Cao Z. (Ed.) Thin Film Growth: Physics, materials science and applications
Подождите немного. Документ загружается.


268 Thin fi lm growth
© Woodhead Publishing Limited, 2011
The changes in the electrostatic potential due to the modulations in the
polarity can even be quanti ed for the three stacking domains of FeO/
Pt(111) (Rienks et al., 2005). For this purpose, the position of the rst
six FER was detected with dI/dV spectroscopy performed in a large bias
window (1–10 V) (Fig. 11.9a). In correspondence to the dI/dV maps, the
series of FER starts in the top domain at 4.25 V (see circle in Fig. 11.8a),
whereas the fcc (square) and hcp (triangle) domains follow at 4.7 and 5.1
V, respectively. It should be noted that due to the limited spatial resolution
of dI/dV spectroscopy at high bias, low-lying FER of neighbouring domains
show up in the spectrum of a selected region as well. Consequently, the dI/
dV peaks of fcc and hcp domains are split into two and three maxima with
the lower ones arising from conductance contributions of the adjacent top
and fcc domains, respectively.
The local surface potential F is determined from tting the experimental
FER positions to a quantum-mechanical model that describes the resonances
as eigenstates in a triangular potential. The well is con ned by the sample
surface and the vacuum barrier that slopes down with the tip-induced electric
eld F (Fig. 11.9b) (Kolesnychenko et al., 2000). Here, F is assumed to be
constant during spectroscopy due to the enabled feedback loop of the STM.
The position of the nth FER calculates to:
eV
e
m
Fn
n
eV
n
eV
2/
3
2/
Fn
2/
Fn
32
Fn
32
Fn
/3
=
+
3
22
22
F
p
Ê
Ë
Á
Ê
Á
Ê
Ë
Á
Ë
ˆ
¯
˜
ˆ
˜
ˆ
¯
˜
¯
with m being the free electron mass (Kolesnychenko et al., 2000). A t of
all experimental FER except the rst one, whose energy position is altered
by other effects, yields the surface potential. The derived F values increase
from 3.5 eV in the top region to 3.65 and 3.85 eV in the fcc and hcp domains,
in good agreement with the results of the dI/dV maps. Conclusively, the
hcp and top domains are the most and least polar ones, corroborating the
predictions from the hard sphere model.
Complementary information on the surface polarity is obtained by measuring
the effective barrier height F
eff
that is experienced by electrons tunnelling
into the FeO lm (second approach). The barrier height is measured via dI/dz
spectroscopy, detecting the current response I to a change in the tip-sample
distance z (Chen, 1993). The relationship between those quantities can be
derived from a one-dimensional model for electron tunnelling through a
square-shaped barrier:
F
ef
f
effef
2
=
ln
8
2
m
dI
lndIln
dz
Ê
Ë
Á
Ê
Á
Ê
Ë
Á
Ë
ˆ
¯
˜
ˆ
˜
ˆ
¯
˜
¯
Although the barrier height is not identical to the real surface potential, it is
a monotonous function of F (Olesen et al., 1996). It will thus be largest on
ThinFilm-Zexian-11.indd 268 7/1/11 9:43:28 AM
269Electronic properties and adsorption behaviour of thin films
© Woodhead Publishing Limited, 2011
the most polar oxide region, as the electrons have to overcome a substantial
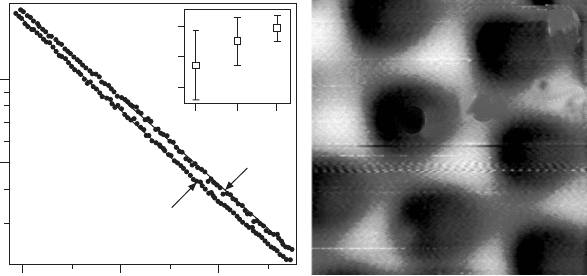
surface dipole. Figure 11.10 shows two approaches to probe the effective
barrier height of the FeO thin lm. In Fig. 11.10(a), the logarithmic current
response to a linear distance ramp is recorded and F
eff
is extracted from the
slope of the ln(I)-Dz curves. In Fig. 11.10(b), the d(lnI)/dz signal is mapped
directly with lock-in technique by adding a small z-modulation to the STM
feedback loop. In both cases, spatial variations in the effective barrier height
are detected on the FeO surface. Here, the top domains exhibit the smallest
slope in ln(I)-Dz curves and the lowest intensity in d(lnI)/dz maps, indicating
a low barrier height. The hcp domains, on the other hand, are characterized
by a large F
eff
value, in agreement with their more polar nature. The two
techniques discussed above, namely evaluating the FER energies and probing
the effective barrier height, therefore reveal similar modulations in the local
surface potential and provide unmatched insight into the polarity of the three
FeO stacking domains.
