χ(T )
3
/
TG
2
v
is a straight
line with an intercept on the y axis equal to log ϑ. Using estimates of X from the different
types of theories described above, Gránásy and Iglól (1997) computed the corresponding
prediction for ϑ in each case. Since ϑ should be less than unity by definition, its values
obtained for the various theoretical models are useful for judging the appropriateness of
the corresponding theory.
