Canale L.C.F., Mesquita R.A., Totten G.E. Failure Analysis of Heat Treated Steel Components
Подождите немного. Документ загружается.

Sintering. During sintering of ferrous PM
parts, the following reactions occur:
Metallurgical bonds are developed from the
mechanical interlocks betwee n the powder
metal particles in the compact.
Metal oxides in the powder compact are
reduced by reaction with the carbon from the
blended graphite or with the combustible
constituents of the atmosphere.
Desired final level of combined carbon in the
microstructure is obtained by diffusion of
carbon from graphite in the powder mix.
Densification of PM compacts can be
achieved in sintering. The degree of densific-
ation depends on the sintering parameters and the
alloys involved. Generally, higher sintering
temperatures and longer sintering times promote
densification of ferrous sinter ed parts.
For conventional sintering, which generally is
conducted at 1120
C, mesh belt conveyor fur-
naces are widely used. These furnaces can be
used up to temperatures of 1150
C, primarily
due to the temperature limitation of the belt and
metallic muffle material.
For stainless steels, proper sintering is critical
for proper corrosion resistance (Ref 2).
Case Hardening
Powder metallurgy steel parts can be case
hardened by several processes, although various
available processes are not equally suited to
every application. A clear case/core relationship
can be obtained only with parts having a density
of at least 7.2 g/cc.
Carburizing. Powder metallurgy parts with
relatively low combined carbon contents of up to
0.20 wt% can be carburized by conventional
pack or gas methods. Liquid carburizing is
not reco mmended because of the difficulty of
washing the parts free of salt.
Gas carburizing is more practical for PM parts
than pack carburizing. For this process to be
successful, however, density as well as the pre-
cise composition of the parts should be known.
Low-density parts should not be subjected to
gas carburizing, because the carburizing gases
penetrate the voids. Consequently, a distinct
case is not achieved compare d with the case
developed on wrought parts under the same
conditions. Instead, the carbon penetration in
PM parts is generally deeper and relatively
nonuniform. The extent of this condition varies
with densi ty. In parts that have been repressed
and resintered, this condition may be tolerable or
even negligible, but for parts of lesser density,
the depth of carbon penetration may be so great
that these sections of quenched parts will be
brittle.
Another reason that conventional gas carbur-
izing enjoys only limited use is because it does
not increase hardenability. Thus, plain carbon
grades usually must be quenched in an aqueous
medium. This may result in cracking, especially
if carbon penetration is excessive. As with
wrought parts, the depth of carburized case of the
PM parts depends on time and temperature.
Carbonitriding is a modified form of car-
burizing. The principal process modification
consists of introducing ammonia into the gas
carburizing atmosphere, which results in the
addition of nitrogen to the carburized case as
it is produced. Nascent nitrogen forms at
the workpiece surface by dissociation of the
ammonia in the furnace atmosphere. Nitrogen
diffuses into the steel surfaces simultaneously
with the carbon, where the austenite stability is
greatly enhanced by nitrogen in solution. This
way, the necessary quenching rate to form
martensite is reduced, and a martensitic micro-
structure is obtained without expensive alloying
elements. Typically, carbonitriding of PM parts
is carried out at 790 to 880
C for a duration of
30 to 60 min.
Carbonitriding is widely used for case hard-
ening of PM parts made of ferrous powders.
Densities of the sintered compacts vary from
approximately 6.8 to 7.9 g/cc. Parts may be
infiltrated with copper prior to carbonitriding.
Carbonitriding is extremely effective for case
hardening high-density (7.2 g/cm
3
) parts made
from sintered iron compacts (Fig. 2). Addition-
ally, it is reasonably effective for case hardening
parts of lower density.
Equipment and Techniques. Procedures for
carbonitriding PM parts are essentially the same
as those used for similar wrought parts. Control
of temperature and time is generally more cri-
tical than for wrought parts because of porosity.
Lower temperatures are avoided to minimize the
potential danger of explosion, and higher tem-
peratures are avoided because case depth control
is more difficult.
The processing cycle, including composition
of the atmosphere, is critical. The ammonia con-
tent (usually 1 to 5% of carrier gas by volum e)
increases hardenability and affects dimensional
stability. Because dimensional changes in heat
Failure Analysis of Powder Metal Steel Components / 397
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_395-415.pdf/Chap_12/ 18/8/2008 3:52PM Plate # 0 pg 397

treating are often crucial to the economic justi-
fication of producing parts by PM, gas compo-
sition, temperature, and quenching medium
must be closely controlled.
When processing to a new specification,
establishment of processing parameters is
usually made on small production quant ities,
thereby requiring sacrifice of only a few parts to
arrive at optimal conditions. Such adjustments
are then recorded so they can be used when
processing the next lot of similar parts. Tem-
pering of carbonitrided parts requires special
consideration, largely because the quenching oil
they contain will partially evaporate and pollute
the environment. Toughness of PM parts in
the harde ned condition (either by oil quenching
or through sinter hardening) is significantly
improved on tempering.