The experimental results obtained on the polar FeO/Pt(111) lm have been
corroborated by DFT calculations (Giordano et al., 2007b). Due to the large
size of the real FeO-Pt coincidence cell, they were performed with a smaller
computational cell that still contains Fe atoms in characteristic top, fcc and
hcp binding congurations (Fig. 11.11a). While the hcp and fcc regions
display similar properties in the simulations, the top domain sticks out in
various aspects. It is characterized by the largest interfacial separation from
top
fcc
hcp
0 0.4 0.8
Dz (Å)
(a) (b)
I (nA)
1.0
0.5
hcp
top
top fcc hcp
F (eV)
3.5
3.3
3.1
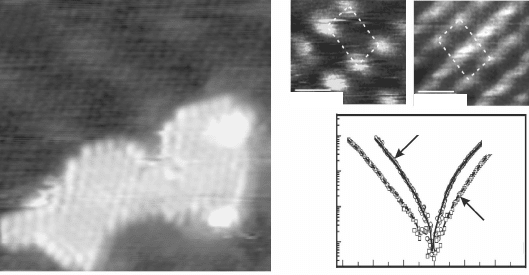
11.10 (a) Tunnel current versus tip-sample distance recorded for the
top and hcp domain of the FeO coincidence cell. From the current
slope, the effective barrier height is determined and displayed in the
inset. (b) d(lnI)/dz image of FeO/Pt(111) taken with lock-in technique
and closed feedback loop. (Image size 5.7 ¥ 5.7 nm
2
, V
s
= 4.5 V, Dz
rms
= 1 Å.) Reprinted with permission from Rienks et al. (2005), Copyright
(2010) by the American Physical Society.
ThinFilm-Zexian-11.indd 269 7/1/11 9:43:29 AM
270 Thin film growth
© Woodhead Publishing Limited, 2011
the Pt(111) surface and therefore mimics the properties of a free-standing
FeO layer. Additionally, the vertical separation between Fe and O planes
(0.5 Å) is 15–20% smaller in the top than in adjacent hcp and fcc domains
(0.8 Å). The top region therefore has the smallest Fe
d+
-O
d–
surface dipole
and hence the lowest surface potential. Its F value is 0.29 and 0.23 eV lower
than those of the hcp and fcc regions, respectively, in good agreement with
the measured potential modulations in the FeO coincidence cell of 0.35
eV.
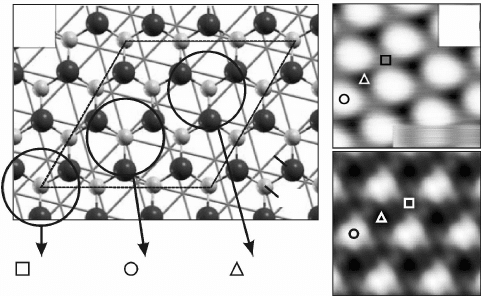
Based on the computed surface potentials, STM images have been
simulated for bias voltages in the eld emission mode (Giordano et al.,
2007b). As discussed above, the contrast in this regime is governed by the
availability of FER, which in turn depends on the local surface potential. A
small F gives rise to a bright contrast, as low-lying FER with high electron
transmissibility promote the electron transport, while regions with large F
appear dark. Calculated F maps, depicted with inverted contrast, indeed
reproduce the high-bias STM images of the FeO/Pt system (Fig. 11.11b). In
agreement with our earlier interpretation, the brightest region corresponds to
the one with the lowest surface potential and is assigned to the top domain
that has the lowest polarity of all FeO stacking domains.
