Das Sh.P. Statistical Physics of Liquids at Freezing and Beyond
Подождите немного. Документ загружается.


3.2 A simple nonclassical model 131
3.2 A simple nonclassical model
We now discuss the process of formation of the critical nucleus in terms of a simple
schematic model (Bagdassarian and Oxtoby, 1994) in which the solid–liquid interface is
described in terms of a single order parameter. The present model is treated analytically and
also captures the important aspects of a more realistic density-functional approach to be
described in subsequent sections. The theory involves choosing simple approximate forms
for the free-energy profile of the undercooled liquid having minima corresponding to the
stable and metastable bulk phases. The single order parameter ψ(r, t) used in the present
model is assumed to have ψ(r, t) = 0 for the metastable bulk liquid phase and some char-
acteristic constant value, say ψ
s
, for the stable bulk solid phase. The relative depth of the
deeper minima denotes the extent of supercooling of the system. The time evolution of this
order parameter is written in the standard time-dependent Ginzburg–Landau (TDGL) (Ma,
1976)form
∂ψ(r, t)
∂t
=−
0
δ[ψ]
δψ
, (3.2.1)
where [ψ] is the grand-canonical free energy expressed as a functional of the order
parameter ψ(r, t) and
0
is the bare kinetic coefficient. The term δ/δψ on the RHS of
(3.2.1) acts like a driving force (de Groot and Mazur, 1984) controlling the time variation
of the order parameter. Hence the equation
δ[ψ]
δψ
= 0 (3.2.2)
corresponds to a stationary nucleus. Now we write the grand potential functional of the
inhomogeneous solid liquid in the square-gradient approximation (Cahn and Hilliard, 1958)
of the order-parameter fluctuation in terms of the simple expression
[ψ]=
1
2
dr
#
ω[ψ]+|∇ψ(r, t)|
2
$
. (3.2.3)
Using this form of free energy in eqn. (3.2.1), the time evolution of ψ(r, t) becomes
−
−1
0
∂ψ(r, t)
∂t
=
δω[ψ]
δψ
−∇
2
ψ(r, t). (3.2.4)
For the free-energy density ω(ψ) we choose the suggestive form
ω(ψ) = min
3
1
2
λ
0
ψ
2
,
1
2
λ
s
(ψ − ψ
s
)
2
+
T
4
(3.2.5)
which represents two intersecting parabolas as shown in Fig. 3.4, with their respective
minima at ψ = 0 and ψ = ψ
s
. The depths of the two minima differ by an amount
T
,
which is a measure of the undercooling of the system.
T
= 0 corresponds to the sit-
uation in which the two minima in ω are equal, signifying the coexistence of the liquid
and crystalline phases. The curvatures of the two parabolas corresponding to the liquid
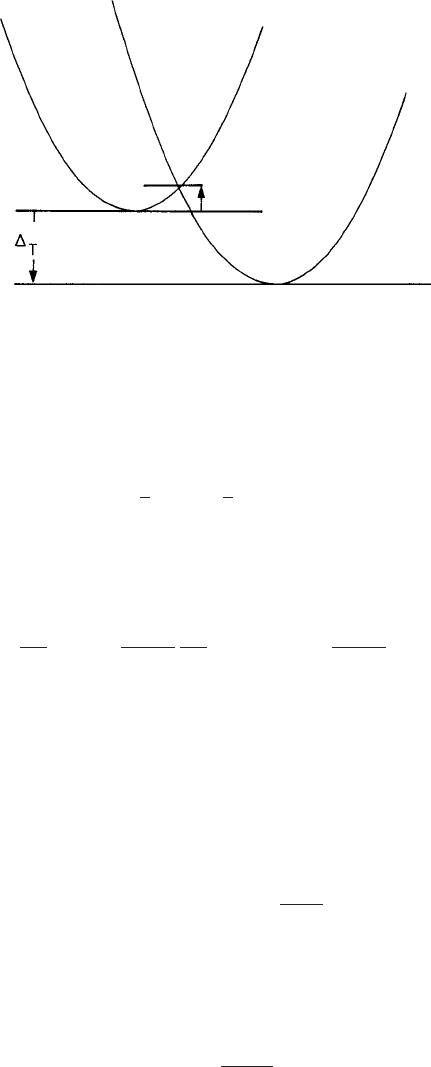
132 Crystal nucleation
Fig. 3.4 The schematic form of the potential energy taken as a combination of two parabolas as given
by eqn. (3.2.5). The height
T
of the free-energy barrier between the two minima of the free energy
represents the extent of supercooling of the system.