Induction Hardening. Induction heating is
a method of h eating electrically conductive
materials by the application of a varying mag-
netic field whose lines of force are intersected
by the workpiece. In this process, the varying
magnetic field induces an electric potential,
which in turn results in generation of electric
current depending on the geometry, the fre-
quency, and the electrical characteristics of
the workpiece. The induced current, termed
eddy current, generates heat that makes it
amenable for use in many different heating
applications, of which the induction hardening
of steels and cast irons is one of the most pre-
dominant.
Induction hardening of PM parts has several
differences compared to hardening wrought
steels and cast irons. Chemical composition,
microstructural heterogeneity, and low density
due to the porosity are the causes of the different
induction-hardening responses of PM parts. The
results of hardening are more sensitive to chem-
ical composition and the prior microstructure
when compared with alternative processes. The
electrical resistivity, thermal conductivity, and
magnetic permeability strongly depend on the
porosity of the PM part. Low density negatively
affects the hardenability of powder metal parts
(Table 1). It is recommended that the part
selected for induction hardening has a density
of at least 7 g/cc. Carbon, copper, nickel, and
molybdenum are the most commonly used
alloying elements in PM parts. The stresses due
to high carbon content or large pores aggravate
cracking. Segregation of alloying elements,
foreign inclusions, or large pores can serve as
stress raisers, making the powder metal part
susceptible to cracking.
It is quite common for PM parts to absorb
oil. Thus, intensive ventilation is required, and
one must ensure that the reused quenchant pro-
vides the required quench severity. Water-based
polymer fluids are the most common quenchants
used for induction hardening of PM parts.
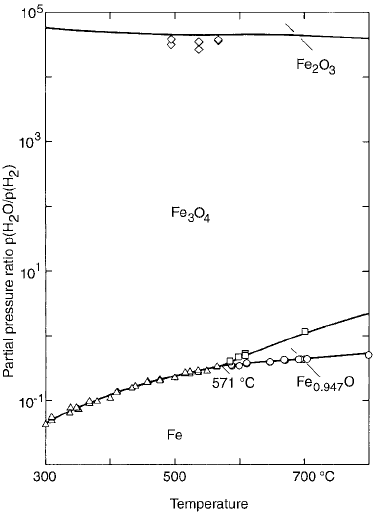
Steam Treatment. Sintered parts are sub-
jected to steam treatment, wherein controlled
oxidaton of the ferrous part is carried out in
an atmosphere of superheated steam. As a result,
a layer of Fe
3
O
4
forms on the surface as well
as in the pores. This oxide is hard and has
excellent wear resistance (hardness of 450
HV
0.05 kg
), increases density, and improves
corrosion resistance. The iron oxide also serves
to seal the pores, which results in parts that
are impermeable to gases. This characteristic of
Table 1 Effect of low density on properties and
induction hardening process parameters
Property Change
Effect on induction
hardening parameters
Thermal
conductivity
Decreases with
decrease in
density
Inefficient conduction
heat transfer
Larger temperature
gradients(a)
Electrical
resistivity
Increases with
decrease in
density
Larger current
penetration depth
Magnetic
permeability
Decreases with
decrease
in density
Larger current
penetration depth
Lower coil electrical
efficiency
Structural
homogeneity
Decreases with
decrease in
density
Wide scatter in
apparent hardness
Wide scatter in case depth
(a) The penetration of the quenchant into open porosity overcompensates the
effect of lower conductivity. As a result, low-density parts cool faster.
Fig. 2
Effect of density on the case depth as measured through
a hardness traverse from the surface. Courtesy of
P. Beiss, University of Aachen, Germany
398 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_395-415.pdf/Chap_12/ 18/8/2008 3:52PM Plate # 0 pg 398
steam-treated PM products has enabled its
widespread use in pistons and valve plates for
compressors. The sealing of pores also helps the
PM part to be amenable to corrosion-preventive
coatings (galvanic as well as barrier coatings)
and, in some cases, to gas and salt bath nitriding.
The formation of Fe
3
O
4
is exothermic, and thus,
a further increase in product temperature by
10 to 20
C is observed. Steam treatment is
typically carried out at 500 to 550
C for a time
of 30 to 300 min. The upper limit of 550
C
is chosen so that the products do not exceed
570
C, which in turn would result in FeO for-
mation (Fig. 3).
Failure Analysis Techniques
Failure analysis techniques can be broadly
classified as preventive methods or corrective
methods. Preventive methods of failure analysis
are typically carried out during product or pro-
cess development. These refer to the standard
techniques for failure mode and effects analysis
(FMEA), which are specified in the various
certifications, such as TS16949 and ISO
9000:2000. The engineer anticipates the failures
of the product on the basis of known failure
modes and designs the process and product
parameters accordingly.