Measurements of the surface potential with an STM have proven to be
an adequate means to probe the polarity of FeO thin lms (Rienks et al.,
2007). Similar results were obtained for other polar systems, e.g. MgO(111)
(a)
(b)
O
Fe
4500 mV
fcc top hcp
11.11 (a) Top view of a model structure for the FeO/Pt(111) system
that contains all relevant Fe binding configurations but is much
smaller than the real coincidence cell. (b) STM image of the
polar FeO film taken at +4.5 V sample bias (top, 7 ¥ 7 nm
2
) and
corresponding surface potential map calculated with the model
shown in (a) (bottom). The map depicts the -F signal, and regions
with low surface potential. Reprinted with permission from Giordano
et al. (2007b), Copyright (2010) by the American Physical Society.
ThinFilm-Zexian-11.indd 270 7/1/11 9:43:29 AM
271Electronic properties and adsorption behaviour of thin films
© Woodhead Publishing Limited, 2011
lms grown on Au(111) (Myrach et al., 2011). Also here, a considerable
surface dipole develops from the stacking of a surface O
2–
layer on top
of an interfacial Mg
2+
layer. This layer sequence is compatible with the
electronegative character of gold, which promotes a charge transfer out of
the cationic plane to reduce the vertical dipole moment (Fig. 11.5a). Similar
to the FeO/Pt(111) system, the surface polarity is not homogeneous on the
MgO(111) surface, but for a different reason. The oxide polarity quickly
vanishes with lm thickness, as three-dimensional MgO islands with zero
dipole develop on the surface. The initial building blocks that are responsible
for polarity healing are nano-pyramids of a few layers height (Wolf, 1992),
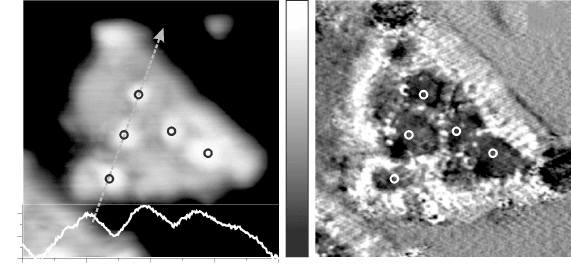
which are readily observed in STM images of the oxide lm (Fig. 11.12a).
Their effect on the oxide polarity has been deduced from the barrier height
images again (Fig. 11.12b). As expected, the F
eff
value turns minimal above
the surface protrusions, demonstrating the effective quenching of the surface
dipole due to the island growth. It should be noted that this morphology-driven
removal of the polarity bears similarities to the octopolar reconstruction that
is the dominant healing mechanism on bulk MgO(111) (Barbier and Renaud,
1997; Barbier et al., 2000).
In general, the STM is able to provide spatially resolved information on
the distinct properties of polar surfaces. The technique is not only suited to
characterize the various polarity healing mechanisms from a topographic
point of view, but enables a local determination of the surface potential and
its correlation to the oxide polarity. Although STM-based approaches do not
reach the same quantitative accuracy in determining surface potentials as,
(Å)
4
2
0
0 20 40 60 80
Position (Å)
Low d(lnI)/dz High
(a) (b)
11.12 (a) Topographic image of a bilayer MgO(111) island grown on
Au(111) (–0.25 V, 9 ¥ 9 nm
2
). A height profile taken across the island
is shown in the inset. (b) Effective barrier height (d(lnI)/dz) image
of the same surface region. Particularly low values of the barrier
height are revealed on the ad-structures that cover the oxide island,
indicating their importance in quenching the surface dipole.
ThinFilm-Zexian-11.indd 271 7/1/11 9:43:29 AM
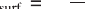
272 Thin fi lm growth
© Woodhead Publishing Limited, 2011
for instance, Kelvin–Probe spectroscopy, they feature an unmatched spatial
resolution with respect to alternative techniques.