and crystalline states are denoted by λ
0
and λ
s
, respectively. The parabolas intersect at the
order parameter value ψ = ψ
c
, which can be related to the undercooling
T
by
T
=
1
2
λ
0
ψ
2
c
−
1
2
λ
s
(ψ
s
− ψ
c
)
2
. (3.2.6)
ψ(r, t) is the order parameter through the solid–liquid interface. The critical nucleus is rep-
resented by eqn. (3.2.2), which represents a saddle point for the free energy [ψ] (Bender
and Orszag, 1978). Note that
∂
∂t
=
dr
δ[ψ]
δψ
∂ψ
∂t
=−
0
dr
δ[ψ]
δψ
2
, (3.2.7)
so that always decreases while the optimum order-parameter profile is being determined
by the condition (3.2.2). In solving this equation for ψ, we note that for the part ψ>ψ
c
we need to use the solid part of the free-energy profile (the parabola with its minimum at
ψ
c
), whereas for ψ<ψ
c
the liquid part (the parabola with its minimum at 0) is to be used.
In the above choice of the free energy in the double-parabola form there is automatically
the limit in which the liquid loses its metastability with respect to the solid, i.e., ψ
c
= 0.
This limiting situation is characterized by the undercooling
T
obtained from (3.2.6) as
sp
≡
T
|
ψ
c
=0
=−
λ
s
ψ
2
s
2
(3.2.8)
and represents a spinodal in the present model.
The basic equation describing the critical nucleus therefore follows from (3.2.4) in the
form of a second-order differential equation for the order-parameter field in the steady
state,
∇
2
ψ =
δω[ψ]
δψ
. (3.2.9)

3.2 A simple nonclassical model 133
For representing the spherical nucleus we consider a spherically symmetric order-parameter
profile. Using the corresponding spherically symmetric form of the ∇
2
operator, we obtain
the following differential equations for the order parameter from eqn. (3.2.2):
d
2
ψ(r)
dr
2
+
2
r
dψ(r)
dr
−[ψ(r) − ψ
s
]=0, for ψ<ψ
c
, (3.2.10)
d
2
ψ(r)
dr
2
+
2
r
dψ(r)
dr
−¯ν
2
ψ(r) = 0, for ψ>ψ
c
. (3.2.11)
In writing the above two differential equations we express length r in units of 1
√
λ
s
(i.e.,
√
λ
s
r → r) and define the parameter ¯ν =
√
λ
0
/λ
s
. The two differential equations stated
above are obtained by using in the equation (3.2.2) the respective parabolic form for the
grand potential function as stated in eqn. (3.2.5). The parameter ¯ν denotes the relative
curvatures of these two parabolas representing the order-parameter dependence of the free
energy . The crossover value of the order parameter ψ = ψ
c
marks the proximity to
the stable liquid or the crystalline minimum. The crystalline nucleus is identified with the
order-parameter value ψ>ψ
c
, since in this case the system is closer to the free-energy
minimum at ψ = ψ
s
. The order-parameter values ψ<ψ
c
, on the other hand, correspond
to the liquid state, which is characterized by the minimum at ψ = 0.
3.2.1 The critical nucleus
Equations (3.2.10) and (3.2.11) are solved to obtain the following relation among ψ
c
, ψ
s
,
and r
c
for the critical nucleus:
ψ
c
= ψ
s
1 −r
−1
c
tanh r
c
1 +¯ν tanh r
c
. (3.2.12)
The solution of eqns. (3.2.10) and (3.2.11) under appropriate boundary conditions to reach
the above result (3.2.12) is given in Appendix A3.1. The boundary r = r
c
therefore is a
marker for the crystalline phase in this case and is a natural identification of the radius
of the critical nucleus corresponding to the condition (3.2.2).Asweseeineqn. (A3.1.4)
of Appendix A3.1.1, ψ
in
(0) →ψ
s
, i.e., the order parameter at the center of the nucleus
approaches the bulk-solid value. The relation (3.2.12) between ψ
c
and ψ
s
is useful in deter-
mining how the critical radius changes with the undercooling
T
. Two limiting cases are
of interest here.
