Das Sh.P. Statistical Physics of Liquids at Freezing and Beyond
Подождите немного. Документ загружается.

A2.3 The weighted-density-functional approximation 111
a defining relation for the density ¯n
0
(x) reduces to the form (2.2.2), with the corresponding
weight function w(x
1
− x
2
) obtained as
w(x − x
;¯n
0
(x)) =
W[x, x
;n
0
(x), ¯n
0
(x)]
6
dx
W[x, x
;n
0
(x), ¯n
0
(x)]
. (A2.3.9)
The weighting function w obtained above automatically satisfies the normalization condi-
tion (2.2.3). Equation (A2.3.9) also demonstrates the dependence of the weighting function
on the direct correlation function of the liquid.
The homogeneous liquid
Next, we obtain the relation (2.2.6) for the weighting function in the uniform-density limit.
By virtue of the definition of the weighted density function ¯n(x), the excess free energy is
defined as
F
WDA
ex
[n
0
(x)]=
dx n
0
(x) f
ex
( ¯n
0
(x)), (A2.3.10)
where f
ex
(n
0
) is the free-energy density for the homogeneous liquid of density n
0
.On
taking a functional derivative of (A2.3.10) with respect to n
0
(x), we obtain
δF
WDA
ex
δn
0
(x)
= f
0
[¯n
0
(x)]+
dx
1
f
ex
[¯n
0
(x
1
)]n
0
(x
1
)
δ ¯n
0
(x
1
)
δn
0
(x)
, (A2.3.11)
where the prime on f
ex
implies a partial derivative with respect to the corresponding
density n
0
. On taking a second functional derivative we obtain
δ
2
F
WDA
ex
δn
0
(x)δn
0
(x
)
= f
ex
[¯n
0
(x)]
δ ¯n
0
(x)
δn
0
(x
)
+ f
ex
[¯n
0
(x
)]
δ ¯n
0
(x
)
δn
0
(x)
+
dx
1
n
0
(x
1
)
δ ¯n
0
(x
1
)
δn
0
(x)
f
ex
[¯n
0
(x
1
)]
δ ¯n
0
(x
1
)
δn
0
(x
)
+
dx
1
f
ex
[¯n
0
(x
1
)]n
0
(x
1
)
δ
2
¯n
0
(x
1
)
δn
0
(x)δn
0
(x
)
. (A2.3.12)
Now, in order to simplify further the above equation, we need to evaluate the first and sec-
ond functional derivatives of the weighted density ¯n
0
(x) with respect to the actual density
n
0
(x). Starting from the defining relation
¯n
0
(x) =
dx
w(x − x
, ¯n
0
(x))n
0
(x
), (A2.3.13)
we obtain the following results for the functional derivatives:
δ ¯n
0
(x
)
δn
0
(x)
= w(x
− x;¯n
0
(x))
+
dx
1
n
0
(x
1
)w
(x
− x
1
;¯n
0
(x
))
δ ¯n
0
(x
)
δn
0
(x)
. (A2.3.14)
112 Appendix to Chapter 2
On taking a second derivative we obtain
δ
2
¯n
0
(x
1
)
δn
0
(x
)δn
0
(x)
= w
(x
1
− x;¯n
0
(x))
δ ¯n
0
(x)
δn
0
(x
)
+ w
(x
1
− x;¯n
0
(x
1
))
δ ¯n
0
(x
1
)
δn
0
(x
)
+
dx
2
n
0
(x
2
)w
(x
1
− x
2
;¯n
0
(x
1
))
δ ¯n
0
(x
1
)
δn
0
(x)
δ ¯n
0
(x
1
)
δn
0
(x
)
+
dx
2
n(x
2
)w
(x
1
− x
2
;¯n
0
(x
1
))
δ
2
¯n
0
(x
1
)
δn
0
(x)δn
0
(x
)
. (A2.3.15)
We now evaluate the above functional derivatives in the homogeneous-liquid limit
n
0
(x) → n
0
and correspondingly the weighted density ¯n
0
(x) → n
0
(obtained using the
normalization (2.2.3)). For the first derivative we obtain, from eqn. (A2.3.10),
δ ¯n
0
(x
)
δn
0
(x)
*
*
*
*
n
0
= w(x
− x;n
0
), (A2.3.16)
with the second term on the RHS going to zero, since
dx
1
w