However, in practice, failures are observed in
spite of carrying out the measures that are
recommended by an FMEA. This can be due to
the fact that assumptions which go into carr ying
out the FMEA may change over a period of time,
or the basis of the assumptions is incorrect, but
more commonly, the failures occur due to the
breakdown in one or more so-called 4M para-
meters (man, machine, method, material). Thus,
there is a need for corrective methods of failure
analysis, which form the bulk of this chapter.
However, the techniques used in the FMEA are
useful for analyzing failures, and a few are very
relevant to heat treated products. In the case
of heat treated powder metal parts, the failure
modes and their causes are limited in number
and can be quantified easily. The common fail-
ure modes of heat treated powder metal parts
are:
Wear
Fracture
Dimensional instability because of plastic
deformation
Dimensional instability because of phase
transformation
Corrosion
These can be related to deficiencies in mechan-
ical or metallurgical properties. Properties that
play a role in failure of heat treated PM steel
include:
Metallurgical properties: microstructure;
case depth; coating adhesion, hardness, and
thickness for wear-resistant coatings; salt
spray life in the case of coatings for corro-
sion prevention; density; and chemical
composition
Mechanical proper ties: tensile strength,
elongation, impact strength, fatigue strength,
fracture toughness, and hardness
Deficiencies in properties that can result in a
failure are caused by a combination of one
or more of the 4M parameters. For example,
low apparent hardness after carbonitriding may
be associated with carbonitriding or due to
low density in compaction. Thus, in analyzing
the failure, one needs to consi der the fact that
the cause of the failure may be associated with
more than one stage of component production.
Fig. 3
Fe-O-H
2
O diagram indicating the zones in which
various oxides of iron are stable. Courtesy of P. Beiss,
University of Aachen, Germany
Failure Analysis of Powder Metal Steel Components / 399
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_395-415.pdf/Chap_12/ 18/8/2008 3:52PM Plate # 0 pg 399
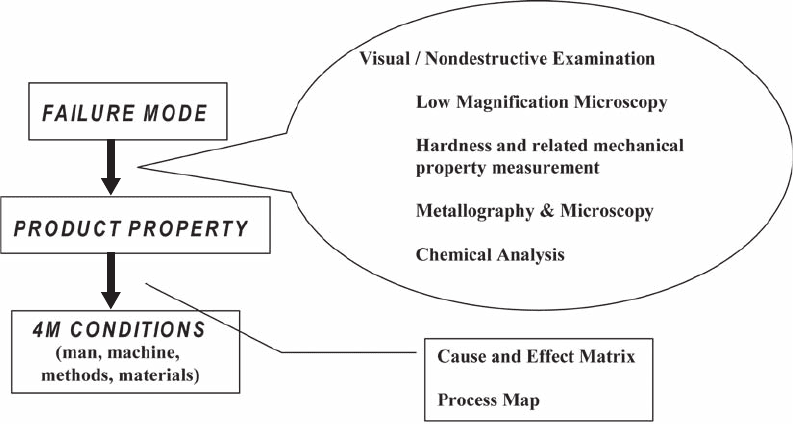
Failure Analysis Tools. The objective of the
failure analysis is to relate the failure to a pro-
duct property attributed to one or more 4M
parameters of processing. The steps that are
typically carried out in the failure analysis of
heat treated powder metal parts are shown in
Fig. 4. Examination of the product involves one
or more of the following techniques:
Visual or any other method of non-
destructive examination
Fractography or examination under a low-
magnification stereomicroscope
Hardness and related mechanical prope rty
measurements
Metallography under an optical or scanning
electron microscope
Chemical analysis
The failure can occur because of a non-
conforming property in the product even though
the product meets the specifications. The cause
of the former is related to the manufacturing
process, and the latter is related to the design.
However, both failures involve determining the
4M conditions that cause the failure and cor-
recting the same. In the case of failure of a heat
treated powder metal part, the failure mode as
well as the causes of failure are limited. As
mentioned previously, a principal activity is an
operation-wide 4M analysis to determine the
cause of the failure. Two tools are invaluable
aids in this regard. These are process maps and a
cause-and-effect matrix.
Process Maps. In charting a process map,
the entire manufacturing process is considered.
The implicit assumption is that the cause of
failure can be related to not only the final
operation but also to prior operations. Every
operation in the manufacturing process is
regarded as a process with inputs and outputs.
The inputs consist of the 4M conditions and the
output of the product requirements. All possible
4M conditions that can result in a nonconform-
ing product are listed as inputs, with the non-
conforming properties of the products as output.
Table 2 is an example of a detailed process
map for the PM process. This exercis e is carried
out for all stages in the manufacturing process.
Once the process map is charted, the potential
causes at different manufacturing steps are
identified.