11.4 Adsorption properties of polar fi lms
Residual polarity gives rise to unusual adsorption and chemical properties of
thin oxide lms (Goniakowski et al., 2008; Sun et al., 2009). The difference
to non-polar systems lies in the electrostatic contribution to the surface free
energy that originates from the uncompensated surface dipole (see discussion
in Section 11.1). The binding behaviour of adsorbates is therefore not only
governed by the usual physisorption and chemisorption effects, but includes
changes in the electrostatic energy of the system as adsorbates might reduce its
polarity. Given the magnitude of the energies involved, the binding potential
of polar surfaces can be substantially higher compared to non-polar ones. A
direct manifestation of this effect is the wetting growth of metals on polar
surfaces, whereas mainly three-dimensional deposits form on non-polar oxide
materials (Goniakowski and Noguera, 2002; Meyer and Marx, 2004).
Two mechanisms have to be considered in conjunction with polarity
healing via adsorbates. In a rst scenario, the ad-species become charged
upon adsorption and alter the electron density on the surface. As discussed
in Section 11.1, depolarization of the system takes place when the surface
charge density equals the bulk density times the ratio between interlayer
distance d and unit cell height D:
ss
su
ss
su
ss
rf
ss
rf
ss
ss
=
ss
ss
ss
d
D
[11.4]
This condition can now be ful lled by adsorbing the required number of
charged species to the surface. The most prominent example of this mechanism
is the attachment of protons (H
+
) that often originate from the heterolytic
splitting of water to polar surfaces (hydroxylation). For rocksalt (111), every
surface site needs to be occupied by a hydroxyl group in order to quench
the polarity, although d/D = 0.5. The reason is that each H
+
carries only half
the charge of an oxide ion (Mg
2+
, O
2–
). In the case of wurzite (0001), on the
other hand, 50% surface coverage would be suf cient. The hydroxylation
of polar oxide surfaces has been intensively studied with infrared re ection
absorption and high resolution electron energy loss spectroscopy (HREELS),
as discussed for instance in Rohr et al. (1994), Poon et al., (2006) and Wang
(2008). At the local scale, proton attachment was investigated with the STM
on Cr
2
O
3
/Cr(110) lms (Maurice et al., 2001) and more recently on FeO/
Pt(111) (Merte et al., 2009; Knudsen et al., 2010). Although dipole removal
via hydroxylation is observed most frequently, any other adsorbate that is
easily ionized or polarized can be used instead. Examples for the adsorption
of charged ad-species on polar oxide lms are given in Section 11.4.1.
ThinFilm-Zexian-11.indd 272 7/1/11 9:43:29 AM
273Electronic properties and adsorption behaviour of thin films
© Woodhead Publishing Limited, 2011
A second mechanism for dipole quenching is the metallization of polar
surfaces. In this case, the adsorbates create electron bands that cross the
Fermi energy of the system and can be lled with the required number
of electrons to satisfy Eq. 11.4. Those electronic states might be intrinsic
to the ad-species or induced into the polar material upon adsorption. The
rst case is typically realized with metallic adsorbates that possess states
around the Fermi level. For instance, a single Pd layer attached to a Mg-
terminated MgO(111) slab was found to charge up negatively by lling a
Pd-like interface state (Goniakowski and Noguera, 2002). The computed
charge density in the Pd layer hereby adopts 50% of the MgO bulk value
in accordance with Eq. 11.4. Further thickening of the metal lm does not
change this situation, indicating that a single layer is sufcient to cancel the
oxide polarity. Experimentally, the wetting growth of metals on polar oxide
materials has been studied for Cu on Zn(0001) (Koplitz et al., 2003; Dulub
et al., 2005; Kroll and Köhler, 2007). Especially, on the O-terminated surface,
a layer-by-layer growth of Cu is observed that reects, however, not only
the impact of oxide polarity but also the generally higher adhesion for this
surface termination. A similar result was obtained for the polar FeO/Pt(111)
lm, where extended 2D islands form upon Pd deposition (Fig. 11.13a). It
should be noted that metals usually develop 3D particles when deposited
0.4 nm
0.4 nm
H-covered
Clean
–3 –2 –1 0 1 2 3
Sample bias (V)
Log I (nA)
1
0.1
0.01
1e–3
(b)(a)
11.13 (a) STM image of a planar Pd island grown on a FeO/Pt(111)
film (9.0 ¥ 9.0 nm
2
). The two-dimensional growth mode is triggered
by the polarity of the system. (b) STM images of a clean (left)
and a hydrogen covered ZnO(10-10) surface (right). The dashed
rectangle marks the (1¥1) surface unit cell. H adsorption leads to a
metallization of the ZnO(10-10) surface, as shown by the vanishing
band gap in I-V curves taken on the H-covered surface. Reprinted
with permission from Wang et al. (2005), Copyright (2010) by the
American Physical Society.