(x
− x
1
;¯n
0
(x
))
*
*
*
*
n
0
≡
δ
δ ¯n
0
(x
)
dx
1
w(x
− x
1
;¯n
0
(x
))
*
*
*
*
n
0
= 0, (A2.3.17)
as a result of the normalization condition (2.2.3). Similarly, the second functional derivative
is evaluated from (A2.3.15), giving the following result in the homogeneous-liquid limit:
δ
2
¯n
0
(x
1
)
δn
0
(x
)δn
0
(x
)
*
*
*
*
n
0
= w
(x
1
− x
;n
0
)w(x
1
− x;n
0
) + w
(x
1
− x;n
0
)w(x
1
− x
;n
0
),
(A2.3.18)
since the last two terms on the RHS are equal to zero, using similar arguments regard-
ing normalization of the weight function w tothoseusedin(A2.3.17). We now evalu-
ate (A2.3.12) for the second functional derivative of F
WDA
ex
in the homogeneous-liquid
limit by substituting results (A2.3.17) and (A2.3.18) for the first and second derivatives,
respectively, of the weighted density function and obtain
δ
2
F
WDA
ex
δn
0
(x)δn
0
(x
)
*
*
*
*
n
0
= 2 f
ex
(n
0
)w(x − x
;n
0
)
+ n
0
f
ex
dx
1
w(x − x
1
;n
0
)w(x
1
− x
;n
0
)
+ n
0
f
ex
dx
1
w(x − x
1
;n
0
)w
(x
1
− x
;n
0
)
+ w
(x − x
1
;n
0
)w(x
1
− x
;n
0
)
. (A2.3.19)

A2.4 The modified weighted-density-functional approximation 113
In writing eqn. (A2.3.19) we assume that the weighting function w(x;n
0
) in the homoge-
neous liquid depends only on |x|. Upon identifying the functional derivative on the RHS as
a two-point direct correlation function we obtain for the homogeneous liquid the relation
−β
−1
c
(2)
(x − x
;n
0
) = 2 f
ex
(n
0
)w(x − x
)
+ n
0
f
ex
(n
0
)
dx
1
w(x − x
1
)w(x
1
− x
)
+ n
0
f
ex
(n
0
)
dx
1
w(x − x
1
)w
(x
1
− x
)
+ w
(x − x
1
)w(x
1
− x
)
, (A2.3.20)
where w(x) is the weighting function in the uniform liquid of density n
0
.
A2.4 The modified weighted-density-functional approximation
The formulation of the MWDA approach in terms of eqns. (2.2.10) and (2.2.11) can be
be justified at a formal level. The analysis is similar to what has been presented above for
the basic equations of the WDA in Appendix A2.3. We take the reference density to be a
constant, ¯n
0
(x) =ˆn
0
by setting the second term in eqn. (A2.3.7) equal to zero. This leads
to the following expression for the effective liquid density ˆn
0
in the MWDA,
ˆn
0
=
dx
dx
n
0
(x)n
0
(x
) ˜w[x, x
;n
0
(x), ˆn
0
],
where the weighting function ˜w is now expressed in a form equivalent to (A2.3.9) for the
WDA case,
˜w(x − x
) =
˜
W[x, x
;n
0
(x), ¯n
0
(x)]
6
dx n
0
(x)
6
dx
n
0
(x
)
˜
W[x, x
;n
0
(x), ¯n
0
(x)]
, (A2.4.1)
the corresponding function
˜
W being
˜
W[x, x
;n
0
(x), ˆn
0
]≡
1
0
dλλ
1
0
dλ
c
(2)
!
x, x
;λ{ˆn
0
+ λ
[n
0
(x) −ˆn
0
]}
"
. (A2.4.2)
Note that the weighting function ˜w in the present case of the MWDA is different from the
weighting function w (see eqn. (A2.3.9)) for the WDA. However, it also satisfies a similar
normalization condition to (2.2.3), namely
dx
˜w(x − x
, ˆn
0
) = 1 (A2.4.3)
at all densities n
0
.