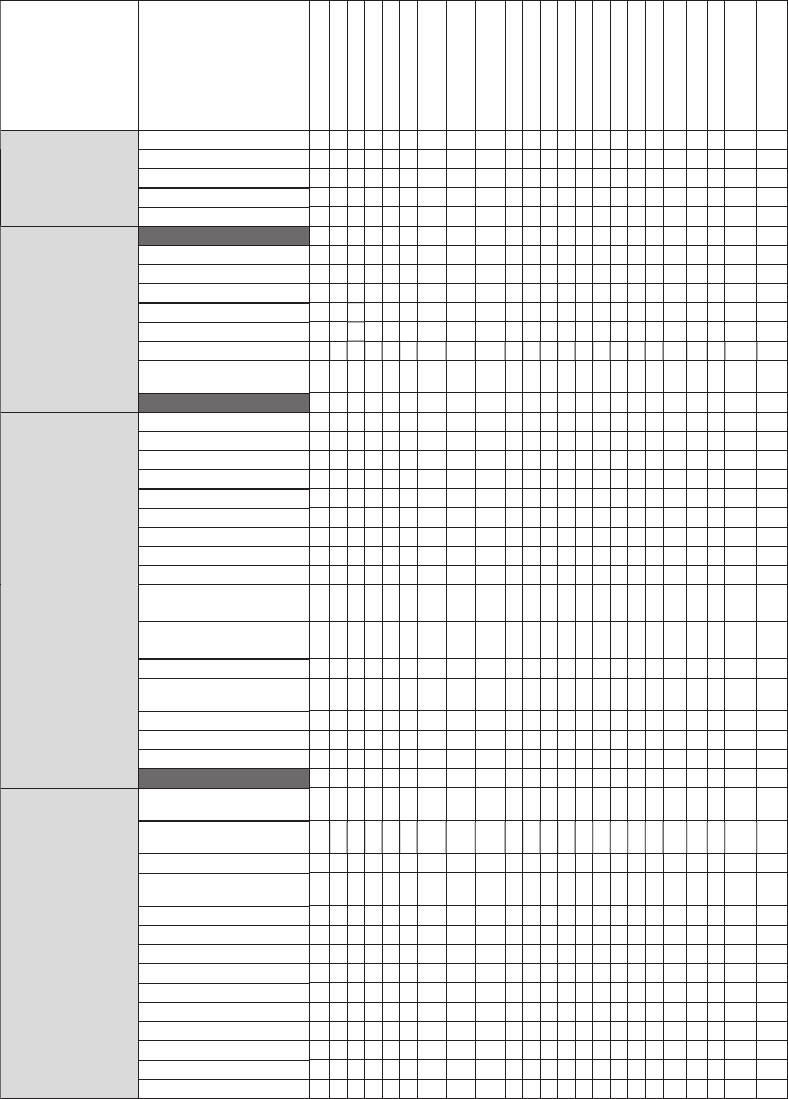
Cause-and-Effect Matrix. The process map
is an effective tool that eliminates the possibility
of ignoring an operation that can result in failure
of the product. However, information presented
by the process map requires sorting for easy and
meaningful interpretation. The cause-and-effect
(CE) matrix ensures this. In the CE matrix, the
4M conditions that can cause the failure are
listed in the y-axis and the defective properties
Fig. 4 Steps in a failure analysis
400 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_395-415.pdf/Chap_12/ 18/8/2008 3:52PM Plate # 0 pg 400

on the x-axis. These are usually transferred from
the process map. A coordinate in this matrix
links the potential cause, that is, the 4M con-
ditions, to the failure, that is, the defective pro-
duct characteristic. Once all the coordinates are
filled, all the potential causes of a failure are
listed in a user-friendly format. An example of
a process map and CE matrix is illustrated in
Fig. 5.
Case Studies of PM Steel Failures
Case Study 1: Wear after Sinter Hardening
Sinter-hardened bushes were developed for
an application that required high wear and
impact fatigue resistance. The bushes were sin-
ter hardened to a martensitic-bainitic micro-
structure using a prealloyed Fe-Cr-Mo powder.
Accelerated rig testing for 24 h yielded no wear,
but the bushes were observed to fail in the field.
