Cao Z. (Ed.) Thin Film Growth: Physics, materials science and applications
Подождите немного. Документ загружается.


258 Thin fi lm growth
© Woodhead Publishing Limited, 2011
moment in a slab of N unit cells can be approximated with the help of a
plate capacitor model:
VN
VN
d
tota
VN
tota
VN
l
VN
l
VN
0r
VN =VN
1
VN
1
VN
·
·
ee
0r
ee
0r
·
ee
·
0r
·
0r
ee
0r
·
0r
s
[11.2]
whereby e
0
and e
r
are the vacuum permittivity and the dielectric constant of the
material, respectively (Kittel, 1996). To get an idea of the energies involved,
the electrostatic potential is calculated for a free-standing MgO(111) bilayer
(e
r
~ 10). The system consists of a positively-charged Mg
2+
and a negative-
charged O
2–
plane (s = 2|e|/7.8 Å
2
) separated by the bulk lattice parameter
of 1.8 Å and already has an electrostatic energy of 8.3 eV. Apparently, the
values for thicker slabs easily reach the lattice energy in ionic crystals of
20–30 eV, rendering polar materials energetically unstable. Bulk-like structures
with uncompensated polarity should therefore not exist in nature.
The latter statement seems to be in con ict with the experimental
observation of systems with polar character. Moreover, polar terminations
are often characterized by a lower surface free energy than their non-polar
counterparts. For example, MgO cubes terminated by non-polar (100) surfaces
were found to develop polar (111) facets in a humid environment, although
this should be accompanied by a dramatic increase of the electrostatic energy
(Hacquart and Jupille, 2007). Similarly, a (111)-type NiO surface has been
identi ed as the one with the lowest free energy even with respect to the
non-polar (100) and (110) planes (Barbier et al., 2000). This discrepancy
can be solved by considering the various polarity-healing mechanisms that
are able to remove the electrostatic dipole and therewith the polarity of the
respective surface (Goniakowski et al., 2008). From a purely mathematical
viewpoint, polarity cancellation is achieved by adjusting a distinct charge
density at the surface that compensates the bulk dipole. For simple systems,
the required surface charge density is given by:
ss
su
ss
su
ss
rf
ss
rf
ss
ss
=
ss
ss
ss
d
D
[11.3]
d
D
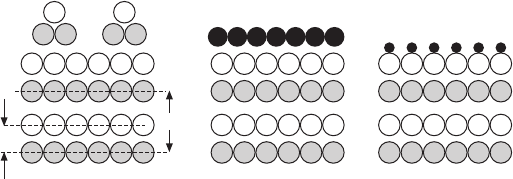
(a) (b)
0 sd 2sd
Thickness
2D
D
d
11.2 (a) Vertical cut through a polar system. The layer distance d
and the size of the unit cell D are indicated. (b) Dependence of the
electrostatic energy on the thickness of the polar slab.
Energy
ThinFilm-Zexian-11.indd 258 7/1/11 9:43:26 AM
259Electronic properties and adsorption behaviour of thin films
© Woodhead Publishing Limited, 2011
with D being the height of the unit cell and d the interlayer distance in the
crystal. As an example, layers with 25% and 50% of the bulk charge density
need to be formed on the wurzite (0001) (d/D = 0.25) and the rocksalt (111)
surface (d/D = 0.5) in order to remove the polarity in the whole system. In
simple terms, the polarity annihilation might be understood in these cases
as the result of an additional dipole that is created between the upper and
lower surface of the slab and has exactly the same size but an opposite sign
as the bulk moment.
Several mechanisms have been identied that enable an adjustment of
the required charge density at the surface (Noguera, 1996; Goniakowski
et al., 2008). A rst possibility is the restructuring of the top-most surface
layers, creating planes with fractional atom lling and hence modied
charge distribution (Fig. 11.3a). The best studied example is the octopolar
reconstruction of the rocksalt (111) surface, which was predicted by
Wolf in 1992 and later veried experimentally through X-ray diffraction
measurements on NiO(111) and MgO(111) (Barbier et al., 2000; Finocchi
et al., 2004). The octopolar reconstruction comprises two surface layers
with 75% and 25% atom lling with respect to the ideal plane, which adds
up to the required 50% charge density at the surface according to Eq. 11.3.