114 Appendix to Chapter 2
The homogeneous liquid
As in the case of the WDA, the weighting function ˜w for the MWDA is determined by con-
sidering the limiting case of the homogeneous liquid. Starting from the basic eqn. (2.2.10)
for the MWDA, we take the functional derivative with respect to n
0
(x) to obtain
δF
MWDA
ex
δn
0
(x)
= f
0
( ˆn
0
) + Nf
ex
( ˆn
0
)
δ ˆn
0
δn
0
(x)
. (A2.4.4)
On taking a second functional derivative, we obtain
δ
2
F
MWDA
ex
δn
0
(x)δn
0
(x
)
= f
ex
( ˆn
0
)
δ ˆn
0
δn
0
(x)
+
δ ˆn
0
δn
0
(x
)
+ Nf
ex
[ˆn
0
(x
)]
δ ˆn
0
δn
0
(x)
δ ˆn
0
δn
0
(x
)
+ Nf
ex
[ˆn
0
(x
)]
δ
2
ˆn
0
δn
0
(x)δn
0
(x
)
. (A2.4.5)
The first and second functional derivatives of the weighted density ˆn
0
with respect to the
actual density n
0
(x) are evaluated starting from the defining relation (2.2.11) as
δ ˆn
0
δn
0
(x)
=
2
N
dx
w(x − x
;ˆn
0
) −
1
N
2
dx
dx
˜w(x − x
;ˆn
0
), (A2.4.6)
δ
2
ˆn
0
δn
0
(x)δn
0
(x
)
=
2
N
w(x − x
;ˆn
0
) −
2
N
2
dx
w(x − x
;ˆn
0
). (A2.4.7)
We now evaluate the above functional derivatives in the homogeneous-liquid limit n
0
(x) →
n
s
( the solid density) and correspondingly the weighted density ˆn
0
→ n
s
(obtained using
the normalization (A2.4.3)). For the first derivative we obtain from eqn. (A2.4.6)
δ ˆn
0
δn
0
(x)
*
*
*
*
n
s
=
2n
s
N
2
−
n
s
N
=
n
s
N
. (A2.4.8)
Similarly, the second functional derivative is evaluated from (A2.4.5), giving the following
result in the homogeneous-liquid limit:
δ
2
ˆn
0
δn
0
(x
)δn
0
(x
)
*
*
*
*
n
s
=
2
N
˜w(x − x
;n
s
) −
2
NV
. (A2.4.9)
We now evaluate (A2.4.5) for the second functional derivative of F
MWDA
ex
in the
homogeneous-liquid limit by substituting results (A2.4.8) and (A2.4.9) for the first and
second derivatives, respectively, of the weighted density function and obtain
−β
−1
c
(2)
(x, x
;n
s
) =
n
s
V
+ 2 f
ex
˜w(x, x
;n
s
), (A2.4.10)
giving the following result for the weighting function:
˜w(x, x
;n
s
) =−
1
2 f
ex
β
−1
c
(2)
(x, x
;n
s
) +
n
s
V
f
ex
( ˆn
0
)
. (A2.4.11)
The self-consistent solution (for the appropriate ˆn
0
)of(A2.4.11) provides the weighting
function for the MWDA.
