Cui Dongmei. Atlas of Histology: with functional and clinical correlations. 1st ed
Подождите немного. Документ загружается.

CHAPTER 8
■
Blood and Hemopoiesis
145
Hemopoiesis
Introduction and Key Concepts
for Hemopoiesis
All formed elements, with the exception of some lymphocytes,
have a fi nite life span in circulation, so there must be an ongoing
replacement throughout the life of an individual. The magnitude
of the task for a given cell type can be appreciated if the approxi-
mate required daily production rate is calculated from estimates
of the total number in circulation and the rate of turnover for the
cell type. For erythrocytes, with a life span of about 120 days,
the daily production rate is roughly 250 billion, for neutrophils,
with a time in circulation of less than a day, the daily production
rate is normally roughly 60 billion, and for platelets, with a life
span of about 10 days, the daily production rate is approximately
150 billion. The development of each type of blood cell involves
numerous cell divisions and a series of differentiation steps so that
a small number of completely undifferentiated stem cells pro-
duce enormous numbers of cells that have the specifi c equipment
necessary for the particular mature cell to perform its functions.
Although all blood cells originate from a common pluripotential
hemopoietic stem cell, each blood cell type has its own lineage of
cell generations committed to proliferate and, at the same time,
differentiate only into that cell type. Cells that can be recognized
morphologically as undertaking differentiation into a particular
blood cell are called precursor cells. Precursor cells are produced
by cells that are committed to a specifi c lineage (i.e., they are
determined to give rise to, e.g., only erythrocytes) but show no
morphological signs of differentiation. These are called progenitor
cells. Some progenitor cells are not restricted in potential to just
one blood cell lineage but rather to two lineages. Progenitor cells
are also termed colony-forming cells (CFCs). A commonly used
abbreviation system for designating specifi c progenitor cells uses
the fi rst letter of the blood cell name after the letters CFC, for
example, CFC-E for erythrocyte colony–forming cell and CFC-B
for basophil colony–forming cell. Development of blood cells
occurs mostly in the specialized environment of the bone marrow.
Because extensive cell proliferation is required, the process is very
vulnerable to irradiation, so protection within the cores of bones
is clearly advantageous. The development of each type of blood
cell involves a series of precursor cells that can be recognized in
smears of the red bone marrow that have been stained with the
same procedures used for peripheral blood smears. Lymphocytes
and monocytes are little differentiated, so the morphological
appearance of their precursors (lymphoblasts and promonocytes,
respectively) is not easily distinguished. By contrast, the precursors
of erythrocytes, granulocytes, and platelets exhibit relatively dis-
tinct features as they differentiate in a series of steps that involve
predictable changes and identifi able stages. The red bone marrow
is a hemopoietic compartment where blood cells (except lympho-
cytes) develop and mature (Fig. 8-15C).
ERYTHROCYTE DEVELOPMENT is called erythropoiesis.
The appearances of the precursors of erythrocytes refl ect the pro-
cesses that must take place to generate, from an undifferentiated
cell, a cell that is essentially a plasmalemma bag of hemoglobin. In
the initial stage, the proerythroblast, the main event is generation of
free ribosomes that will be needed to synthesize the globin that will
combine with heme to form hemoglobin. Therefore, the intense
basophilic staining of the cytoplasm in the next stage, the basophilic
erythroblast, results from a peak concentration of free ribosomes
that begin translation of globin mRNAs. In the polychromatophilic
erythroblast, enough hemoglobin has accumulated to confer some
eosinophilia to the cytoplasm, whereas the concentration of ribo-
somes has decreased from dilution that accompanies cell division.
Continuation of cell division, dilution of ribosomes, and accumu-
lation of more hemoglobin account for the strong eosinophilic
staining of the cytoplasm in the orthochromatophilic erythroblast
(
normoblast). Concurrent with the successive changes in staining of
the cytoplasm during erythrocyte development in the cytoplasm are
changes in the appearance of the nucleus. Production of ribosomes
and transcription of mRNA for globin and other proteins are man-
ifested by a large euchromatic nucleus with prominent nucleoli in
the proerythroblast; subsequent stages have progressively smaller,
less active nuclei, and the nucleus is ultimately extruded at the end
of the orthochromatophilic erythroblast stage. The mitochondrion,
another key organelle, is required to synthesize protoporphyrin and
combine it with iron to form the heme of hemoglobin (Figs. 8-9A
and 8-10A to 8-11C).
PLATELET DEVELOPMENT is called thrombopoiesis.
Although platelets are small (2–4 μm in greatest diameter) frag-
ments of highly organized cytoplasm, they are produced by
very large cells called megakaryocytes. These cells measure up
to 100 μm or more in diameter. Megakaryocytes develop from
precursor cells (called megakaryoblasts) through a series of
incomplete cell cycles (endomitosis) that do not include division
of the nucleus or cytoplasm. The result is that the nucleus of a
mature megakaryocyte has up to 64N chromosomes, instead
of the usual 2N chromosomes. The nucleus is large and lobu-
lar, but it remains one nucleus. The cytoplasm develops numer-
ous mitochondria, a variety of granules, and microfi laments
and microtubules. As the megakaryocyte reaches maturity, its
cytoplasm becomes cordoned off by an elaborate system of
membranes, called demarcation membranes or channels, which
subdivide the cytoplasm into platelet zones—like perforations
in a sheet of stamps (Figs. 8-9B and 8-12A–C).
GRANULOCYTE DEVELOPMENT (GRANULOCYTOPOI-
ESIS) proceeds through an orderly sequence of events that
result in cytoplasm that is packed with granules containing a
wide variety of substances related to infl ammation and destruc-
tion of pathogenic organisms. As is the case with erythrocyte
development, the initial discernible event is generation of com-
ponents (ribosomes and RNA) needed for protein synthesis,
but in granulocyte development, the proteins will be packaged
in vesicles (granules). Accordingly, packaging of the proteins
requires development of an extensive endoplasmic reticulum
and Golgi complex. These events are prominent in the myelo-
blast and promyelocyte stages, both of which have relatively
large, active nuclei with nucleoli and cytoplasm that is baso-
philic owing to its content of ribosomes. Granule generation
occurs sequentially, the nonspecifi c (lysosomal) granules fi rst,
in the promyelocyte stage, and the specifi c granules second, in
the myelocyte stage. Because specifi c granules fi rst appear in the
myelocyte stage, this is the earliest stage at which the precur-
sors of the three granulocytes can be distinguished from each
other. Other notable changes during granulocyte maturation are
progressive condensation, elongation, and segmentation of the
nucleus (Figs. 8-13A to 8-15B).
CUI_Chap08.indd 145 6/17/2010 10:20:04 AM
146
UNIT 2
■
Basic Tissues
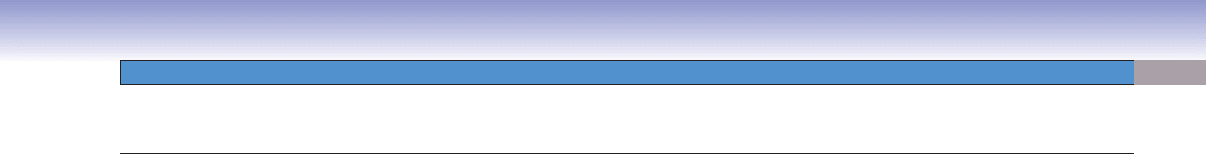
Figure 8-9A. A representation of erythropoiesis (red blood cell formation), bone marrow. Wright stain, 1,569
Erythrocyte formation includes several stages of cell changes during differentiation. Erythrocytes derive from progenitor cells (CFC-Es)
that give rise to the fi rst recognizable erythrocyte precursor, the proerythroblast. The proerythroblast is a large cell, which has a large
active nucleus with nucleoli. Each proerythroblast divides into two basophilic erythroblasts. Each basophilic erythroblast divides into
two polychromatophilic erythroblasts, each of which then divides to form orthochromatophilic erythroblasts, which do not divide.