ThinFilm-Zexian-11.indd 273 7/1/11 9:43:29 AM
274 Thin film growth
© Woodhead Publishing Limited, 2011
on non-polar materials in order to minimize the contact area with the inert
oxide surface (Bäumer and Freund, 1999).
Induction of a metallic surface state in the substrate itself has been
demonstrated with STM conductance spectroscopy and HREELS for the
ZnO(10-10) surface (Wang et al., 2005; Yin et al., 2006). In those experiments,
the oxide band gap was found to disappear after adsorbing one H atom per
unit cell of the ZnO surface (Fig. 11.13b). This result was explained with
the transfer of the hydrogen electron into the initially empty 4s-band of zinc
that subsequently becomes half-lled. Increasing the dosage to two H atoms
per unit cell renders the surface non-metallic again, as a second electron is
added to the surface band that consequently shifts below the Fermi level.
A similar metallization effect has so far not been revealed for the polar
ZnO(0001) surface.
The detailed adsorption properties of polar systems are discussed for the
FeO/Pt(111) lm in the following section. This particular system is selected
again because it has already been introduced in the previous sections.
Furthermore, the FeO lm exhibits spatial modulations of the surface polarity,
which induces a template effect in the arrangement of the adsorbates. It is
therefore an example where uncompensated polarity induces self-assembly
phenomena on an oxide surface.
11.4.1 Adsorption of metal atoms on polar FeO films
As discussed in Section 11.3, the polarity of the FeO lm arises from the
interplay between an Fe
d+
interface and an O
d–
surface plane (Vurens et al.,
1988; Kim et al., 1997). The Fe-O layer separation is not constant, but varies
between 0.52 and 0.78 Å due to different Fe-O stacking congurations on
the Pt(111) surface. Large interlayer distances occur for the fcc and hcp
domains, while small separations are found in the top region of the FeO-Pt
coincidence cell (see Fig. 11.7a for a structure model). As discussed above,
such modulations in the interlayer distance affect the vertical dipole strength
and therefore the polarity of the lm, whereby the hcp and top regions turn
out to be the most and least polar ones, respectively (Rienks et al., 2005).
The same modulations should govern the spatial distribution of adsorbates
on the FeO surface as well.
A rst demonstration of this effect was provided in a low-temperature
STM experiment, where single Au atoms were deposited onto the lm at 10
K sample temperature (Nilius et al., 2005). The incoming atoms perform a
transient diffusion on the FeO surface due to their initial thermal energy, and
are able to reach their preferred adsorption sites. After thermalization, they
can be imaged in the STM as 1.0 Å high, circular protrusions. The adatoms
are not randomly distributed on the surface, but exhibit a large tendency to
attach to the hcp domains of the coincidence cell. From the ve adatoms
ThinFilm-Zexian-11.indd 274 7/1/11 9:43:30 AM
275Electronic properties and adsorption behaviour of thin films
© Woodhead Publishing Limited, 2011
shown in Fig. 11.14(a), only the uppermost one binds to a top region while
the four lower ones sit in hcp domains. At a nominal Au coverage of 0.01
ML, which is close to one atom per coincidence cell, more than 70% of
the hcp domains are occupied with Au atoms, whereas adjacent fcc and top
regions remain nearly adsorbate-free (occupancy below 10%) (Fig. 11.14c).