The parameters that can cause the failure
on listed in the CE matrix for wear of sinter-
hardened bushes, as follows:
Operation 4M parameter Observations
Powder
chemistry
Low carbon Yes
Blending Segregation of carbon and
alloy elements
No
Compaction Low density No
Sintering Low sintering temperature No
Low soaking time No
Oxidizing atmosphere No
Decarburizing atmosphere No
High rapid burnoff temperature No
Bainite in microstructure Yes
Overloading of parts No
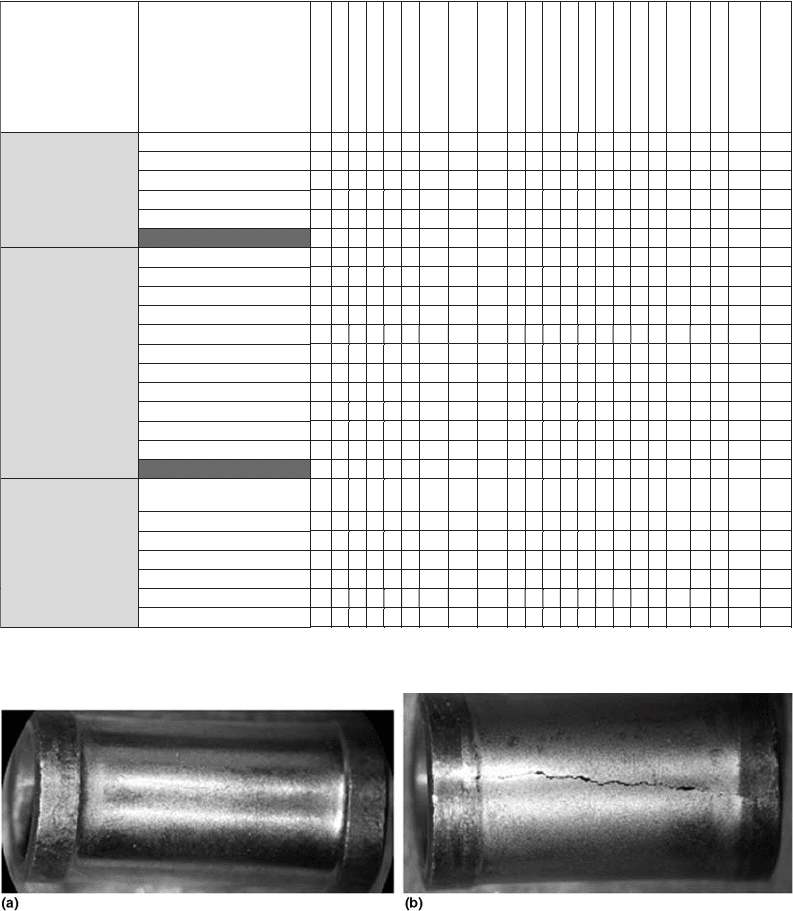
Figures 6(a) and (b) show the failed bush.
Figure 7 shows the microstructure of the failed
bush, which consists of martensite and upper and
Table 2 Detailed powder metallurgy process map
Inputs Outputs
Compaction
Single platen press ...
No pressway Low density
No double action Density difference within
compact exceeding 0.2 g/cc
High apparent density Carbon and lube segregation
Low apparent density Alloy segregation
Segregation of alloys Dimensions not conforming
to specification
Segregation of carbon and
lubricant
...
Sintering
Low cooling rate ...
High cooling rate Low hardness
Low temperature Decarburization
High temperature Oxidation
Low soaking time Excess growth
High soaking time Shrinkage
Oxidizing atmosphere Carburized microstructure
Decarburizing atmosphere Low mechanical properties
Carburizing atmosphere Blistered product
High rapid burnoff
temperature
Pinholes
Low rapid burnoff
temperature
Low sintered density
Low density ...
Carbon-lubricant
segregation
...
Alloy element
segregation
...
Low sectional density ...
Poor sinter hardenability ...
Carburizing/carbonitriding
Low carbonitriding
temperature
...
High carbonitriding
temperature
...
Low soaking time High surface hardness
Inputs Outputs
Carburizing/carbonitriding (continued)
High time of cabon
potential attainment
...
High soaking time Low surface hardness
Oxidizing atmosphere Decarburization
Decarburizing atmosphere Oxidation
Carburizing atmosphere Excess growth
High nitrogen potential Shrinkage
Masking of products ...
Delayed quench Carbide network in case
Hot oil quench Nonmartensitic transformation
product in case
High-viscosity quench oil ...
Delayed tempering Low mechanical properties
Poor hardenability High retained austenite
Low density Low case depth
Carbon segregation High case depth
Alloy segregation High core hardness
Low sectional density Low core hardness
Induction hardening ...
Too high frequency ...
Too low frequency Cracked product
High heating rate High case depth
Low heating rate Low case depth
High heating time Low surface hardness
Delayed quench Localized zones of melting
Severe quench ...
High temperature ...
Low temperature ...
High carbon ...
Low density ...