These, in turn, differentiate into reticulocytes (Fig. 8-11B,C), which fi nally become erythrocytes. There are some general tendencies
accompanying differentiation of erythrocytes: (1) the overall size of the cells decreases, (2) the nucleus size decreases and the condensa-
tion of the chromatin increases, (3) nucleoli disappear, and (4) the color of the cytoplasm changes from blue to gray to pink because
of a reduction of ribosomes and an increase of hemoglobin. When the ribosomes are diluted by cell division and the hemoglobin
concentration rises to a near mature level, the cell becomes an orthochromatophilic erythroblast (or normoblast). When the nucleus
is extruded and only a few organelles (polyribosomes and mitochondria) remain in the cytoplasm, the cell is called a reticulocyte. The
reticulocyte completes maturation and enters the blood circulation to become a mature erythrocyte (red blood corpuscle).
D. Cui
Proerythroblast
Proerythroblast
Basophilic
erythroblast
Basophilic
erythroblast
Polychromatophilic
erythroblast
Polychromatophilic
erythroblast
Erythrocytes
Erythrocytes
Orthochromatophilic
erythroblast (normoblast)
Orthochromatophilic
erythroblast
(normoblast)
Reticulocyte
A
D. Cui
Platelets
Megakaryocyte
Promegakaryocyte
Megakaryoblast
B
Figure 8-9B. Thrombopoiesis (platelet formation process), bone marrow. Wright stain, 843, 586, and 1,570 (from left to
right)
Platelets are very small fragments of cells that have no nuclei. They are also called thrombocytes. Their differentiation from a large
cell, the megakaryocyte, takes place in the bone marrow. Megakaryoblasts are the precursor cells. They have a large, round nucleus,
undergo mitosis, and become promegakaryocytes. These cells have a large, round nucleus and develop through growth and a series
of endomitoses into megakaryocytes. A megakaryocyte has a large, multilobed nucleus with a huge amount of cytoplasm containing
numerous granules. The maturation process includes the development of a demarcation membrane system and the subdivision of
the cytoplasm to form platelets (Fig. 8-12A,B).
CUI_Chap08.indd 146 6/17/2010 10:20:04 AM
CHAPTER 8
■
Blood and Hemopoiesis
147
Erythropoiesis
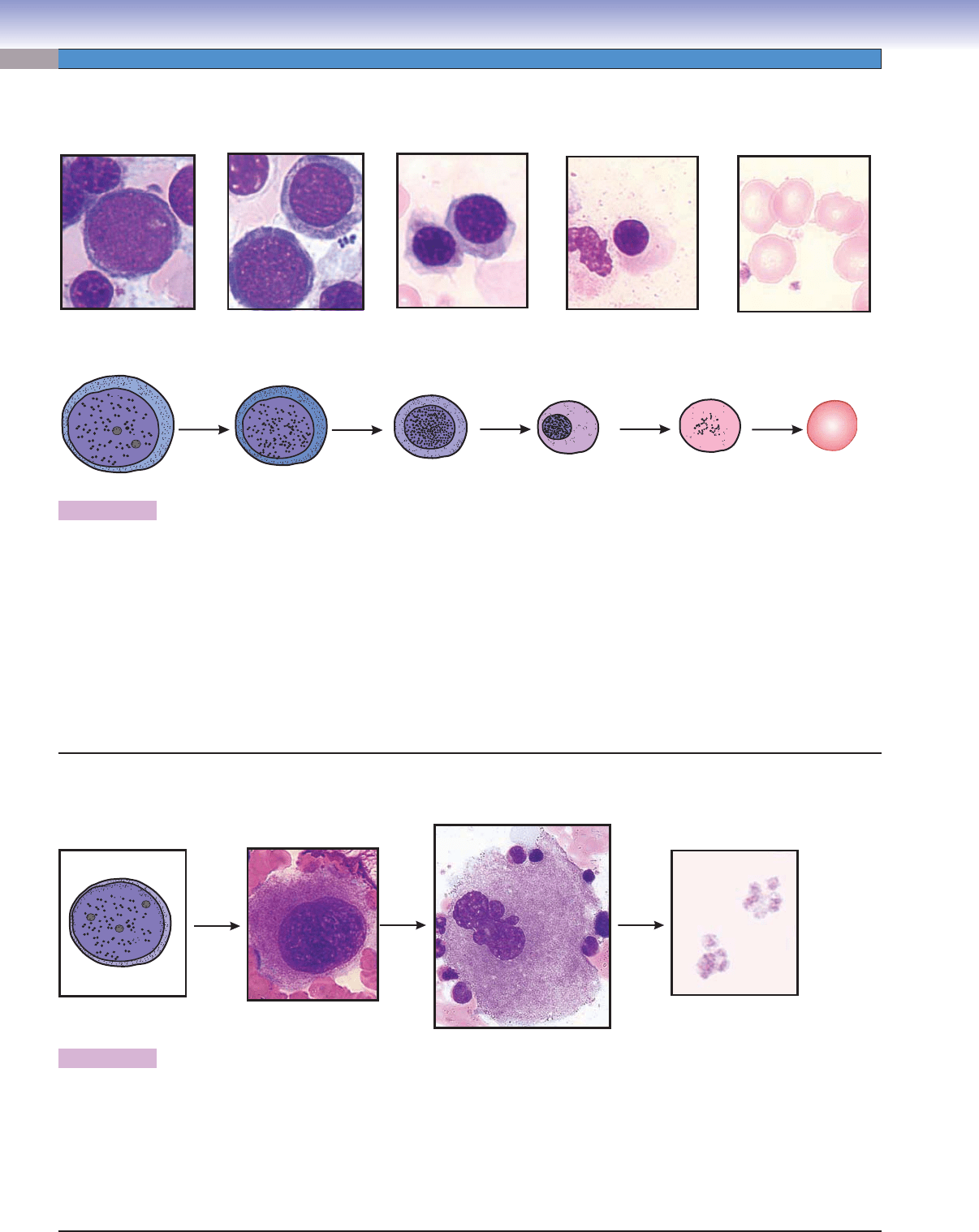
Figure 8-10A. Proerythroblasts, bone marrow smear.
Wright stain, 710; inset 1,569
The proerythroblast is a relatively large cell with a
large, round nucleus containing two to three nucleoli.
The cytoplasm appears basophilic (blue) because of the
presence of a large number of ribosomes. At this stage,
the cell is beginning to accumulate the necessary com-
ponents for the production of hemoglobin. Proeryth-
roblasts are precursor cells, which develop from two
functionally identifi able progenitor cells: burst-forming
unit–erythroid (BFU-E) cells, which take about a week
to mature to become colony-forming unit–erythroid
(CFU-E) cells and another week to become proerythro-
blasts. Proerythroblasts undergo mitosis to produce two
daughter cells that will develop the features of basophilic
erythroblasts.