The dipole compensation in this case is so efcient that the free energy of
the reconstructed surface drops below the value for comparable non-polar
oxide planes (Barbier et al., 2000). Alternative reconstructions have been
identied for other lattice geometries and different chemical environments.
For instance, a spinel-like termination was found to develop on NiO(111) at
low O
2
partial pressures (Barbier and Renaud, 1997; Barbier et al., 1999),
while several non-stoichiometric surface compositions were revealed for
srTiO
3
(111) in a Sr- or O-rich environment (Bottin et al., 2003). For the
Zn-terminated ZnO(0001) surface, polarity healing has been investigated
at the local scale with the help of scanning tunnelling microscopy (STM)
(Parker et al., 1998; Dulub et al., 2003; Ostendorf et al., 2008). The studies
revealed triangular-shaped islands and pits of 1 ML height that cover the
d
D
Reconstruction
(a)
Metallization
(b)
Adsorption
(c)
11.3 Sketch of the three main polarity-healing mechanisms in ionic
systems.
ThinFilm-Zexian-11.indd 259 7/1/11 9:43:26 AM
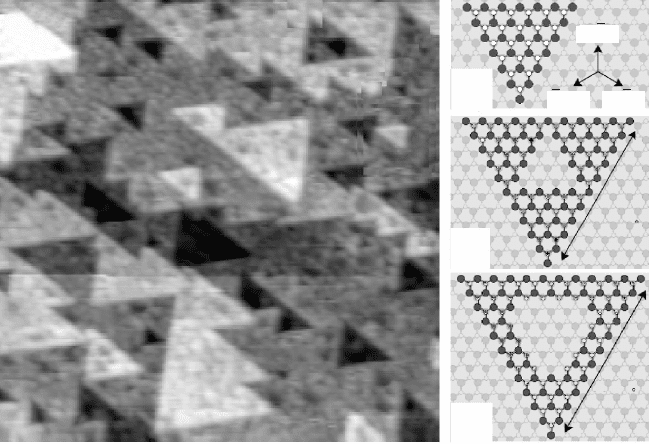
260 Thin film growth
© Woodhead Publishing Limited, 2011
complete oxide surface (Fig. 11.4a). According to density functional theory
(DFT) calculations, the edges of those triangular structures are saturated with
O atoms, rendering the surface oxygen-rich with respect to the bulk (Kresse
et al., 2003). As discussed above, the polarity of wurzite(0001) is cancelled
by creating a surface layer with 25% extra charges, which corresponds to
a local Zn:O stoichiometry of 3:4 with respect to the bulk value of 1:1.
Exactly this ratio is adjusted by introducing the triangular islands and pits
into the ZnO surface. Each equilateral triangle comprises ½ · n · (n + 1)
anions and ½ · n · (n – 1) cations (n being the side length) and has an excess
of n oxygen ions. The desired 3:4 surface stoichiometry is now realized by
forming islands with seven edge atoms (n = 7), a structure that is indeed
frequently found on the surface (Fig. 11.4b). For larger islands, additional
atoms have to be removed from the layer underneath to reach the ideal Zn:O
surface ratio (Fig. 11.4c,d). The patched Zn-terminated ZnO(0001) surface
is therefore an ideal example for polarity healing via surface reconstruction
(Dulub et al., 2003).
(a)
(b)
(c)
(d)
[1010]
[1100] [0110]
11.4 (a) Empty-states STM image of Zn-terminated ZnO(0001) (50 ¥
50 nm
2
). The terraces are covered with triangular islands and pits
that are involved in healing the surface polarity. (b–d) Structure
models of three triangular islands of different size. The island
borders are saturated with oxygen. The resulting non-stoichiometry
produces a charged surface layer that compensates the bulk
dipole moment. Reprinted with permission from Dulub et al., 2003,
Copyright (2010) by the American Physical Society.
ThinFilm-Zexian-11.indd 260 7/1/11 9:43:27 AM
261Electronic properties and adsorption behaviour of thin films
© Woodhead Publishing Limited, 2011
Alternatively, dipole compensation might be achieved via purely electronic
effects, leaving the atomic structure of the surface unchanged (Fig. 11.3b).