Steam treatment
Continous mesh belt
furnace
Low hardness
Batch furnace Leaky product ( pores not well sealed)
Low temperature Low oxide layer
Insufficient time Poor surface appearance
Insufficient steam Loose rust
Oil in pores Red rust
Failure Analysis of Powder Metal Steel Components / 401
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_395-415.pdf/Chap_12/ 18/8/2008 3:52PM Plate # 0 pg 401
stupni ssecorP pets ssecorP
Low apparent hardness
High apparent hardness
Low case depth
High case depth
High core hardness
Low core hardness
High retained austenite
Carbide network in case
Nonmartensitic
transformation product
Free ferrite in core
Oxidation
Decarburization
Cracked product
Melt zones
Thin oxide layer
Thick oxide layer
Red rust
Loose rust
Poor surface apearance
Pinholes
Blistered product
Poor mechanical
properties
Nonconforming
dimensions
emit ssecxE
X
emit tneiciffusnI
XXX
snoitidda yolla latnemelE
XX X
nobrac hgiH
XXX
gnidnelB
tnacirbul hgiH
XX
sserp netalp elgniS
yawsserp oN
X
noitca elbuod oN
X
apparent density hgiH
X
Low apparent density
XX
syolla fo noitagergeS
X
dna nobrac fo noitagergeS
tnacirbul
XX
X
noitcapmoC
etar gnilooc woL
X
etar gnilooc hgiH
X
erutarepmet woL
XXX
erutarepmet hgiH
X
emit gnikaos woL
XXX
emit gnikaos hgiH
X X
Ox erehpsomta gnizidi
XXXXX
erehpsomta gnizirubraceD
X
gniretniS
erehpsomta gnizirubraC
X
High rapid burnoff
temperature
Low rapid burnoff
temperature
XXXX
X
X
ytisned woL
X X
tnacirbul-nobraC
noitagerges
X X X
noitagerges tnemele yollA
X
ytisned lanoitces woL
X
ytilibanedrahretnis rooP
X
gnidirtinobrac woL
erutarepmet
X XX
X
X
gnidirtinobrac hgiH
erutarepmet
X
X
X
XX
emit gnikaos woL
XX
XX X
emit gnikaos hgiH
High time of carbon
potential attainment
XX
Ox erehpsomta gnizidi
XX
erehpsomta gnizirubraceD
XX X
erehpsomta gnizirubraC
XX
X
XX
laitnetop negortin hgiH
X
X
X
stcudorp fo gniksaM
XX
hcneuq deyaleD
X
X
X
hcneuq lio toH
X
lio hcneuq ytisocsiv h-giH
X
gnirepmet deyaleD
X
Carburizing/
carbonitriding
Fig. 5 Cause-and-effect matrix derived from powder metallurgy process map
402 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_395-415.pdf/Chap_12/ 18/8/2008 3:52PM Plate # 0 pg 402
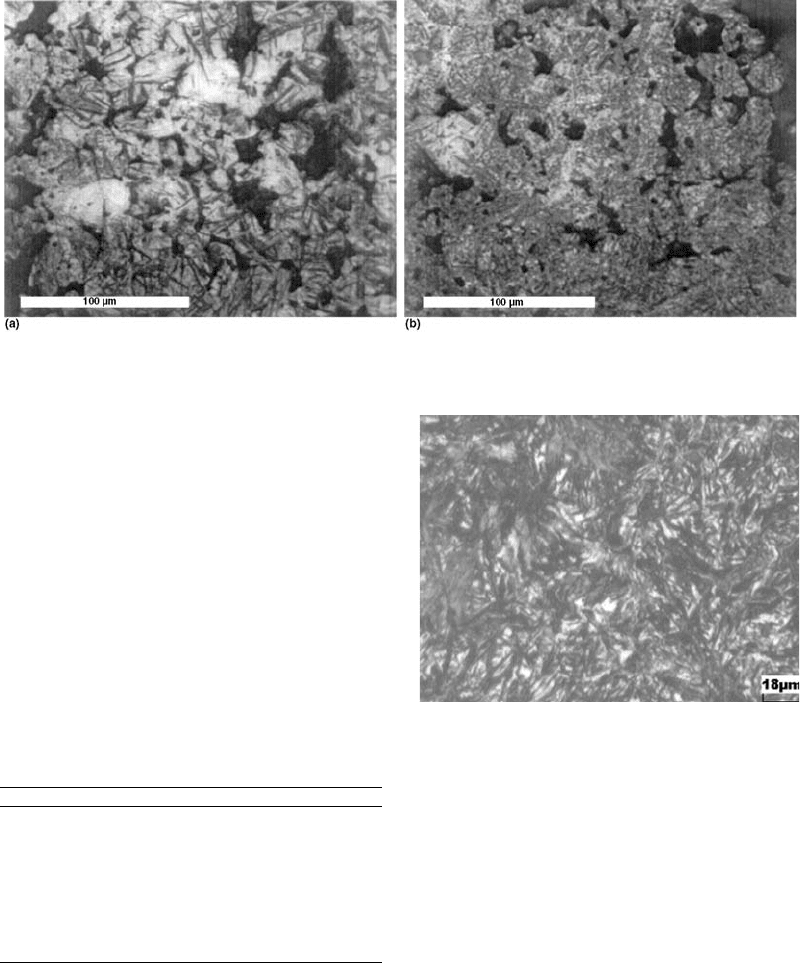
lower bainite. The failure was due to the softer
bainite being worn out, resulting in eventual
accelerated fatigue failure of the bush.
Bainite is an essential component for
toughness. A fully martensitic microstructure
obtained by increasing the cooling rate and the
carbon content has poor toughness. Thus, the
decision was made to use a different material
that can give the required hardness and tough-
ness.