D. Cui
Proerythroblast
Proerythroblast
Proerythroblast
PoE
Proerythroblast
Proerythroblast
Proerythroblast
Nucleoli
Proerythroblast
OE
A
B
D. Cui
Basophilic
erythroblast
Basophilic
erythroblast
Basophilic
erythroblast
Basophilic
erythroblast
Basophilic
erythroblast
PoE
OE
BE
BE
Figure 8-10B. Basophilic erythroblasts, bone mar-
row smear. Wright stain, 710; inset 1,569
The basophilic erythroblast is smaller than the pro-
erythroblast, and its cytoplasm is deep blue because
of a high content of tightly packed ribosomes. Com-
pared to proerythroblasts, basophilic erythroblasts have
smaller nuclei with a coarser texture because most of
the chromatin is in the heterochromatin form. At this
stage, nuclei are less active than in proerythroblasts, and
their nucleoli disappear. These cells undergo mitosis and
divide into daughter cells, which mature to become poly-
chromatophilic erythroblasts. (OE, orthochromatohilic
erythroblast; PoE, polychromatophilic erythroblast; BE,
basophilic erythroblast.)
C
D. Cui
Basophilic
erythroblast
Polychromatophilic
erythroblast
Polychromatophilic
erythroblast
Polychromatophilic
erythroblast
Polychromatophilic
erythroblasts
Orthochromatophilic
erythroblast
Figure 8-10C. Polychromatophilic erythroblasts, bone
marrow smear. Wright stain, 710; inset 1,569
The polychromatophilic erythroblast is smaller than
its parent cell (basophilic erythroblast). The nucleus is
smaller and it has no nucleoli. The condensed nucleus is
densely stained, and it displays a patchy pattern because
of condensation of chromatin. The cytoplasm of the cell
is usually grayish in overall color because, at this stage,
signifi cant amounts of hemoglobin have been produced
and accumulated in the cytoplasm, so that the staining
of the cytoplasm refl ects the presence of both ribosomes
(basophilic) and hemoglobin (eosinophilic). Therefore,
the cytoplasm is mottled with mixed patches of blue and
pink (polychromatophilic means “attracting multiple
colors”). Polychromatophilic erythroblasts undergo
mitosis and divide into daughter cells that develop into
orthochromatophilic erythroblasts.
CUI_Chap08.indd 147 6/17/2010 10:20:07 AM
148
UNIT 2
■
Basic Tissues
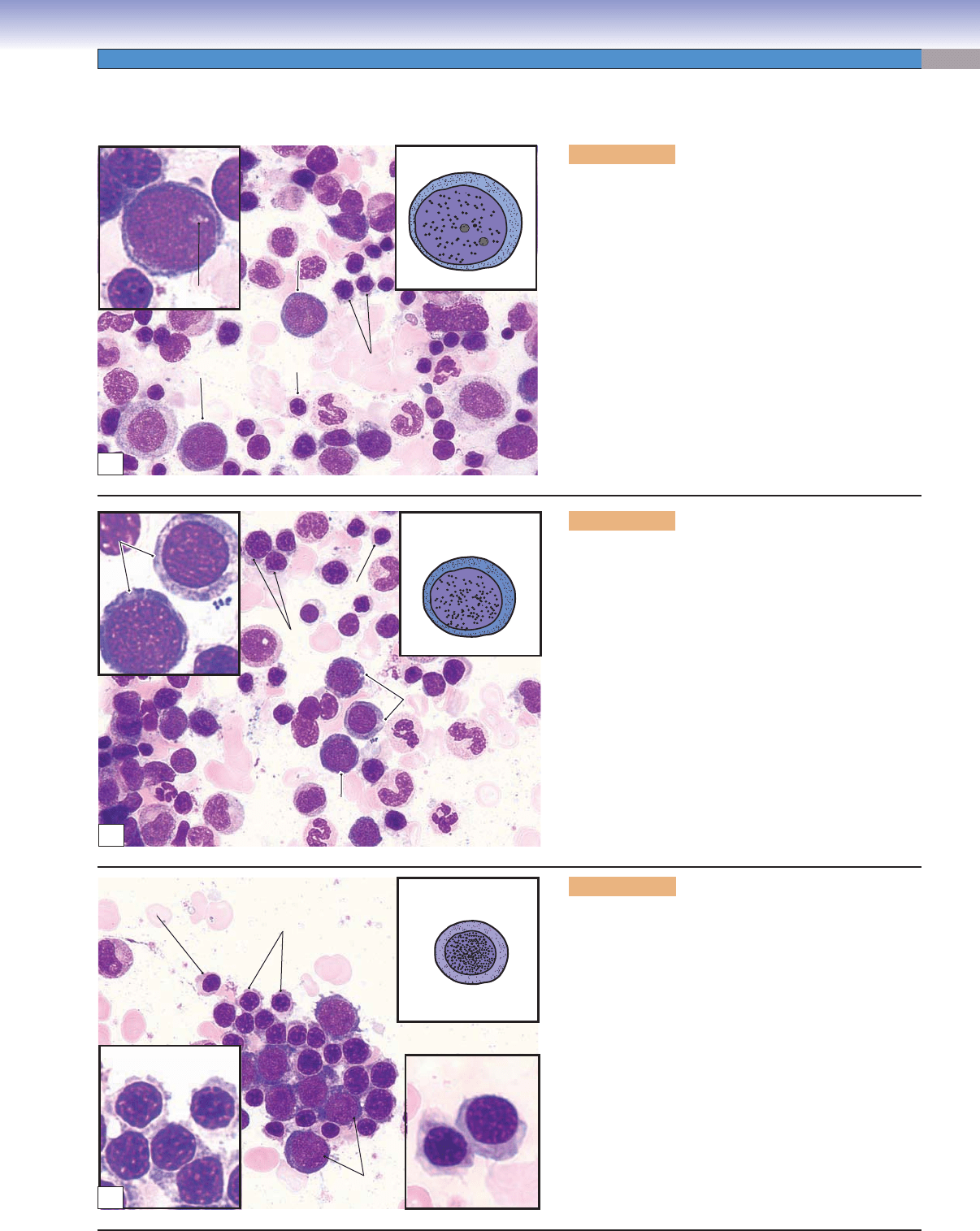
Figure 8-11A. Orthochromatophilic erythroblasts, bone
marrow smear. Wright stain, 710; inset 1,569
The orthochromatophilic erythroblast, also called a
normoblast, is a very small cell, close to the size of an
erythrocyte. The nucleus is small and so condensed that
it looks like a dark dot because of the extreme condensa-
tion of the chromatin. The cytoplasm appears pinker than
that of the polychromatophilic erythroblast. Hemoglobin
production and accumulation are almost complete, with
few ribosomes left in the cytoplasm. At this stage, the cell
is unable to divide. Orthochromatophilic erythroblasts
become reticulocytes (Fig. 8-11B) after losing their nuclei.
D. Cui
OE
Orthochromatophilic
erythroblast
Proerythroblast
Proerythroblast
Proerythroblast
PoE
PoE
Orthochromatophilic
erythroblast
A
CLINICAL CORRELATION
Figure 8-11C.
Reticulocytosis, Peripheral Blood
Smear
. New methylene blue stain, 1,020
Reticulocytosis is a condition characterized by an
increased number of reticulocytes. Reticulocytes are
premature red blood cells. The normal percentage of
reticulocytes is 0.5% to 1.5%. Hemolytic anemia usu-
ally increases erythropoietin production, which in turn
causes the bone marrow to produce more red blood
cells, resulting in a reticulocyte percentage of above
4% to 5%. An increased number of reticulocytes in
peripheral blood is an important indication of hemo-
lysis (red blood cell rupture) or bleeding. It can also
be the consequence of treating the anemia of chronic
kidney disease with erythropoietin. This illustration
shows the increased number of reticulocytes with new
methylene blue stain after hemolytic anemia.