One proposed mechanism is the creation of surface states, the electron lling
of which is adjusted to reach the required charge density for polarity healing
(Goniakowski et al., 2008). The formation of a partly-lled electronic state is
always connected with the metallization of the oxide surface. This particular
mechanism has been predicted for bulk Al
2
O
3
(0001) (Wang et al., 2000)
and thin, unreconstructed MgO(111) lms (Goniakowski et al., 2007). The
formation of dipole-compensating surface states becomes particularly easy
when the polar oxide is capped by a metal lm, as demonstrated for Pd and
Cu over-layers on MgO(111) and ZnO(0001), respectively (Goniakowski
and Noguera, 2002; Meyer and Marx, 2004).
A third way to compensate the polarity of oxide materials is the binding of
ad-species that become charged upon adsorption (Fig. 11.3c). The prototype
adsorbate to heal surface polarity is hydrogen, which forms hydroxyl groups
consisting of a surface oxygen ion and a positively charged H
+
ion. The
hydroxylation of oxide surfaces is often triggered by the heterolytic splitting
of water, which renders this compensation mechanism especially efcient in
an ambient environment. Hydroxylation was predicted to occur spontaneously
on most rocksalt (111) surfaces (Pojani et al., 1997), on ZnO(0001) and
on Al
2
O
3
(0001) (Wang et al., 2000). It has been revealed experimentally
for instance on MgO(111) (Poon et al., 2006; Hacquart and Jupille, 2007),
NiO(111) (Rohr et al., 1994; Kitakatsu et al., 1998) and ZnO(0001) (Wang,
2008) by detecting the O–H vibrational bands. Also combined mechanisms
are reported, where molecular adsorption induces the formation of a partly-
lled surface state at the Fermi level, which in turn removes the surface
polarity (Wang et al., 2005). Adsorbate-mediated polarity healing, in general,
is responsible for the unique binding properties of polar systems and their
enhanced chemical reactivity with respect to non-polar materials (Sun et al.,
2009).
11.2 Polar oxide films
Whereas for bulk materials the polarity needs to be healed in order to
avoid a divergence in the electrostatic energy, thin lms grown on metal
and semiconductor supports can be stabilized even in a polar state. This
difference to bulk materials relies on two effects. First, the electrostatic
energy might be kept below the lattice energy of the lm, as the number
of polar units is small (see Eq. 11.2). As a consequence, reconstruction of
the surface can be avoided and the lm keeps its polar nature. Second, the
substrate contributes to a reduction of the lm dipole, especially when using a
polarizable metal support. In this case, the required charge density that heals
the polarity according to Eq. 11.3 is provided by the substrate and localized
ThinFilm-Zexian-11.indd 261 7/1/11 9:43:27 AM
262 Thin film growth
© Woodhead Publishing Limited, 2011
at the metal–oxide interface (Goniakowski and Noguera, 2002, 2009). The
interplay between a polar lm and a metal support shall be demonstrated in
the following for a simple bilayer structure, consisting of a cationic and an
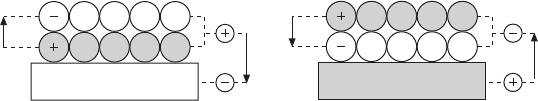
anionic plane (Fig. 11.5).
The preferred adsorption geometry of the lm is given by the electron
afnity of the substrate. Whereas the interface is formed by positively charged
ions on electronegative metals (e.g. Au and Pt), a negatively charged oxygen
layer sits on top of electropositive materials (e.g. Mg and Al) (Goniakowski
and Noguera, 2009). In the rst case, electrons accumulate in the topmost
substrate plane, leading to the following sequence of charged layers: metal
surface(–)/cations(+) / anions(–). In the second scenario, the charge distribution
changes to metal(+)/anions(–)/cations(+), as the electron density in the metal
surface is depleted by the polar lm. In both cases, the tri-layer structure has
a reduced vertical dipole moment and hence low polarity. This substrate-
mediated effect on the polarity might be enhanced by a real charge transfer,
whereby electrons ow out of the polar lm on electronegative metals but
into the ad-layer on electropositive supports. The resulting charge-driven
dipole always aligns in opposite direction to the lm moment and further
quenches the polarity of the metal–oxide system. The various electrostatic
interactions that occur between a metal surface and a polar lm, as well as
the dipole moments involved are summarized in Fig. 11.5.