Corrective Measur es. An improved chem-
istry was derived, where the base iron powder
was prealloyed with nickel and molybdenum,
and the carbon content was increased to 0.9%.
This resulted in a predominantly martensitic
microstructure. The presence of nickel ensured
the toughness requirements, and the increased
carbon ensured the wear requirement. Figure 8
shows the new microstructure, wherein mar-
tensite with some retained austenite is observed.
Fig. 6 Sintered bush. (a) Outside diameter wear. (b) Outside diameter crack
ytilibanedrah rooP
XX X X
ytisned woL
XXX
noitagerges nobraC
X
noitagerges yollA
X
ytisned lanoitces woL
XXX
ycneuqerf hgih ooT
X
ycneuqerf wol ooT
X
etar gnitaeh hgiH
X
etar gnitaeh woL
emit gnitaeh hgiH
XX
hcneuq deyaleD
X XX
X
hcneuq ereveS
X
erutarepmet hgiH
X X
erutarepmet woL
XX
nobrac hgiH
X
ytisned woL
X
X
noitcudnI
gninedrah
tleb hsem suonitnoC
ecanruf
ecanruf hctaB
erutarepmet hgiH
XXX
erutarepmet woL
X
maetS
tnemtaert
emit tneiciffusnI
XXX
maets tneiciffusnI
XXXXX
serop ni liO
X XXX
stupni ssecorP pets ssecorP
Low apparent hardness
High apparent hardness
Low case depth
High case depth
High core hardness
Low core hardness
High retained austenite
Carbide network in case
Nonmartensitic
transformation product
Free ferrite in core
Oxidation
Decarburization
Cracked product
Melt zones
Thin oxide layer
Thick oxide layer
Red rust
Loose rust
Poor surface apearance
Pinholes
Blistered product
Poor mechanical
properties
Nonconforming
dimensions
Carburizing/
carbonitriding
Fig. 5 (continued)
Failure Analysis of Powder Metal Steel Components / 403
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_395-415.pdf/Chap_12/ 18/8/2008 3:52PM Plate # 0 pg 403
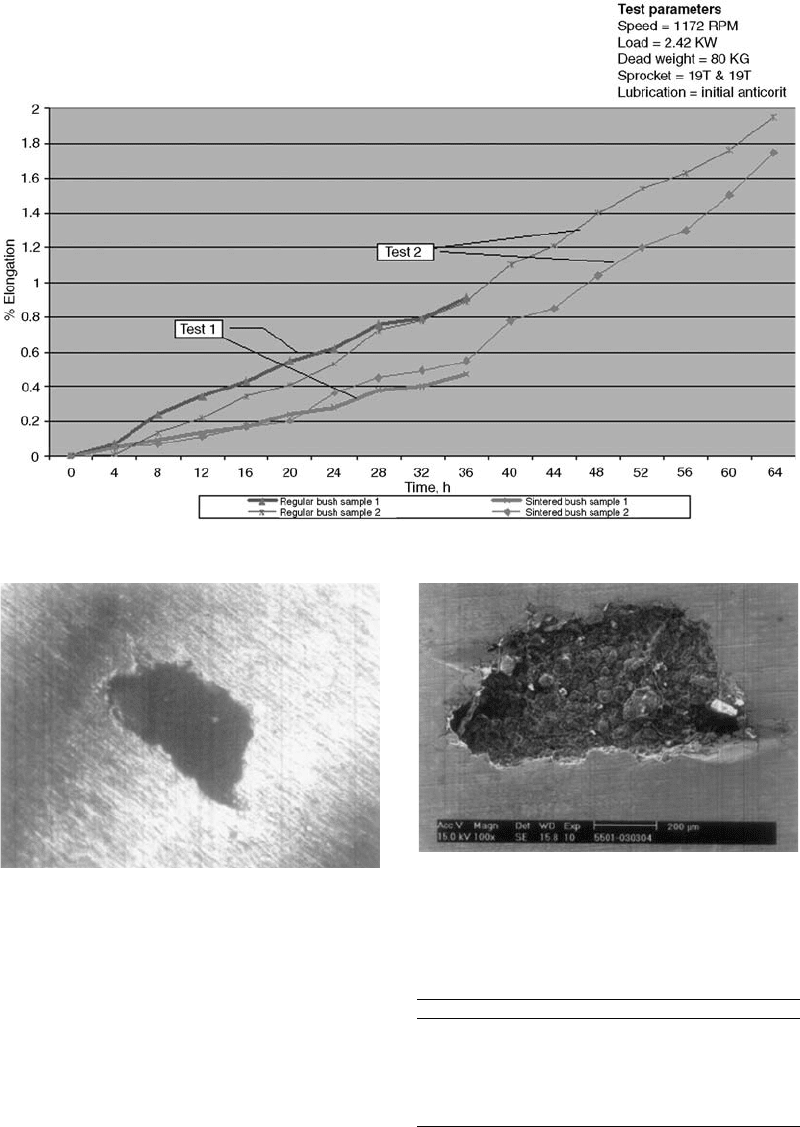
Results. The improved bushes were assem-
bled in chains and tested in a rig as well as in the
field. Figure 9 shows the comparative evaluation
of the PM and wrought chains after the change in
material of the PM bush. It is observed that in
addition to withstanding the tests, the chains
with PM bushes have less elongation compared
to chains with wrought bushes.