Erythrocyte
Reticulocytes
C
D. Cui
Capillaries
Mature
erythrocyte
(inside of capillary)
Reticulocyte
Orthochromatophilic
erythroblast
(normoblast)
B
Figure 8-11B. Reticulocytes: the fi nal step of erythro-
cyte formation.
Orthochromatophilic erythroblasts have small and highly
condensed nuclei. In the next stage, the nucleus is extruded
and phagocytosed by macrophages. Although the cells
loose their nuclei, they retain some polyribosomes in their
cytoplasm. When stained supravitally with cresyl blue or
new methylene blue, the ribosomes aggregate into a blue
reticular network; therefore, the cells are called reticulo-
cytes. Reticular cells appear the same as mature erythro-
cytes with Wright stain (Fig. 8-11C). Reticulocytes enter
the blood circulation through the bone marrow sinusoidal
capillaries and become mature erythrocytes in one or two
days. Mature erythrocytes have neither nuclei nor organ-
elles and appear as a biconcave disk.
CUI_Chap08.indd 148 6/17/2010 10:20:12 AM
CHAPTER 8
■
Blood and Hemopoiesis
149
Thrombopoiesis
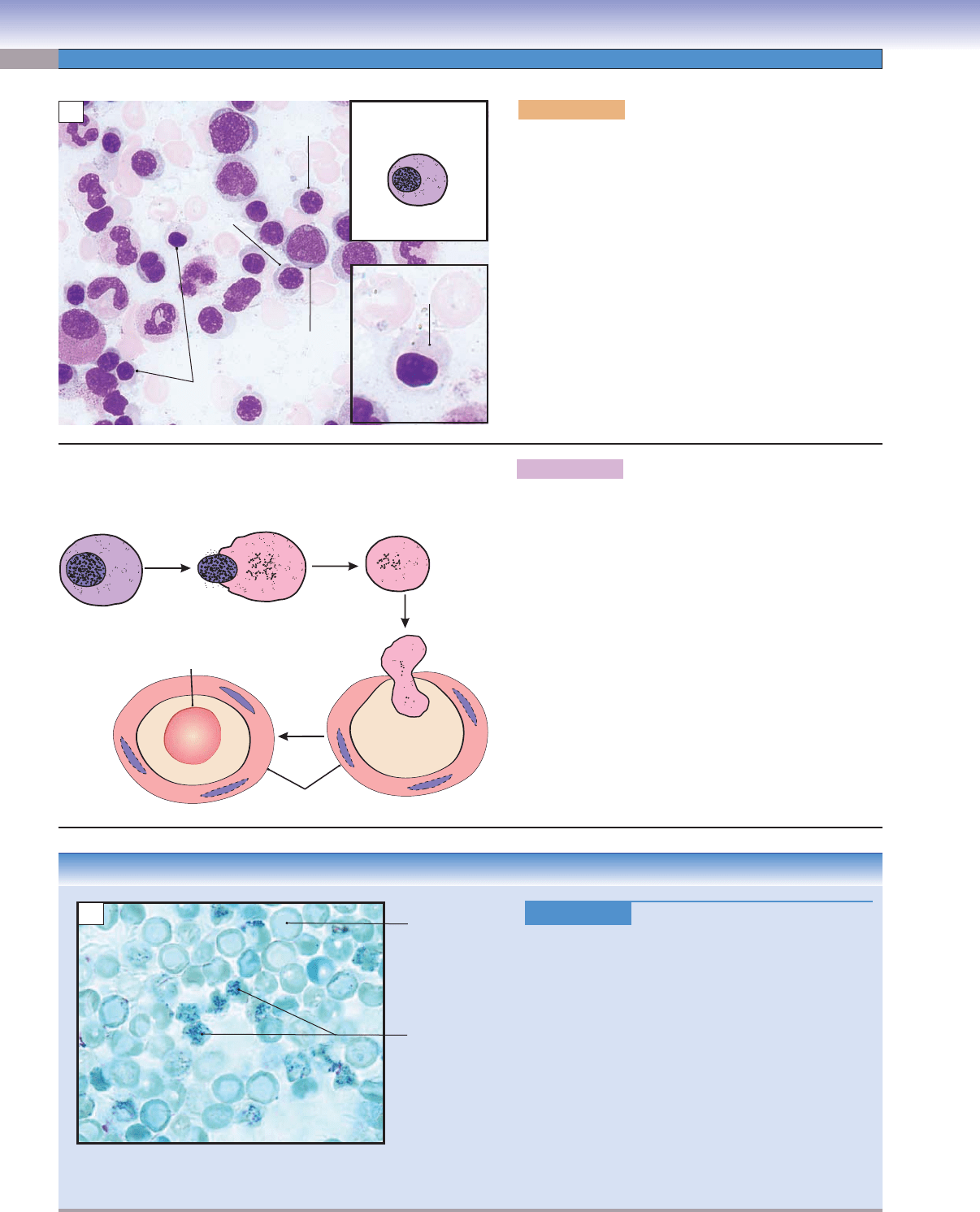
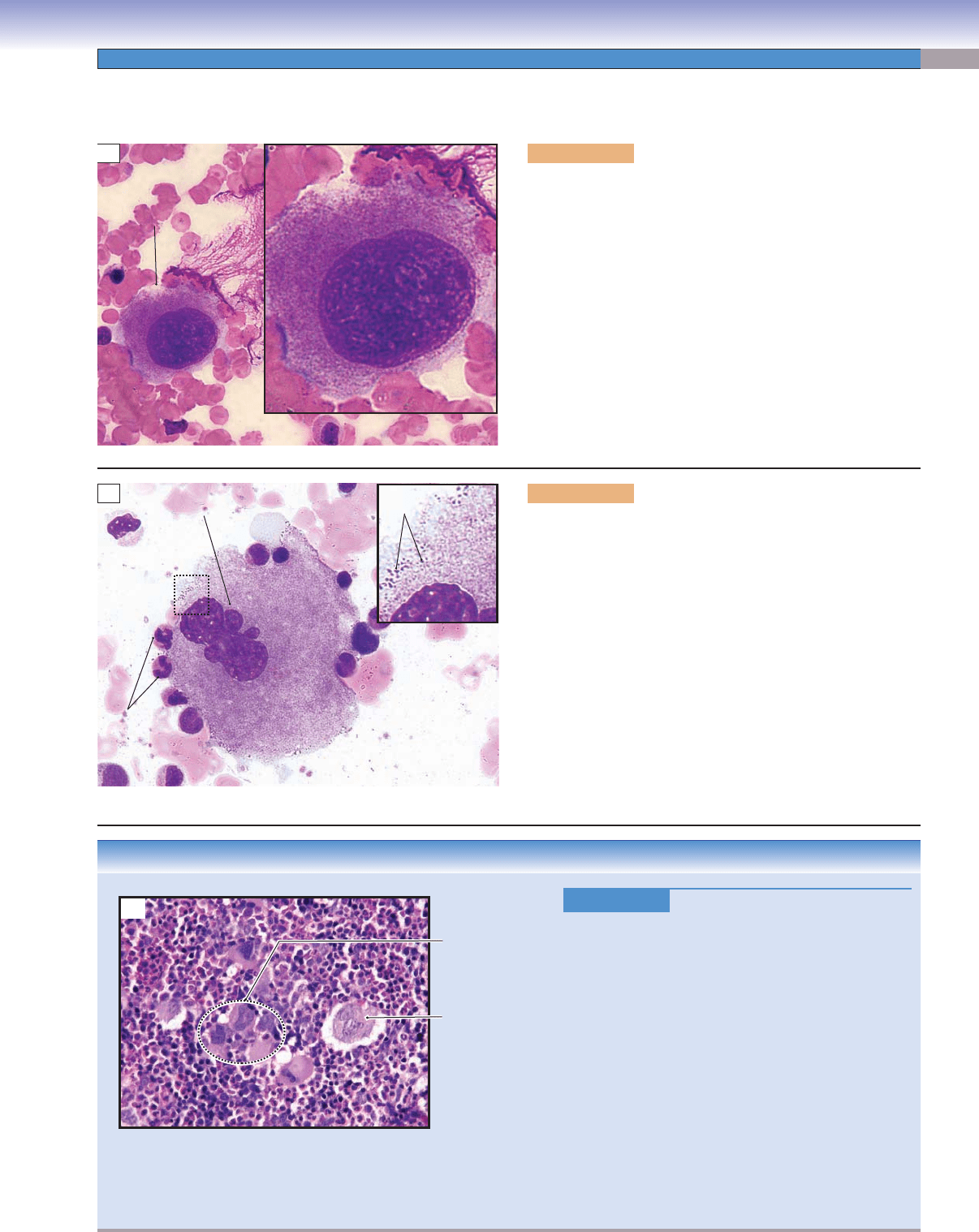
Figure 8-12A. Promegakaryocytes (immature megakaryo-
cytes), bone marrow smear. Wright stain, 754; inset 1,605
Promegakaryocytes develop from megakaryoblasts in the bone
marrow. Megakaryoblasts lose their ability to undergo cytokine-
sis but undergo a series of incomplete cell cycles called “endomi-
tosis” that results in replication of DNA up to 64N without
division of nuclei or cytoplasm. Each megakaryoblast has a
large nucleus, multiple nucleoli, and basophilic cytoplasm (Fig.