Near coexistence
In this limit the undercooling
T
→0 and it then follows from (3.2.6) that ψ
s
→ψ
c
(1+¯ν). Identification of the same relation from (3.2.12) requires tanh r
c
→ 1 and r
−1
c
→0.
This corresponds to r
c
→∞. In order to find the leading-order relation between r
c
and
T
,
we simplify the relation (3.2.6) to leading order in
T
as
ψ
s
− ψ
c
=¯νψ
c
1 −
T
λ
0
ψ
2
c
. (3.2.13)

134 Crystal nucleation
Upon inverting the above relation expressing ψ
c
in terms of ψ
s
with the approximation
ψ
s
≈ (1 +¯ν)ψ
c
, we obtain
ψ
c
=
ψ
s
1 +¯ν
+
T
¯νλ
s
ψ
s
+ O
(
2
T
)
. (3.2.14)
For large r
c
, to leading order (3.2.12) reduces to
ψ
c
= ψ
s
1
1 +¯ν
−
r
−1
c
1 +¯ν
. (3.2.15)
On comparing (3.2.14) and (3.2.15) we obtain the relation
r
c
T
=−
¯ν
1 +¯ν
λ
s
ψ
2
s
. (3.2.16)
In the above limit of coexistence
T
→ 0(r
c
→∞)wehave
r
c
=
2
˜
T
¯ν
1 +¯ν
, (3.2.17)
where
˜
T
=
T
/
sp
is the undercooling of the system relative to that at the spinodal point.
The spinodal limit
This is characterized by the situation ψ
c
→0, with the corresponding undercooling
T
being given by (3.2.8). In this case it follows from (3.2.12) that tanh r
c
→r
c
, i.e., r
c
→0,
implying that the liquid state is always unstable. In the limit r
c
→0 the relation (3.2.12)
reduces to the form ψ
c
≈ ψ
s
r
2
c
/3. On using this in the definition (3.2.8) for the undercool-
ing
T
we obtain
T
=−
λ
s
ψ
2
s
2
1 −
2ψ
c
ψ
s
=−
λ
s
ψ
2
s
2
1 −
2
3
r
2
c
. (3.2.18)
The radius of the critical nucleus r
c
approaches zero with the supercooling
sp
correspond-
ing to the spinodal point as,
r
c
∼
1 −
˜
T
1/2
. (3.2.19)
3.2.2 The free-energy barrier
The free energy for the formation of the critical nucleus relative to the liquid is computed
in the present schematic model as
NC
= 4π
∞
0
dr r
2
ω[ψ(r, t)]+
|∇ψ(r, t)|
2
2
, (3.2.20)
where the subscript NC refers to a nonclassical quantity to distinguish it from the corre-
sponding quantity in classical nucleation theory. In Appendix A3.1.1 we demonstrate that

3.2 A simple nonclassical model 135
evaluating the RHS of eqn. (3.2.20) corresponding to the critical nucleus profile
leads to
NC
=
4πr
3
c
3
T
− 4πr
2
c
sp
1 −r
−1
c
tanh r
c
1 +¯ν tanh r
c
(
¯ν +r
−1
c
)
. (3.2.21)
The last equality above is reached by using the relation (3.2.12) for ψ
c
/ψ
s
.Usingthe
leading-order expansion of tanh r
c
≈ 1 − 2exp(−2r
c
) for large r
c
the second term on the
RHS can be further simplified to obtain
NC
=
4πr
3
c
3
T
− 4πr
2
c
¯ν
1 +¯ν
+r
−1
c
sp
. (3.2.22)
Upon identifying the RHS of (3.2.22) with the surface term in the classical expression
(3.1.9) for the free energy of the critical nucleus, we obtain the following expression for
the surface free energy:
γ
s
=−
sp
¯ν
1 +¯ν
≡
λ
s
ψ
2
s
2
¯ν
1 +¯ν
. (3.2.23)
The free energy
NC
and radius r
c
of the optimum cluster in the present schematic
model can be compared with the corresponding results of the CNT. To make such a com-
parison we need to identify the relevant quantities in the present model with their corre-
sponding counterparts in the CNT. This is done primarily on the basis of the similarities
between the expression (3.2.22) for the free energy in the schematic model and the classical
expression for the same quantity as given by (3.1.17). Thus r
c
is taken as the radius of the
critical nucleus and
T
as the free-energy difference G
v
in the CNT. The surface tension
γ
s
is chosen from (3.2.23). Using the expression for the (3.1.17) for G
∗
we obtain the
free energy (in units of
sp
)as
˜
G
∗
=
16π
3
˜
2
T
¯ν
1 +¯ν
3
. (3.2.24)
The radius of the critical nucleus in the CNT is obtained from (3.1.18) as
R
∗
=−
2γ
s
T
=
2
˜
T
¯ν
1 +¯ν
, (3.2.25)
which agrees with the corresponding result (3.2.17) in the schematic model close to coex-
istence. For a chosen free-energy landscape (characterized by ¯ν) and a given undercooling
(determined by
˜
T
), the CNT expressions for the free energy and the radius of the critical
nucleus are given by (3.2.24) and (3.2.25), respectively.