Thanks to efcient polarity stabilization schemes, thin oxide lms with
residual dipole moment can be prepared on metal surfaces, even if the respective
bulk oxides are thermodynamically unstable. Well-studied examples for polar
oxide lms are MgO(111) on Ag(111) and Au(111) (Kiguchi et al., 2003;
Arita et al., 2004; Mantilla et al., 2008; Myrach et al., 2011), ZnO(0001)
on Ag(111) (Tusche et al., 2007), CoO(111) on Ir(100) (Giovanardi et al.,
2006) as well as FeO(111) on Pt(111) (Vurens et al., 1988; Ritter et al., 1998;
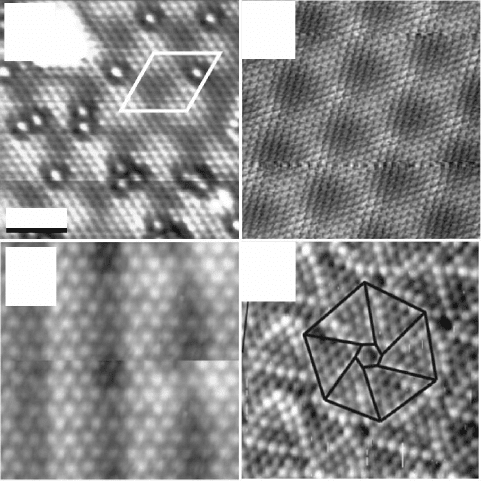
Rienks et al., 2005). STM topographic images of the respective lms are
displayed in Fig. 11.6. The residual polarity of the various systems depends
µ
film
µ
film
µ
supp
µ
supp
Electronegative support Electropositive support
(a) (b)
11.5 Sequence of oxide layers at the interface to an electronegative
(a) and an electropositive (b) support. In both cases, the intrinsic
dipole of the film is oriented in opposite direction to the interface
dipole that results from a polarization/charge transfer between oxide
and metal support. This compensation effect partly quenches the
polarity of the combined system.
ThinFilm-Zexian-11.indd 262 7/1/11 9:43:27 AM
263Electronic properties and adsorption behaviour of thin films
© Woodhead Publishing Limited, 2011
largely on the lm structure and the nature of the metal–oxide interactions
and is rather different in all cases. Rocksalt MgO(111) is only polar in the
limit of ultrathin lms, but becomes non-polar with increasing thickness due
to the formation of 3D oxide islands (Myrach et al., 2011). In ZnO(0001)/
Ag(111), on the other hand, the polarity is suppressed already in the rst
atomic planes, which adopt a hexagonal boron nitride structure with Zn and
O ions lying in the same layer (Tusche et al., 2007). The wurzite structure
of bulk ZnO is only restored in thicker lms, which are, however, subject
to considerable surface roughening that quenches the reappearing surface
dipole. In rocksalt CoO(111) and spinel Co
3
O
4
(111), polarity healing is
achieved by a substantial decrease in the interlayer distance between the
top-most O and Co planes and a concomitant reduction of the ionicity of
the surface species (Giovanardi et al., 2006). A similar means to reduce the
surface polarity has been identied for FeO(111) on Pt(111). Using X-ray
diffraction techniques, the Fe-O layer separation in the lm was determined
(a)
(c)
(b)
(d)
22.5 Å
11.6 STM topographic images of polar oxide films prepared on
metal supports. (a) ZnO/Ag(111) (9 ¥ 9 nm
2
, 1.2 V). Reprinted with
permission from Tusche et al. (2007), Copyright (2010) by the
American Physical Society. (b) FeO/Pt(111) (9 ¥ 9 nm
2
, 0.65 V). (c)
CoO/Ir(100) (4.5 ¥ 4.5 nm
2
, 0.15 V). Reprinted with permission from
Giovanardi et al. (2006), Copyright (2010) by the American Physical
Society. (d) VO/Rh(111) (6 ¥ 6 nm
2
, 2.0 V). Reprinted with permission
from Parteder et al. (2008), Copyright (2010) by Elsevier.
ThinFilm-Zexian-11.indd 263 7/1/11 9:43:27 AM
264 Thin film growth
© Woodhead Publishing Limited, 2011
as 0.68 Å, which is 50% smaller than the bulk value and substantially
decreases the surface dipole (Kim et al., 1997). As a result of this vertical
contraction, the FeO layer is expanded within the surface plane and the Fe
nearest-neighbour distance increases from its bulk value of 3.0 Å to 3.1 Å
in the lm. A similar tetragonal distortion has been revealed for other polar
lms, e.g. for VO/Rh(111) (Schoiswohl et al., 2005; Parteder et al., 2008)
and TiO
x
/Pt(111) (Sedona et al., 2005), indicating the universal nature of
this polarity healing mechanism.