8-9B). Promegakaryocytes can be recognized by their large size,
round (or oval) nuclei, and large amount of cytoplasm. Devel-
opment of a demarcation membrane system is an important
feature of thrombopoiesis and begins at a very early stage. The
demarcation membrane system is produced by the invagination
of plasma membranes to form branched interconnected chan-
nels through the cytoplasm. This system may play an important
role in later subdivision of the cytoplasm into platelet zones.
Megakaryoblast
Megakaryoblast
Megakaryoblast
A
CLINICAL CORRELATION
Figure 8-12C.
Essential Thrombocytosis, Bone
Marrow
Smear. Wright stain, 304
Essential thrombocytosis, also called essential throm-
bocythemia, is one of the myeloproliferative disorders,
characterized by overproduction of platelets by mega-
karyocytes in the bone marrow without an identifi able
cause. The platelet counts exceed 600,000/μL, but the
platelets do not function properly. Symptoms and signs
include headache; bleeding from gums, nose, and gas-
trointestinal tract; throbbing and burning pain of the
hands and feet caused by thrombosis of small arteri-
oles; and splenomegaly (enlarged spleen). Treatment
includes using low-dose aspirin to control headache
and other vasomotor symptoms and anticancer agents
such as hydroxyurea to maintain proper platelet count.
Bone marrow smears show increased numbers of mega-
karyocytes. Large platelets, similar in size to red blood
cells, may be found in the peripheral blood.
Megakaryocytes
Megakaryocyte
C
Nucleus of the
megakaryocyte
Incipient
platelets
Neutrophils
B
Figure 8-12B. Megakaryocytes, bone marrow smear. Wright
stain, 754; inset 2,827
Because of their very large size, megakaryocytes are sometimes
called giant cells. They have a large indented (partially lobulated)
nucleus and an extremely voluminous cytoplasm containing a
variety of granules. In this stage, the demarcation membrane
system is complete, and the megakaryocytes are ready to release
platelets. Megakaryocytes tend to move toward and attach to
the marrow sinusoids (capillaries in the bone marrow) after
they become mature. The platelets are released into the blood
circulation through the wall of the marrow sinusoids. Note the
neutrophils on the surface of the megakaryocyte for size com-
parison. The inset shows incipient platelets (fragments of cyto-
plasm beginning to become platelets) in the surface region of the
megakaryocyte that are ready to be released. Thrombopoietin,
a humoral factor produced in the liver, is believed to regulate
megakaryocytes and the production of platelets.
CUI_Chap08.indd 149 6/17/2010 10:20:16 AM
150
UNIT 2
■
Basic Tissues
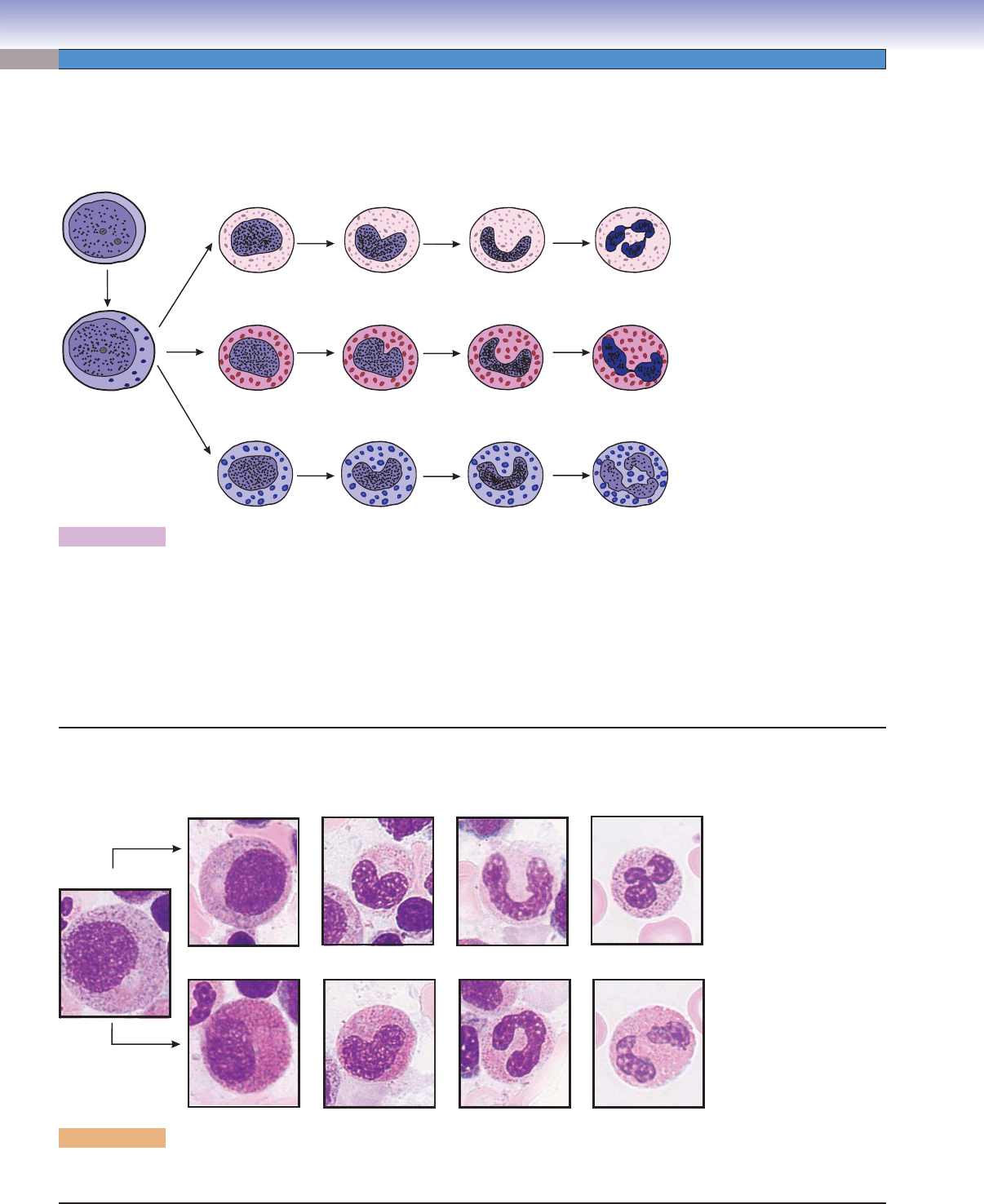
Promyelocyte
Eosinophilic
myelocyte
Eosinophilic
metamyelocyte
Eosinophil
Eosinophilic
stab cell
Neutrophil
Neutrophilic
myelocyte
Neutrophilic
metamyelocyte
Neutrophilic
stab cell
B
Figure 8-13B. Overview of stages of granulocytes in development, bone marrow smear. Wright stain, 1,569
These are examples of microphotographs that show various stages of granulocyte maturation in the neutrophilic and eosinophilic series.