In the nonclassical schematic model, on the other hand, estimates of the free energy and
radius of the critical nucleus are obtained by directly solving eqns. (3.2.12) and (3.2.6).
These two equations can be conveniently written as
136 Crystal nucleation
ϒ ≡
ψ
c
ψ
s
=
1 −r
−1
c
tanh r
c
1 +¯ν tanh r
c
, (3.2.26)
where ϒ is obtained from the solution of the equation
ϒ
2
(
1 −¯ν
2
)
− 2ϒ +
(
1 −
˜
T
)
= 0. (3.2.27)
For a given value of ¯ν the critical radius r
c
is obtained as a function of the relative under-
cooling
˜
T
. The free-energy barrier
∗
NC
(expressed in units of
sp
) to formation of the
critical nucleus is obtained from (3.2.21) as
∗
NC
=
4πr
3
c
3
˜
T
− 4πr
2
c
ϒ
(
¯ν +r
−1
c
)
. (3.2.28)
Therefore the critical radius r
c
(¯ν,
˜
T
) and
∗
NC
(¯ν,
˜
T
) are determined simultaneously.
The radius r
c
of the critical nucleus in the present model can be either higher or lower than
its counterpart R
∗
in the CNT depending on the value of the parameter ¯ν and the extent
of undercooling
˜
T
. Results for the radius of the critical nucleus from these two theories
agree in the limit of zero supercooling, i.e., at coexistence, at which r
c
→∞.Thesame
behavior is observed with respect to the height of the free-energy barrier to critical-nucleus
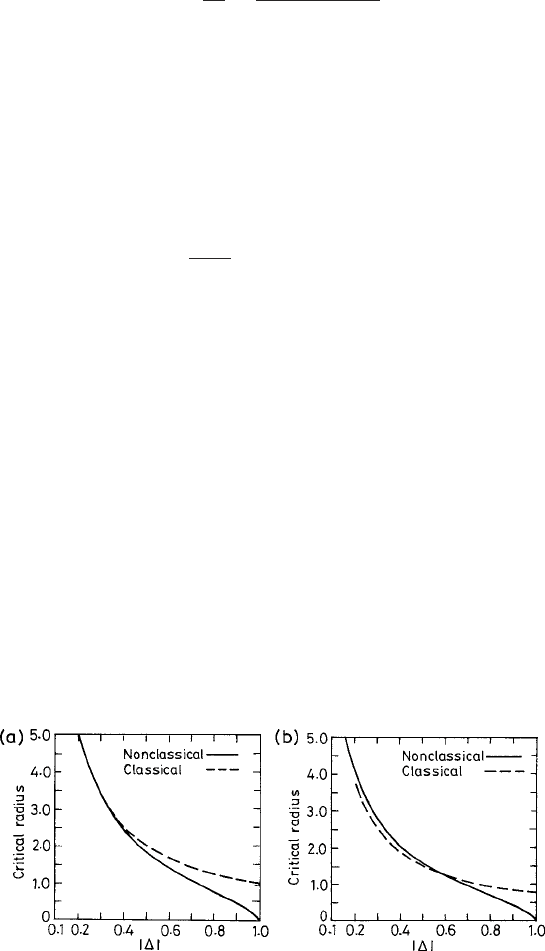
formation computed in the above two theories. The dependence of the critical radius r
c
on the degree of undercooling
˜
T
is shown in Fig. 3.5. We choose for the parameter
¯ν = 0.5 here. In Fig. 3.5, we see that the predictions of the nonclassical theory can be
higher and lower than the corresponding CNT predictions, depending on the undercool-
ing. Both trends are also predicted within the different types of DFT models discussed in
the next section. We have focused here primarily on the formation of the critical nucleus.