The site-specic adsorption behaviour of Au therefore leads to the formation
of a hexagonal adatom array, the lattice parameter of which matches the size
of the FeO/Pt(111) coincidence cell. The long-range order in the adatom
arrangement becomes evident in a 2D pair-distribution function n(x, y),
calculated from more than 700 atom positions (Fig. 11.15). The highest
probability to nd a neighbouring Au atom is in the hcp domain of the
next FeO/Pt coincidence cell; however, also second and third neighbouring
hcp domains show substantial occupancy due to the perfect ordering of the
adsorbates. A distinct atom distribution is discernible even within each hcp
region, as marked by seven, hexagonally-arranged spots with 3.1 Å mutual
distance. This ne structure reects the few atomic binding sites in the
centre of each hcp domain that are actually populated by Au. Based on the
pair distribution function, the modulation of Au binding energies within the
coincidence cell DE can be estimated by assuming a Boltzmann distribution
for the occupation probability: n(x, y)µ exp(
–DE
/
kT
) (Silly et al., 2004; Kulawik
top
top
fcc
fcc
hcp
hcp
Occupancy
0.6
0.4
0.2
0.0
(a) (b)
(c)
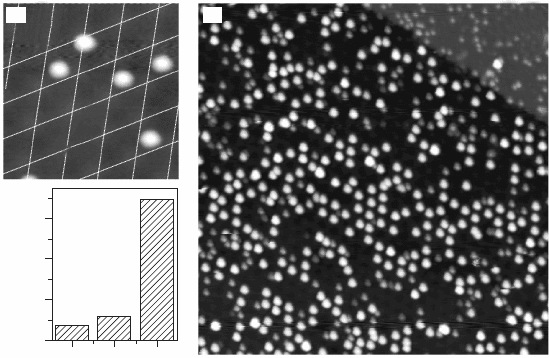
11.14 (a) STM topographic image of Au atoms on FeO/Pt(111) (0.5 V,
13 ¥ 13 nm
2
). Four out of five adatoms occupy hcp domains in the
FeO coincidence cell. (b) Overview STM image demonstrating the
ordering of Au atoms into a hexagonal lattice (0.5 V, 60 ¥ 60 nm
2
).
(c) Histogram for the probability of finding an Au adatom on the
different FeO stacking domains. The plot is based on the evaluation
of ~700 atom positions. Reprinted with permission from Nilius et al.
(2005), Copyright (2010) by the American Physical Society.
ThinFilm-Zexian-11.indd 275 7/1/11 9:43:30 AM
276 Thin film growth
© Woodhead Publishing Limited, 2011
et al., 2005). As the self-assembly is observed for temperatures as high as 50
K, this temperature is used to calculate a lower bound for DE. The analysis
yields an energetic preference of (10 ± 2) meV for Au binding to the hcp
domains with respect to adjacent fcc and top regions. It is interesting to
note that Au atoms remain essentially monomeric upon adsorption onto the
oxide lm even at relatively high coverage. No aggregation sets in before
the majority of hcp domains is lled, which suggests a repulsive, most likely
Coulomb-type interaction between the adatoms that inhibits cluster formation
(Nilius et al., 2005).
The self-assembly of Au atoms on the FeO/Pt(111) surface can be traced
back to the polar nature of the lm. The adatoms preferentially attach to the
hcp domains having the largest surface dipole (Giordano et al., 2007b). The
uncompensated polarity in this region enables an efcient polarization of the
Au, which in turn leads to an electrostatic coupling between the adatoms and
the dipole eld of the polar lm. This binding contribution will be smaller
on the adjacent fcc and top domains, where the dipole strength is reduced.