In all these experiments, changes in the oxide stoichiometry and parasitic
adsorption processes have been excluded as means to heal the oxide polarity.
Thin oxide lms that can be prepared on various metal supports are therefore
well suited to study the adsorption behaviour of polar materials. However,
before this issue is addressed in Section 11.4, experimental techniques
to quantify the amount of oxide polarity will be introduced in the next
Section.
11.3 Measuring polarity of thin oxide films
In most experiments, the polar nature of thin lms is concluded from indirect
evidence, for instance from a reconstruction of the surface (Dulub et al.,
2003; Ostendorf et al., 2008), unusual electronic properties (Kiguchi et al.,
2003; Arita et al., 2004) or an adsorption behaviour that strongly deviates
from the non-polar case (Rohr et al., 1994; Wang et al., 2005; Poon et al.,
2006). Such indirect indications give no information on the nature of the
actual polarity healing mechanism and on the size of the remaining surface
dipole. However, this kind of data can be obtained with STM, a technique
that is usually employed to probe the surface topography. In the following,
two spectroscopic applications of STM are discussed that enable detection of
the local surface potential as a measure for the polarity. The techniques are
based on the evaluation of (i) energy positions of eld emission resonances
(FER) and (ii) effective barrier heights for electron-tunnelling into the
polar lm. They are demonstrated using the example of the FeO(111) lm
mentioned above (Rienks et al., 2005), but can be applied to any other polar
system with sufcient conductivity to perform STM.
The FeO lm has a bilayer structure, consisting of a hexagonal O layer
at the surface and a hexagonal Fe plane at the interface to the Pt(111) (Fig.
11.7). It has a polar character due to the ionic nature of the Fe
d+
and O
d–
species and belongs to type III materials in the Tasker scheme (Tasker,
1979). The FeO lm has an in-plane lattice parameter of 3.1 Å, being 11%
larger than that of Pt(111) (2.76 Å). This mismatch leads to the formation of
a coincidence lattice with 25 Å edge length and a crystallographic relation
of (√91 ¥ √91)R ± 5.2° with respect to the Pt support (Ritter et al., 1998).
Within the coincidence cell, three stacking domains are distinguishable that
ThinFilm-Zexian-11.indd 264 7/1/11 9:43:27 AM
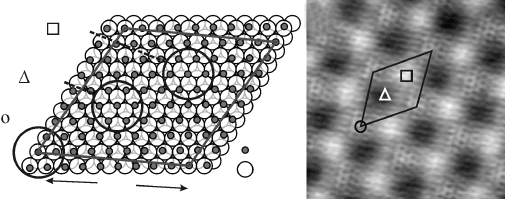
265Electronic properties and adsorption behaviour of thin films
© Woodhead Publishing Limited, 2011
differ in their Fe binding geometry on the Pt(111) surface (Fig. 11.7a). In the
top domain, the interfacial Fe atoms bind on top of Pt, while the O atoms in
the layer above occupy fcc hollow sites. In the fcc (hcp) domains, Fe binds
to fcc (hcp) hollow sites while O sits in hcp (top) positions of the Pt(111)
lattice. The different binding congurations give rise to a distinct contrast of
the FeO lm in low-bias STM images (Fig. 11.7b). The discernible regions
have been assigned to the underlying stacking domains with the help of
model calculations based on DFT (Giordano et al., 2007b) and a quantum-
chemical scattering approach (Galloway et al., 1996).
The binding of Fe and O atoms either to Pt hollow or top sites modulates
the interlayer distance and hence the surface dipole in the three oxide domains.
The resulting change in polarity is an observable quantity and produces a
strong bias-dependent contrast of the FeO lm in STM images taken at
high sample bias (Fig. 11.8b). At 4.25 V, one region of the coincidence
cell (marked with a circle) is imaged with a particularly bright contrast.
The contrast is not related to a geometric effect, as the low-bias corrugation
of the lm that reects its true morphology is very small (0.3 Å). It is, in
fact, of electronic origin and caused by a strong increase in the conductance
through the STM junction at this particular bias. To verify this statement, the
differential conductance (dI/dV) through the FeO/Pt(111) system has been
measured directly as a function of bias voltage, as shown in Fig. 11.8a. In
correspondence to the topographic image, high dI/dV intensity at 4.25 V
is observed in the domain marked by the circle. However, with increasing
bias the dI/dV maximum moves to an adjacent domain, being agged by
the square, and nally appears in the triangle region at 4.9 V sample bias.