The dynamics of its evolution is also obtained (Bagdassarian and Oxtoby, 1994) by solv-
ing the corresponding TDGL equations (3.2.1) for specific choices of the free-energy
landscape defined in terms of ¯ν.
Fig. 3.5 The critical radius r
c
in units of λ
−1/2
s
(see the text) vs. the undercooling
˜
T
(see the text)
for two values of the parameter ¯ν (see text): (a) 1.0 and (b) 0.6. The corresponding values of the
critical radius obtained from the CNT in the respective cases are also shown for comparison. From
Bagdassarian and Oxtoby (1994).
c
American Institute of Physics.

3.3 The density-functional approach 137
3.3 The density-functional approach
The density-functional theory outlined in the previous chapter has been used for under-
standing the nucleation phenomena starting from basic principles of statistical mechanics.
Compared with the model described in the previous section, here a more realistic descrip-
tion of the crystal and the liquid state is chosen. In this formulation the interaction potential
between the constituent particles in the system is the primary input. As we have seen
in Chapter 2 regarding studying the bulk crystalline phase or the interfaces, the grand-
canonical potential is minimized with respect to the inhomogeneous density function in
order to identify the critical nucleus. In the DFT formulation, unlike in the CNT described
above, the effect of curvature of the interface in the computation of the free energy of the
clusters is taken into account. The difference between the grand-canonical potentials
of the inhomogeneous state (consisting of the nucleating bubble) and of the homogeneous
liquid state is optimized with respect to the inhomogeneous density function. This auto-
matically identifies the critical nucleus, i.e., corresponding to the optimum density function
n
0
(x) representing the crystal nucleus in the melt, we have
[n
0
(x)]=
l
+ G
∗
, (3.3.1)
where G
∗
denotes the free energy required for the formation of the critical nucleus (see
eqn. (3.1.15) in the discussion of the CNT above). In formulating the appropriate DFT
for the present case it is important to notice that the critical nucleus is in fact dynami-
cally unstable. However, this instability is with respect to the variation of the number of
monomers in the critical nucleus, which can either grow or shrink as a result of fluctuations
in the number of monomers. This is therefore relevant when the grand-canonical ensemble
is used to describe the critical nucleus (with a fixed number of particles, on the other hand,
the critical nucleus is the state of minimum free energy and hence corresponds to a ther-
modynamically stable state). The critical nucleus represents a saddle point in the function
space of density, having one unstable direction with respect to the particle number. Hence
the optimum density distribution of the critical nucleus is determined from the solution of
the Euler–Lagrange equation
δ
δn(r)
n(r)=n
0
(r)
= 0. (3.3.2)
The identification of the critical nucleus and its size as depicted through the optimum den-
sity profile follows in a natural way from the optimization of the thermodynamic function
in the DFT approach to this problem. However, it is still a mean-field approach and the
treatment of the smallest clusters containing a few molecules can only be approximate.
3.3.1 The square-gradient approximation
A crucial ingredient of the DFT is the choice of the test density function with respect
to which the appropriate thermodynamic functional is minimized. We have discussed in
138 Crystal nucleation
the previous chapter (see, for example, eqn. (2.4.32)) how the density-functional model is
extended to study the plane interface of the liquid and the crystal. The parametrization of
the density function for the critical nucleus is done in a manner similar to that done in the
DFT studies for the plane interface of the liquid and crystal. The spatial dependences of the
parameters in the inhomogeneous density function characterizing the nucleus are chosen
in such a manner that, away from the surface of the nucleus, near the center, the structure is
crystalline, while at large radial distances the uniform liquid state of the melt is obtained.