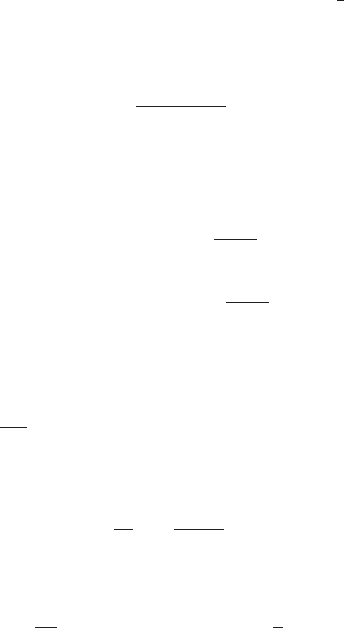
A2.5 The Gaussian density profiles and phonon model 115
A2.5 The Gaussian density profiles and phonon model
Note that the Gaussian approximation (2.2.14) for the density of the inhomogeneous solid
used in the liquid-based DFT here can be linked to a phonon description of the solid in
a self-consistent Debye model, a one-parameter (T
D
) theory for the dynamic crystal. In
the Debye model (Huang, 1987) the phonon frequency ω = ck for wave number k < k
D
or, correspondingly, can be defined for the Debye temperature T
D
using k
B
T
D
= hω
D
=
hck
D
. The speed of sound in the crystal, both for longitudinal and for transverse waves, is
assumed here to be c. The density of states g(ω) in the Debye solid (the number of modes
between ω and ω + dω is g(ω)dω) is proportional to ω
2
and, in the normalized form, is
expressed as
g(ω) =
3
9N ω
3
D
/ω
2
, for ω ≤ ω
D
,
0, for ω>ω
D
,
(A2.5.1)
where ω
D
is the Debye frequency. For high temperatures, T
D
/T 1, so that quantum
effects are negligible. The energy of the solid in terms of the 3N normal modes is obtained
in terms of the occupation numbers {n
i
} as E{n
i
}=
5
i
(
n
i
+
1
2
)
ω
i
, ignoring any
constant contribution to the energy. The partition function for the 3N phonon modes is
obtained as
Q =
%
i
e
−βω
i
/2
1 − e
−βω
i
, (A2.5.2)
where the frequencies range from 0 up to a maximum of ω
D
. On taking the corresponding
logarithm of the partition function, we obtain
ln Q =−
i
ln{1 − e
−βω
i
}+
βω
i
2
=−
ω
D
0
ln{1 − e
−βω
i
}+
βω
i
2
g(ω)dω. (A2.5.3)
Using the standard thermodynamic identities, we obtain the entropy of the phonon gas in
terms of the dimensionless parameter x
D
= T
D
/T as
−TS =−β
∂ F
∂β
V
= Nβ
−1
!
3ln(1 −e
x
D
) − 4I(x
D
)
"
, (A2.5.4)
where I is the Debye integral,
I(x) =
3
x
3
x
o
t
3
e
t
− 1
dt. (A2.5.5)
The energy of the phonons is obtained as
U =−
∂
∂β
ln Q = 3Nβ
−1
I(x
D
) +
3
8
x
D
. (A2.5.6)
116 Appendix to Chapter 2
In the high-temperature limit (x
D
1), the integral I has the asymptotic behavior
I(x
D
) ≈ 1 −
3
8
x
D
+···. (A2.5.7)
In the high-temperature limit the energy goes to the value 3Nk
B
T . For the free energy of
the noninteracting phonons we take only the kinetic-energy contribution E = 3Nk
B
T /2.
Using the result (A2.5.4) for the entropy in this high-temperature limit, we obtain for the
scaled free energy per particle the result
β
E − TS
N
= β f = 3lnx
D
−
5
2
+ O
(
x
2
D
)
. (A2.5.8)
This form reduces to the ideal-gas free-energy expression (2.2.21) if we identify
x
D
=
9
α
π
0
with the Gaussian model of eqn. (2.2.14),
0
being the thermal wavelength. At higher
temperatures (hence smaller x
D
),
0
∼ T
−1/2
becomes smaller and thus refers to smaller
values of α, indicating a lower degree of mass localization in the crystalline state.
3
Crystal nucleation
If the liquid is cooled beyond the corresponding freezing point T
m
at which the liquid and
crystalline phases coexist in equilibrium, a thermodynamic driving force builds up towards
forming the crystal. In this chapter we will discuss how the liquid transforms into a crystal,
focusing on how the changes in the liquid are initiated and on the nature of the crystalline
region that is formed. This process is referred to as nucleation. The thermodynamic force
favoring the formation of the crystal seed in the supercooled liquid competes with the pro-
cess of forming an interface between the solid and the liquid. The cost of the interfacial
free energy therefore presents a barrier to the formation of the new phase. Only when the
driving force is made large enough by moving deep into the supercooled state does crystal-
lization occur on laboratory time scales. Thus pure water can be cooled to −20
◦
C or below
without freezing. Our focus here will be mainly on the process of crystallization of solid
from the melt. The condensation of vapor into liquid is a very thoroughly studied process
that has been discussed in various reviews (Stanley, 1971; Evans, 1979; ten Wolde et al.,
1998). For condensation from a low-density gas or crystallization from dilute solution, it
is easier to identify the nucleating bubbles since they differ widely in composition from
the surrounding phase. In a crystal forming from a melt the nucleating droplet is far less
distinct. It is somewhat problematic to define the cluster in the bulk of the melt in terms of
a certain set of particles. One possible route is to identify the coordination numbers, which
differ between the liquid and the crystalline phase. We will simplify our discussion here
by limiting the discussion to one-component systems and also focus on what is termed
homogeneous nucleation (Oxtoby, 1992a, 1992b; Gunton, 1999), which occurs in the bulk
of the pure liquid phase. We also briefly explore here the issue of heterogeneous nucleation
(Turnbull, 1969) started by impurities or occurring on the surfaces.