These bias-dependent conductance changes are caused by electron transport
through conned states that develop in the classical part of an STM junction
and become accessible for electrons at elevated bias (Binnig et al., 1982;
fcc
hcp
top
25 Å
–Fe
–Pt
(a) (b)
11.7 (a) Structure model of the coincidence cell formed between FeO
and Pt(111). The O-top layer is omitted for the sake of clarity. (b)
STM topographic image taken at 65 mV (7 ¥ 7 nm
2
). The different
stacking domains are assigned in accordance to the DFT calculations
presented in Giordano et al. (2007b).
ThinFilm-Zexian-11.indd 265 7/1/11 9:43:27 AM

266 Thin film growth
© Woodhead Publishing Limited, 2011
Becker et al., 1985). Those states may be considered as eigenstates in a
triangular potential, being conned by the sample surface on one side and the
down-sloping vacuum barrier on the other (Fig. 11.9b). Electrons penetrating
this classical region are able to form standing waves, if multiples of half
their electron wavelength match the distance between the two boundaries.
In this case, propagating and reected waves interfere constructively and
quasi-bound electronic states with a high transmission probability develop in
the STM junction. These states are termed eld emission resonances (FER)
and dominate the STM image contrast at elevated bias. Interestingly, they
also contain information on the local surface potential F and might therefore
be used to probe oxide polarity (Fig. 11.9b). The interplay between FER
and F can be explained with a simple picture. The electrostatic energy due
to uncompensated polarity (Eq. 11.2) produces an offset on the surface
potential that shifts the FER to higher energy and modulates their availability
for electron transport through the oxide lm. Up-shifted FER are therefore
indicative for polar regions, while down-shift states occur in areas with
compensated polarity.
Already this crude model is sufcient to connect the contrast changes
observed in the dI/dV maps of FeO/Pt(111) to spatial modulations of the
surface polarity (Rienks et al., 2005). The domain that turns bright at 4.25 V
is the one with lowest surface potential, as the rst FER becomes available
at relatively low bias (Fig. 11.8a). Based on a simple hard-sphere model of
the lm, this region is assigned to the FeO top domain, in which Fe and
O atoms occupy top and hollow sites of the Pt surface, respectively, and
the Fe-O interlayer distance and hence the surface dipole are small. At 4.5
V, the FER become available in the square regions, which have a slightly
higher F as indicated by the up-shifted resonances. The region marked by the
(a)
(b)
4.3 V 4.5 V 4.7 V 4.8 V 4.9 V
11.8 (a) Conductance and (b) topographic images of FeO/Pt(111)
taken as a function of the bias voltage (9 ¥ 9 nm
2
). The contrast
change reflects the varying contributions of field emission
resonances to the electron transport through the different stacking
domains. Reprinted with permission from Rienks et al. (2005),
Copyright (2010) by the American Physical Society.
ThinFilm-Zexian-11.indd 266 7/1/11 9:43:28 AM

267Electronic properties and adsorption behaviour of thin films
© Woodhead Publishing Limited, 2011
triangle turns bright only at 4.9 V and consequently has the highest F and
hence the highest degree of polarity. The hard-sphere model connects this
region to the hcp domains, as the vertical distance between the hollow-bound
Fe atoms and the top-bound O atoms is expected to be largest there. The
sequential availability of FER in the different domains is not so evident in the
topographic images, which contain information on the integral conductance
probed over a large bias window (Fig. 11.8b).
top
fcc
hcp
Conductance
Energy
1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0
Sample bias (V)
(a)
E
vac
f
Tip
f
Sample
E
F
-eV
Tip
FeO
Barrier
Pt(111)
E
F
e
–
FER
levels
n = 5
n = 4
n = 3
n = 2
n = 1
(b)
11.9 (a) Conductance spectra obtained with closed feedback loop
on the three stacking domains of the FeO film, as indicated in the
STM image shown in the inset. Each maximum marks the position
of a field emission resonance (FER) in the tip-sample junction. (b)
Potential diagram visualizing the formation of FER at high bias
voltage and their dependence on the local work function. Reprinted
with permission from Rienks et al. (2005), Copyright (2010) by the
American Physical Society.
ThinFilm-Zexian-11.indd 267 7/1/11 9:43:28 AM