A more detailed picture of the Au ordering effect on the FeO is obtained
from DFT calculations (Giordano et al., 2008). In this study, the Au interaction
with O-top, Fe-top, bridge and hollow sites has been analysed for all three
stacking domains of the coincidence cell. The largest adsorption energy of
0.6 eV was computed for the O-top sites in hcp domains, where the binding
y Displacement (Å)
–40
–20
0
20
40
fcc
hcp
top
–40 –20 0 20 40
x Displacement (Å)
16
12
8
4
0
8
4
0
Energy (meV) Counts
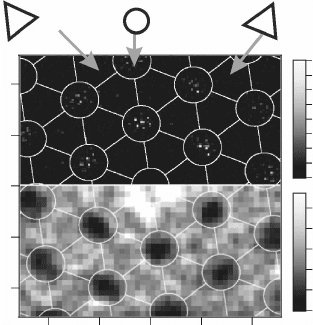
11.15 Two-dimensional pair-distribution function of Au atoms on
the FeO film (top). Most events occur in the hcp domains; the sharp
maxima within those regions reflect the atomic binding sites of the
adsorbates. The bottom part depicts the potential landscape for Au
adsorption, being calculated by assuming a Boltzmann distribution
according to the Au binding energy. Reprinted with permission
from Nilius et al. (2005), Copyright (2010) by the American Physical
Society.
ThinFilm-Zexian-11.indd 276 7/1/11 9:43:30 AM
277Electronic properties and adsorption behaviour of thin films
© Woodhead Publishing Limited, 2011
is 30% stronger than on Fe sites in the hcp and O sites in the fcc and top
domains. This binding preference explains the observed self-assembly of Au
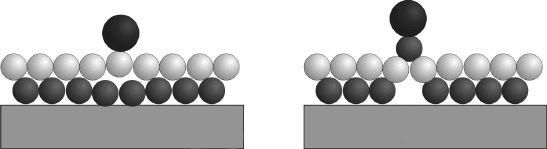
adatoms on the FeO lm. It should be noted that theory nds an even more
favourable adsorption conguration, in which an interfacial Fe atom ips
above the O-plane (Fig. 11.16). The hence under-coordinated Fe atom is able
to bind the surface Au species with ~1.5 eV, much stronger than in the regular
geometry. Due to a concomitant negative charging of the Au adatom, also
the lm polarity is locally quenched in this conguration, as the resulting
O
d–
-Fe
d+
-Au
d–
stacking carries no dipole moment (Fig. 11.16b). A similar
binding conguration has been predicted for other electronegative metals,
such as Pt (Goniakowski et al., 2009). However, the inverted adsorption
geometry could not be conrmed experimentally, as neither the height nor
the electronic properties of a potential Au-Fe ad-species was compatible
with the STM data. Apparently, the energy barrier to break a Pt-Fe bond at
the interface and swap the local Fe-O stacking sequence is not overcome at
the low temperature of the experiment (Nilius et al., 2005).
The experimentally conrmed Au binding to O atoms in the hcp domains
leads to a 20% increase of the Fe-O layer distance around the adsorption site,
which enhances the surface dipole and therewith the electrostatic Au–FeO
interaction (Giordano et al., 2008). The local lattice distortion is initiated
by a charge transfer from the Au atom into the Pt crystal. The Coulomb
interaction of the Au
+
lifts the O
d–
ion underneath above the surface plane,
but repels the three adjacent Fe
d+
ions, thereby inducing a polaronic distortion
of the oxide lattice. The formation of charged adsorbates combined with a
polaronic distortion of the surrounding lattice is a common binding scenario
on ionic oxide lms (Pacchioni et al., 2005; Giordano et al., 2007a). The
cationic nature of Au atoms on the FeO lm is further supported by state
density calculations (Fig. 11.17b). The Au 6s orbital of hcp-bound adatoms
is located at +0.3 eV above the Fermi level and thus empty, in contrast to
its half-lled nature in gas-phase gold. This nding indicates that the 6s
electron has been transferred into the support, rendering the Au positively
– Au
d+
– Au
d–
O
d–
– Fe
d+
Fe
d+
Pt(111)
(a) (b)
11.16 (a) Experimentally observed and (b) energetically favoured
binding configuration of Au on FeO/Pt(111). In the optimum
geometry, a Fe atom flips above the oxide plane in order to increase
the interaction with the Au. The energy barrier for this restructuring
is not known.
ThinFilm-Zexian-11.indd 277 7/1/11 9:43:30 AM