Case Study 2: Pinholes after Sintering
Valve seats have a chemistry that is highly
alloyed and a carbon content greater than 1%.
Because most of the elements are admixed, se-
gregations of these elements are not uncommon.
The parameters that can cause pinholes in valve
seats one:
Operation 4M parameter Observations
Blending Segregation of carbon and lubricant Yes
Copper agglomeration No
No inspection for agglomerates
in powder
Yes
Compaction Not applicable ...
Sintering Low sintering temperature No
Low soaking time No
Machining Pullout of nonmetallic inclusions
in machining
No
In all of these above cases, the pinholes that
are observed after sintering or machining are in
the regions where the agglomerates were pre-
sent, prior to sintering or machining. The copper
melts and diffuses into the iron matrix, carbon
diffuses in the matrix, and the lubricant burns
off, leaving the pinhole in each case. In the case
of nonmetallic inclusions, they are pulled out
during machining, thus leaving a pinhole.
Scanning electron microscopy/electron dis-
persive x-ray analysis was carried out to deter-
mine the cause. Figures 10 to 13 reveal
high carbon content near the pinhole. Thus, the
likely cause of the pinholes was concluded to
be carbon-lubricant agglomeration during
blending.
Corrective Measures. Binder-t reated pre-
mixes that minimize carbon-lubricant segre-
gation were recommende d for the products.
An inspection procedure was evolved to check
the premix for the presence of agglomerates and
coarse particles, wherein the premix was sieved
and the +150 mm fraction was inspected for
agglomerates under a stereomicroscope. This
ensured that a premix with agglomerates is not
issued for compacting the products.
Fig. 8
Martensite and some retained austenite in the modified
chemistry
Fig. 7 Micrographs of the failed bush. (a) Martensite and lower bainite. (b) Upper bainite
404 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_395-415.pdf/Chap_12/ 18/8/2008 3:52PM Plate # 0 pg 404
Results. The rejections due to pinholes
decreased significantly as a result of using bin-
der-treated premix.
Case Study 3: Blistered Sintered Products
Synchronizer keys used in an automobile gear
box were produced with Fe-3%Ni-0.5%C. Ele-
mental nickel and carbon were mixed with the
iron powder. Blistering of the products after
sintering was observed in ~5% of the products.
The parameters that can cause blistered sintered
products are:
Operation 4M parameter Observations
Powder chemistry High nickel content Yes
Blending Segregation of nickel powder No
Elemental nickel additions Yes
Compaction Not applicable ...
Sintering Low rapid burnoff temperature No
H
2
in hot zone Yes
It was observed that delubing the keys was not
completed in rapid burnoff, and the lubricant
Fig. 10 Pinhole in the valve seat at a magnification of 40 : 1
Fig. 11
SEM micrograph of the pinhole of Fig.10 indicating a
suspected graphite particle
Fig. 9 Comparison of sintered versus regu lar bush in 12B model chain
Failure Analysis of Powder Metal Steel Components / 405
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_395-415.pdf/Chap_12/ 18/8/2008 3:52PM Plate # 0 pg 405

was carried into the hot zone, where the atmo-
sphere consists of N
2
-10%H
2
. In the presence
of H
2
, nickel acts as a catalyst favoring the
cracking of the lubricant. This results in the
lubricant exploding, and consequently, blistered
products.
Corrective Measures. Two possible solu-
tions to the problem were considered:
Ensure complete removal of the lubricant
prior to contact with H
2
Reduce the catalytic activity of nickel
Due to the limitations of sintering in a con-
tinous mesh belt furnace, the former could not be
completely ensured. Thus, it was resolved that
reduction in catalytic activity of nickel could be
an effective solution. Sulfur effectively poisons
nickel and limits its catalytic properties, so
0.03% S was added to the blend.
Results. The addition of sulfur to the blend
effectively reduced the rejections from 5%
to nil.
Case Study 4: Dimensional Instability
during Shrink Fitting
Exhaust valve seats with tool steel powder
as a major constituent were shrink-fitted in a
Fig. 12 Region of suspected graphite agglomeration in the pinhole (Fig. 11) at higher magnification
Fig. 13
High carbon in electron-dispersive x-ray analysis, confirming the likely cause of the pinhole (Fig. 10–12) as carbon-lubricant
segregation in blending
406 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_395-415.pdf/Chap_12/ 18/8/2008 3:53PM Plate # 0 pg 406