Granulocytopoiesis
D. Cui
Neutrophilic
myelocyte
Neutrophilic
metamyelocyte
Neutrophilic
stab cell
Mature
neutrophil
Eosinophilic
myelocyte
Eosinophilic
metamyelocyte
Mature
eosinophil
Eosinophilic
stab cell
Basophilic
myelocyte
Basophilic
metamyelocyte
Mature
basophil
Basophilic
stab cell
Myeloblast
Promyelocyte
A
Figure 8-13A. A representation of granulocytopoiesis.
In addition to erythropoiesis, leukopoiesis also occurs in the bone marrow. Here are examples of the development of granular
leukocytes (granulocytopoiesis). The myeloblast is the earliest morphologically recognizable precursor cell. Cell division occurs in
myeloblasts, promyelocytes, and myelocytes. The myelocyte is the last stage that is capable of dividing. The generation of nonspe-
cifi c granules occurs in the promyelocyte stage and specifi c granules in the myelocyte stage. Maturation of granulocytes follows
this sequence: myeloblasts, promyelocytes, myelocytes, metamyelocytes, and stab (band) cells. The following morphologic changes
occur during granulocyte maturation: (1) nucleoli are present only before and during the promyelocyte stage; (2) the nucleus takes
the following shapes in different developmental stages: oval, elongated, indented, arched, and then segmented (lobed); and (3) spe-
cifi c granules are fi rst present at the myelocyte stage.
CUI_Chap08.indd 150 6/17/2010 10:20:23 AM
CHAPTER 8
■
Blood and Hemopoiesis
151
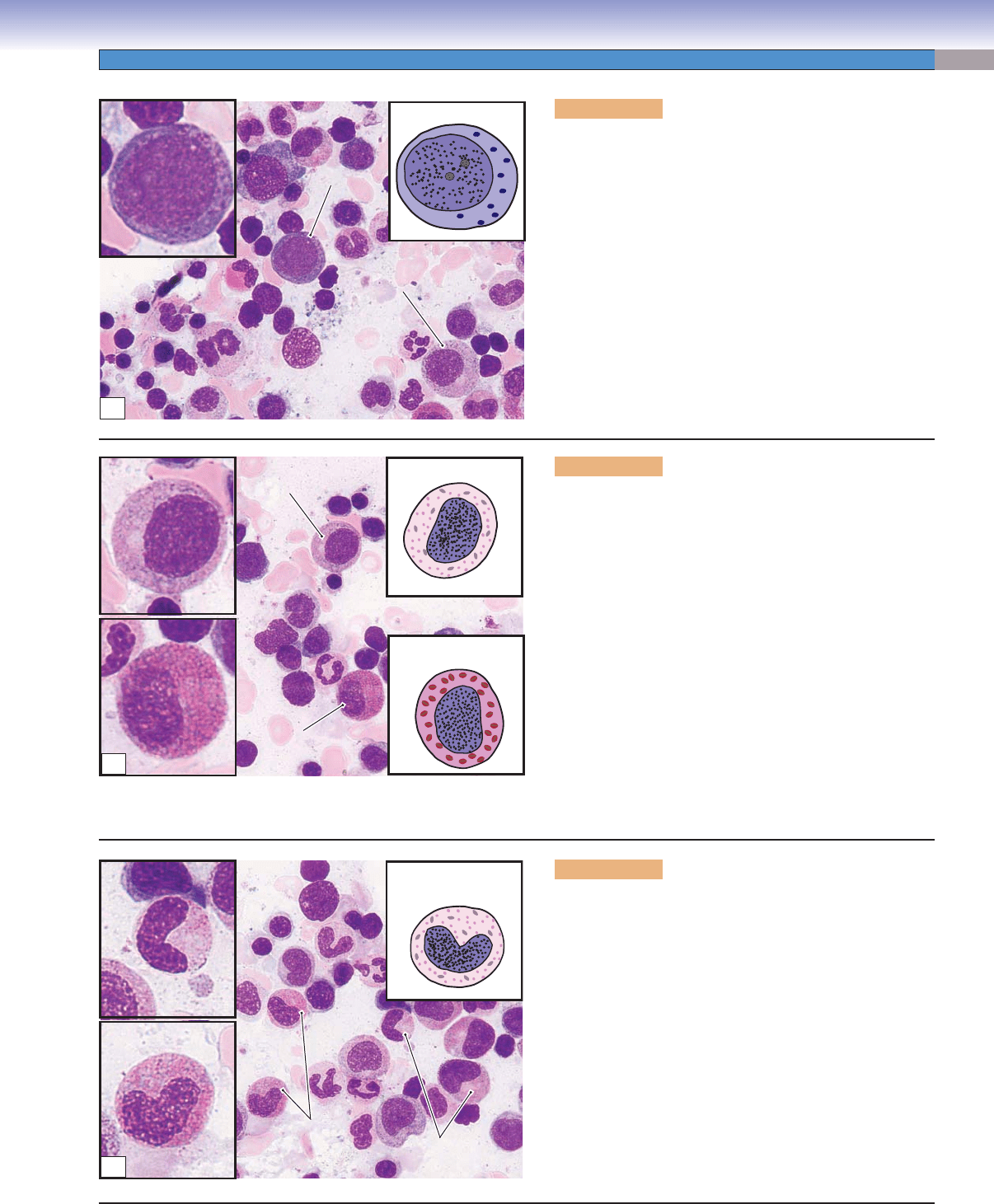
Figure 8-14A. Promyelocytes, bone marrow smear. Wright
stain, 710; inset 1,569
Promyelocytes (also called progranulocytes) have a round
or oval-shaped nucleus with one to three nucleoli. The cyto-
plasm is light blue, containing some dark purple granules
(azurophilic granules). At this stage, specifi c granules have
not been produced. Promyelocytes vary in size and are pro-
duced by the division of myeloblasts. Their nuclei have a
smooth, fi ne texture because of the fact that most of the
chromatin is euchromatin, which is delicately dispersed.
Promyelocytes divide to form myelocytes.