3.1 Classical nucleation theory
In the classical nucleation theory we associate a number of particles with the nucleus of
the new phase having a sharp interface with the bulk liquid. In this theory of the nucleation
process the nucleus of the crystal, no matter how small, is treated using macroscopic ther-
modynamic principles. Let us first consider the formation of N
i
clusters, each consisting
of i monomers, and N single monomers (N N
i
). Assuming that clusters mix ideally
117
118 Crystal nucleation
with the monomers, the number of equivalent states is (N + N
i
)!/(N!N
i
!). Hence the total
change in free energy on forming the clusters, at temperature T ,is
G =
i
N
i
G
i
+ k
B
T
N
i
ln
N
i
N
i
+ N
+ N ln
N
i
N
i
+ N
, (3.1.1)
where G
i
is the free energy of formation of a single cluster of i molecules from the bulk.
On minimizing the free-energy cost G, we find that the equilibrium number of clusters
of size i is given by
N
i
= N exp
!
−G
i
/(k
B
T )
"
. (3.1.2)
3.1.1 The free-energy barrier
Next we compute the free-energy cost G
i
for a cluster of size i from the bulk liquid. We
assume here that the clusters are spherical in shape and consist of i particles. The pressure
difference between the two phases at the interface with principal radii of curvature R
1
and
R
2
is given by
P = γ
s
1
R
1
+
1
R
2
, (3.1.3)
where γ
s
is the surface tension. For a spherical interface R
1
= R
2
= R, eqn. (3.1.3) becomes
P
S
r
− P
L
=
2γ
s
R
, (3.1.4)
where P
S
r
and P
L
, respectively, denote the pressures in the crystalline phase at the spherical
interface of radius r and in the bulk liquid phase. By applying the Gibbs–Duhem relation
(1.1.9) discussed in Chapter 1, dμ
S
= vdP
S
for the crystalline phase, at a constant tem-
perature, we obtain for the difference between the chemical potential on the interface at r
and that of the bulk
μ
S
r
− μ
S
=
P
S
r
P
S
v dP
S
= v
m
(
P
S
r
− P
S
)
, (3.1.5)
assuming that the volume per particle v
m
for the crystal remains constant in the incom-
pressible limit. In obtaining (3.1.5) we approximate the properties of the nucleus at the
center as being same as those of the bulk solid and the surface tension of the small droplet
with curved surface is taken to be the same as that of a plane interface. This is termed
the capillary approximation. This applies better for large droplets and is a weak approxi-
mation in the case of small droplets. On combining (3.1.5) and (3.1.4) we obtain for the
solid-phase chemical potential μ
S
r
at the interface
μ
S
r
= μ
S
+ v
m
2γ
s
r
+ P
L
− P
S
. (3.1.6)

3.1 Classical nucleation theory 119
Since 2γ
s
/r P
L
− P
S
, the above relation approximates to
μ
S
r
= μ
S
+ v
m
2γ
s
r
. (3.1.7)
For a spherically shaped nucleating bubble of volume V and radius R containing a total
number of molecules i,wehavei v
m
≡ V = (4π/3)R
3
. The total Gibbs free energy of the
nucleating bubble is then given by
G
S
r
=
μ
S
dN +
v
m
2γ
s
¯r
dN
= μ
S
i +
4π ¯r
2
d ¯r
2γ
s
¯r
. (3.1.8)
Here we take the cluster to be stationary and ignore the rotational and vibrational con-
tributions to the free energy. The difference of the Gibbs free energy from that of the
surrounding bulk liquid is obtained as
G
i
≡ G
S
r
− G
L
=
4
3
π R
3
G
v
+ 4π R
2
γ
s
, (3.1.9)
where G
v
is the difference of the bulk values for the Gibbs free energy per unit volume
in the two phases,
G
v
=
(
μ
S
− μ
L
)
/v
m
= μ/v
m
. (3.1.10)
Since the crystalline phase is more stable (i.e., μ
S
<μ
L
), G
v
is negative. In terms of the
number of particles i in the nucleus of volume V ,wehavev
m
i = (4π/3)R
3
. Using this
relation, the surface contribution to the free energy represented by the second term on the
RHS of (3.1.9) is expressed in terms of i, giving for the total free-energy difference of the
nucleus of i molecules in the two phases
G
i
= v
m
G
v
i + aγ
s
i
2/3
, (3.1.11)
where ai
2/3
=
3
;
36πv
2
m
i
2/3
is the surface area of the nucleus containing i monomers.