A typical example follows from the ansatz (2.1.42) for the density function generalized in
the following manner for the spherical nucleus (Harrowell and Oxtoby, 1984):
n
0
(r) = n
l
1 + η(r ) +
m
A
m
(r)e
iK
m
· r
, (3.3.3)
in terms of a set of local order parameters, γ(r) ≡{η(r), A
i
(r)}. The corresponding
boundary conditions are η(r →∞) = 0 for the liquid state and η(r → 0) = (n
s
−n
l
)/n
l
,
which is the fractional change of density on solidification, n
s
being the average density
of the solid state. In the discussion below we generalize this approach. We assume that
the inhomogeneous density function n
0
(r;γ) is parametrized in terms of locally vary-
ing amplitudes denoted by γ ≡{γ
i
(r)} for i = 1,...,m.Theγ
i
form a set of local
order parameters. The grand potential function for the inhomogeneous state is computed
within the square-gradient approximation. The latter is applied to evaluate the nonlocal
contributions to the thermodynamic potential involving the densities at two different spa-
tial points. To illustrate this approximation, we consider the local and nonlocal parts of
[n(r)],
[n]=F
id
+ F
ex
− μ
n(r)dr. (3.3.4)
The subscripts of the first and second terms on the RHS denote the ideal and the excess
parts of the Helmholtz free energy, respectively. The ideal part F
id
is represented in terms
of a local functional of the density and the interaction or the excess part F
ex
involves
nonlocal contributions. For the uniform state with constant order parameters these are given
in Chapter 2 in eqns. (2.1.27) and (2.1.38), respectively. The grand potential of the
inhomogeneous state corresponding to the density function n
0
(x, {γ
i
}) is obtained in terms
of a functional Taylor-series expansion around the uniform liquid state.
β=
dx
1
n
0
(x
1
;γ)ln
n
0
(x
1
;γ)
n
l
−
dx
1
(n
0
(x
1
;γ)− n
l
)
+
1
2
dx
1
dx
2
c(x
1
, x
2
)δn
0
(x
2
;γ(x
2
))δn
0
(x
1
;γ(x
1
)) +···, (3.3.5)
where the direct correlation function c(r) (Ohnesorge et al., 1991) is defined as
c(x
1
, x
2
) =−β
δ
2
F
ex
[n
0
]
δn
0
(x
1
;γ)δn
0
(x
2
;γ)
n
l
. (3.3.6)
The above expression is similar to the expansion around the uniform liquid state shown
in eqn. (2.1.41) in Chapter 2. Note that the RHS of (3.3.5) involves the inhomogeneous

3.3 The density-functional approach 139
densities and hence the corresponding order parameters γ
i
at two different spatial points
x
1
and x
2
in the interaction part. This part is simplified by adding to the local contribu-
tion corrections evaluated within the square-gradient approximation. On expanding the
amplitudes {γ } up to second order in spatial derivatives, we obtain for the ith member of
the set
γ
i
(x
2
) = γ
i
(x
1
) +{x
21
· ∇}γ
i
(x
1
) +
1
2
{x
21
· ∇}
2
γ
i
(x
1
), (3.3.7)
where x
2
−x
1
= x
21
and ∇ indicates the derivatives with respect to the spatial variation of
the local order parameters. The density δn
0
(x
2
;γ(x
2
)) is now expressed within the above
square-gradient approximation as
n
0
(x
2
;γ(x
2
)) = n
0
(x
2
;γ(x
1
)) +
i
∂n
0
(x
2
)
∂γ
i
{x
21
· ∇}γ
i
+
1
2
{x
21
· ∇}
2
γ
i
+
1
2
ij
∂
2
n
0
(x
2
)
∂γ
i
∂γ
j
{x
21
· ∇}
2
γ
i
γ
j
, (3.3.8)
where ∂n
0
(x
2
)/∂γ
i
denotes the partial derivative of the density n
0
(x
2
;γ(x
1
)) with respect
to its dependence on the order parameter γ
i
. We will assume here a linear dependence
of the field γ
i
on the density function (similar to the relation (2.4.32) stated above for the
interface problem) and hence ignore contributions from the last term on the RHS of (3.3.8).