D. Cui
Promyelocyte
Promyelocyte
Promyelocyte
Promyelocyte
Promyelocyte
Promyelocyte
Promyelocyte
A
D. Cui
D. Cui
Neutrophilic
Neutrophilic
myelocyte
myelocyte
Neutrophilic
Neutrophilic
myelocyte
myelocyte
Eosinophilic
Eosinophilic
myelocyte
myelocyte
Eosinophilic
Eosinophilic
myelocyte
myelocyte
Neutrophilic
myelocyte
Neutrophilic
myelocyte
Eosinophilic
myelocyte
Eosinophilic
myelocyte
Neutrophilic myelocyte
Eosinophilic myelocyte
B
Figure 8-14B. Myelocytes, bone marrow smear. Wright
stain, 710; inset 1,569
The myelocyte has an oval or kidney-shaped nucleus that
has no nuclei and a coarse texture because of increasing
heterochromatin content. The cytoplasm contains azuro-
philic granules and specifi c granules. In this stage, the cell
has stopped producing azurophilic granules. In addition,
these granules have also been diluted during cell division,
so they are not as prominent as in the promyelocyte stage.
At the same time, the cell has begun to make and accumu-
late specifi c granules so that the cytoplasm starts to take
the characteristic appearance of a mature granulocyte. The
specifi c granules in neutrophilic myelocytes are small and
appear pinkish, granules in eosinophilic myelocytes are large
and stain red, and granules in basophilic myelocytes are
blue-purple. Basophilic myelocytes are least numerous and,
therefore, diffi cult to fi nd. In general, myelocytes are smaller
than promyelocytes, and their nuclear shape and size may
vary. This stage is the longest in granulocytopoiesis.
D. Cui
Eosinophilic
Eosinophilic
metamyelocyte
metamyelocyte
Neutrophilic
Neutrophilic
metamyelocyte
metamyelocyte
Neutrophilic
Neutrophilic
metamyelocyte
metamyelocyte
Eosinophilic
Eosinophilic
metamyelocyte
metamyelocyte
Eosinophilic
metamyelocyte
Eosinophilic
metamyelocyte
Neutrophilic
metamyelocyte
Neutrophilic
metamyelocyte
Neutrophilic
metamyelocyte
C
Figure 8-14C. Metamyelocytes, bone marrow smear.
Wright stain, 710; inset 1,569
Metamyelocytes are small cells. They have condensed
nuclei, which are elongated with various degrees of inden-
tation and contain clumped chromatin. Metamyelocytes
are unable to divide (myelocytes are the last stage in cell
division). The cytoplasm of metamyelocytes contains both
types of granules. Their cytoplasm and granules are similar
to those of mature granulocytes. At this stage, cells have
their distinguishing features, but their nuclei have not yet
become segmented.
CUI_Chap08.indd 151 6/17/2010 10:20:26 AM
152
UNIT 2
■
Basic Tissues
D. Cui
Neutrophilic
Neutrophilic
stab cell
stab cell
Eosinophilic
Eosinophilic
stab cell
stab cell
Basophilic
Basophilic
erythroblast
erythroblast
Neutrophilic
Neutrophilic
stab cell
stab cell
Eosinophilic
Eosinophilic
myelocyte
myelocyte
Eosinophilic
Eosinophilic
metamyelocyte
metamyelocyte
Neutrophilic
stab cell
Neutrophilic
stab cell
Eosinophilic
myelocyte
Eosinophilic
stab cell
Basophilic
erythroblast
Eosinophilic
metamyelocyte
Neutrophilic
Neutrophilic
myelocyte
myelocyte
Neutrophilic
myelocyte
Neutrophilic
Neutrophilic
stab cell
stab cell
Neutrophilic
stab cell
Neutrophilic stab cells
A
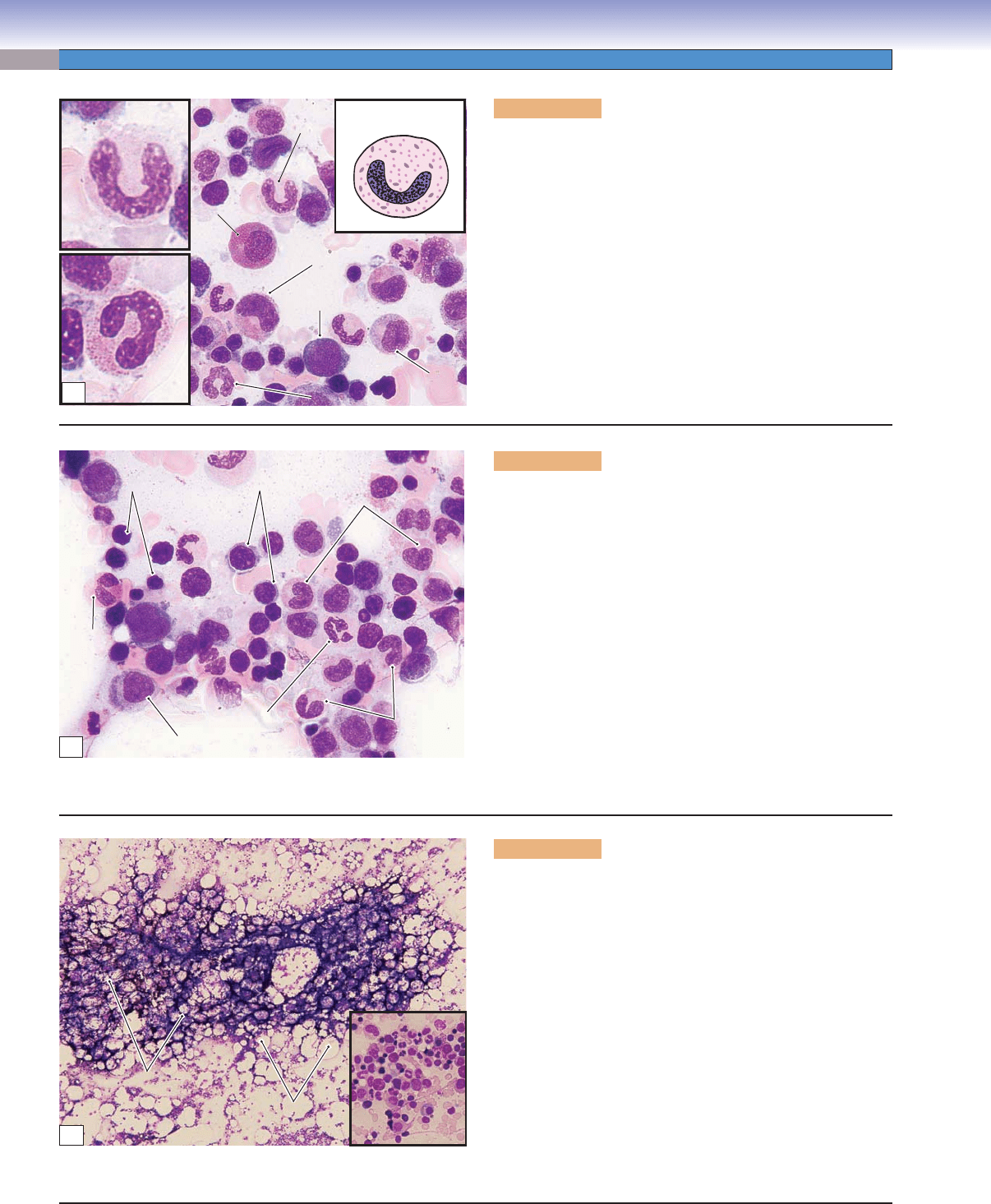
Figure 8-15A. Stab (band) cells, bone marrow smear. Wright
stain, 710; insets 1,569
Granular metamyelocytes mature to become stab cells, which
are also called band cells. The stab cells (mainly neutrophilic
stab cells) can be found in both the bone marrow and the
peripheral blood. Their nuclei are elongated and become band
and arch (or “C”) shaped, and the cytoplasm is the same as
that of mature neutrophils. These cells are the last stage of
granulocyte maturation without division, and in function and
structure, they are very close to mature neutrophils. The nuclei
of mature neutrophils become multilobulated (segmented),
contain dense heterochromatin, and often are described as
polymorphonuclear (or segmented) neutrophils.