If we do not assume a completely spherical shape of the interface, the free energy of the
cluster can be argued to have the form (Turnbull and Fisher, 1949)
G
i
= v
m
G
v
i +
ϕ
(a
ϕ
γ
ϕs
)i
2/3
, (3.1.12)
where the summation in the second term on the RHS is carried out over all facets of
the cluster with corresponding interfacial energy γ
ϕs
and a
ϕ
is a geometric factor (which
will reduce to a for a spherical nucleus). The expression (3.1.12) for the free energy is
schematically written in the form
G
i
k
B
T
= Ai
2/3
− Bi (3.1.13)
120 Crystal nucleation
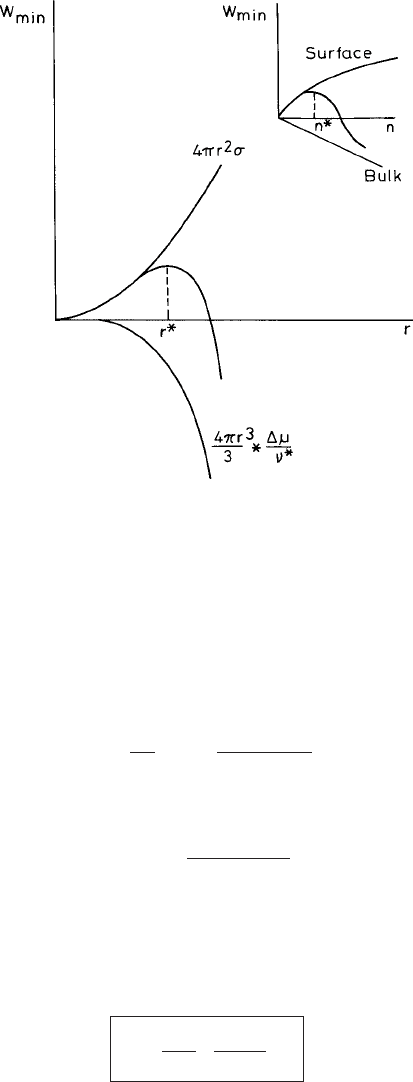
Fig. 3.1 Schematic representations of the surface and bulk contributions to the free energy of the
incompressible embryo in the classical nucleation theory (CNT). The sum of the two terms on the
RHS of (3.1.11) reaches a maximum corresponding to the unstable critical nucleus.
using A =
5
ϕ
(a
ϕ
γ
ϕs
)/(k
B
T ) and B = v
m
|G
v
|/(k
B
T ). Keeping the shape of the nucleus
fixed, the free energy G
i
reaches a maximum for i = i
∗
, i.e., dG
i
/di = 0. We show
this behavior of the free-energy difference G
i
(denoted as w
min
) with the number of
particles i in the cluster schematically in Fig. 3.1.Using(3.1.13) we obtain
i
∗
=
2A
3B
3
=
2
5
(a
ϕ
γ
ϕs
)
3v
m
|G
v
|
3
, (3.1.14)
with the corresponding maximum value of the free energy as
G
∗
=
4
5
(a
ϕ
γ
ϕs
)
3
27v
m
|G
v
|
2
. (3.1.15)
For the special case of the spherical nucleus we have, for all the facets, γ
ϕs
≡γ
s
and
5
a
ϕ
≡a.Theresult(3.1.12) for the barrier in terms of the radius R of the nucleus then
reduces to the form given on the RHS of eqn. (3.1.9). For the critical nucleus the number
of monomers and the barrier heights are respectively obtained as
i
∗
=
32π
3v
m
γ
s
|G
v
|
3
, (3.1.16)