Substituting (3.3.8) for the density fluctuation δn
0
(x
2
;γ(x
2
)) reduces (3.3.5) to the form
β=
dx
1
n
0
(x
1
;γ)ln
n
0
(x
1
;γ)
n
l
−
dx
1
(n
0
(x
1
;γ)− n
l
)
+
1
2
dx
1
dx
2
c(x
1
, x
2
)δn
0
(x
2
;γ(x
1
))δn
0
(x
1
;γ(x
1
))
+
1
4
dx
1
dx
2
i
∂n
0
(x
2
)
∂γ
i
c(x
1
, x
2
)δn
0
(x
1
;γ(x
1
)){x
21
· ∇}
2
γ
i
. (3.3.9)
In obtaining the above result we have set the contribution from the linear term in the
expansion (3.3.7) to zero using the fact that the spatial Fourier transform c(k) of the direct
correlation function c(r) at the RLV is c
(K
n
) = 0 since the K
n
correspond to peaks of the
structure factor. Let us consider the different terms on the RHS of (3.3.9).
(a) The first three terms on the RHS of (3.3.9) constitute to leading orders a contribution
that is expressed in terms of a grand potential density D(x) as
β
u
[γ ]=
dx
1
3
n
0
(x
1
;γ(x
1
)) ln
n
0
(x
1
;γ(x
1
))
n
l
− δn
0
(x
1
;γ(x
1
))
+
1
2
dx
2
c(x
1
, x
2
)δn
0
(x
2
;γ(x
1
))δn
0
(x
1
;γ(x
1
))
4
≡
dx D[γ
i
(x)]. (3.3.10)
140 Crystal nucleation
D involves the order parameter γ
i
(x) only locally. This part of the grand potential is
denoted as
u
with the subscript u signifying the fact that the functional dependence
of
u
is the same as that for the uniform system with constant order parameters. In
(3.3.9)
u
is being evaluated with the Ramakrishnan–Yussouff functional Taylor-series
expansion. Alternatively, this local contribution can be computed using the MWDA in
the same manner as formulated for the bulk phases, for a more accurate evaluation of
the grand potential.
(b) The last term on the RHS of (3.3.9) is further simplified with a partial integration and
using the substitution
∇
α
n
0
(x
1
;γ(x
1
)) =
j
∂n
0
(x
1
)
∂γ
j
∇
α
γ
j
(x
1
), (3.3.11)
where ∇
α
denotes the spatial derivative with respect to the corresponding spatial com-
ponent x
α
. The coefficient of the square-gradient term, i.e., of ∇
α
γ
i
∇
β
γ
j
, is expressed
as a kernel #
αβ
ij
. The latter is evaluated to leading order in terms of the integral per-
formed with V
−1
6
dx
1
and is expressed in terms of the local order parameters γ(x),
#
αβ
ij
[γ(x)]=−
k
B
T
4V
x
α
21
x
β
21
∂n
0
(x
2
)
∂γ
i
∂n
0
(x
1
)
∂γ
j
c(x
1
, x
2
;γ(x))dx
1
dx
2
. (3.3.12)
On combining the above terms we therefore obtain the following result for the differ-
ence between the grand potential of the inhomogeneous state and that of the uniform
liquid:
k
B
T
=
dx
⎧
⎨
⎩
D[γ
i
(x)]+
αβ
i, j
#
αβ
ij
[γ(x)]∇
α
γ
i
(x)∇
β
γ
j
(x)
⎫
⎬
⎭
. (3.3.13)
Thus, in summary, the first term on the RHS of (3.3.13) represents the thermody-
namic potential obtained as a local functional of the order parameter γ(x) while the
second term represents the contribution (evaluated within the square-gradient approxi-
mation) due to the variation of the local amplitudes γ
i
through the interface region. The
critical nucleus is identified by optimizing the thermodynamic grand-canonical poten-
tial , i.e., solving the corresponding Euler–Lagrange equation (3.3.2). In the present
context these equations simplify to ordinary differential equations,
δ
δγ
i
(r)
= 0. (3.3.14)