Proerythroblast
Proerythroblast
Proerythroblast
Neutrophil
Neutrophil
Neutrophil
Neutrophilic
Neutrophilic
stab cell
stab cell
Neutrophilic
stab cell
Neutrophilic
Neutrophilic
metamyelocyte
metamyelocyte
Neutrophilic
metamyelocyte
Polychromatophilic
Polychromatophilic
erythroblasts
erythroblasts
Polychromatophilic
erythroblasts
Orthochromatophilic
Orthochromatophilic
erythroblast
erythroblast
Orthochromatophilic
erythroblast
Eosinophil
Eosinophil
Eosinophil
B
Figure 8-15B. Bone marrow cells, bone marrow smear. Wright
stain, 710
This is an example of blood cells at various stages of develop-
ment in the bone marrow, which includes both the erythro-
cyte and the granulocyte series. These cells, at various stages
of development, are densely packed together and can be found
randomly distributed in the bone marrow. During the matura-
tion process, the cell size becomes smaller and nuclei become
denser. In the erythropoiesis series, the cytoplasm of cells
becomes light blue and then more pink, and nuclei become
much denser and smaller and fi nally disappear. In the granu-
locytopoiesis series, the cytoplasm becomes less blue, primary
(nonspecifi c) granules are produced, and then specifi c granules
are produced and are present in myelocytes, which gives these
cells the appearance characteristic of their identity as a neutro-
phil, eosinophil, or basophil. In other changes, nuclei become
progressively denser, and the shape changes from round to
oval, elongated, indented, and then lobed (segmented).
Reticular tissue
Reticular tissue
Reticular tissue
Adipocyte
Adipocyte
spaces
spaces
Adipocyte
spaces
Blood cells
Blood cells
Blood cells
C
Figure 8-15C. Bone marrow, bone marrow smear. Wright
stain, 35; inset 184
Bone marrow is a specialized example of a reticular connec-
tive tissue, a loose connective tissue in which numerous cells are
supported by a delicate network of reticular fi bers. It resides in
cavities within bones (see Figs. 5-8, 5-10, and 5-11). Bone mar-
row can be categorized into red bone marrow and yellow bone
marrow. The term red bone marrow denotes active hematopoi-
esis; yellow bone marrow refers to a marrow composed chiefl y
of adipocytes (fat cells). Pictured is a smear of red bone marrow,
which contains many developing blood cells, a few adipocytes,
and some thin-walled blood vessels (sinusoidal capillaries). The
red bone marrow is organized into a hemopoietic compartment
and a vascular compartment. The hemopoietic compartment is a
network of reticular fi bers in which immature and mature blood
cells are suspended. The vascular compartment is composed of
mainly sinusoidal capillaries, which allow mature blood cells to
enter the blood circulation.
CUI_Chap08.indd 152 6/17/2010 10:20:33 AM

CHAPTER 8
■
Blood and Hemopoiesis
153
Hematopoietic
Hematopoietic
compartment
compartment
Hematopoietic
Hematopoietic
compartment
compartment
Sinusoidal
Sinusoidal
capillary
capillary
Hematopoietic
compartment
Hematopoietic
compartment
Sinusoidal
capillary
Leukocyte granules
Mature neutrophili
or neutrophilic
metamyelocyte
Leukocyte precursors
Endothelial cell
Sinusoidal
Sinusoidal
capillary
capillary
Sinusoidal
capillary
Figure 8-16. Developing blood cells in the bone marrow. EM, 5,000
The two compartments of the red (hematopoietically active) bone marrow can be distinguished here. The vascular compartment is
composed of sinusoidal capillaries, which in this view contain numerous mature erythrocytes. The hematopoietic compartment is
composed of blood cells and the precursors and progenitors of blood cells suspended in a loose network of support cells and reticu-
lar fi bers. The cells seen in the hematopoietic compartment here appear to be mostly developing or mature granulocytes. The exact
stage of differentiation of individual cells is not as clear here as it is in a bone marrow smear.
CUI_Chap08.indd 153 6/17/2010 10:20:41 AM
154
UNIT 2
■
Basic Tissues
SYNOPSIS 8-2 Hematopoiesis
Stem, Progenitor, and Precursor cells
Stem cells
■ are capable of differentiating into multiple cell lineages and can undergo proliferation indefi nitely.
Progenitor cells
■ are only capable of differentiating into a single cell lineage (restricted to one or two blood cell types) and
are morphologically undifferentiated.
Precursor cells
■ can be recognized morphologically as undergoing differentiation along a particular blood cell lineage.
Erythropoiesis
Cytoplasm
■ becomes progressively less basophilic because of dilution of ribosomes during the erythropoiesis process.
Nucleus size
■ progressively decreases because of increased condensation of chromatin.
Cell size
■ progressively decreases during the erythroid differentiation.
Cytoplasm
■ becomes progressively more eosinophilic because of increased accumulation of hemoglobin.
The nucleus
■ retains a round shape and no indentation occurs before it disappears.
Granulocytopoiesis
Cell size
■ decreases and nucleus becomes more condensed as in erythropoiesis.
Nucleus shape
■ changes from round or oval (promyeloblasts) to kidney shaped/slightly indented (myelocytes) and then
changes from deeply indented (metamyelocytes) to band shaped (band cells) and fi nally to lobed (mature granulocytes).
Promyelocytes
■ do not have specifi c granules (only azurophilic granules); at this stage, it is too early to tell which granular
leukocytes they will become.
Myelocytes
■ are the last developmental stage capable of dividing; specifi c granules accumulate in this stage.
SYNOPSIS 8-3 Pathological and Clinical Terms for Mature and Developing Blood Cells
Gray platelet syndrome ■ : This condition is characterized by a defi ciency or absence of the alpha granules and contents in
blood platelets, giving platelets a gray appearance in a Wright stain smear (Fig. 8-2B).
Platelet storage pool defi ciency
■ : Disorder caused by a decrease or absence of platelet delta granules (dense bodies),
which normally store and release adenine nucleotides and 5HT. “Platelet-type” bleeding is common with this defi ciency
(Fig. 8-2B).
Petechiae
■ : Minute red or purple spots on the skin or mucous membranes caused by capillary hemorrhage; common causes
include physical trauma and decreased platelets (thrombocytopenia).
Smudge cell
■ : Damaged lymphocytes seen on a peripheral blood smear caused by mechanical stress in the process of
producing the smear; although nonspecifi c, smudge cells are encountered more frequently on blood smears of patients with
chronic lymphocytic leukemia (Fig. 8-4C).
Reticulocytosis
■ : Increased reticulocytes in the blood, often in response to blood loss, stimulation by erythropoietin treat-
ment, or treatment of iron defi ciency anemia with iron supplementation (Fig. 8-11C).
Thrombocytosis
■ : Increased platelet count in the blood, which may be reactive or neoplastic, as in the disease essential
thrombocytosis (Fig. 8-12C).
CUI_Chap08.indd 154 6/17/2010 10:20:44 AM
