Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


metal and fifth most abundant element (after oxygen,
carbon, hydrogen, and nitrogen) in the human body.
In both plants and animals, calcium plays key struc-
tural and metabolic roles.
0006 In plants, calcium forms salt bridges between pectin
molecules in the middle lamella, providing the ‘glue’
that holds the walls of adjacent cells together. Calcium
in solution regulates the activity of many enzymes in
plant cells, and protoplasmic calcium concentrations
are regulated by sequestration of calcium in vacuoles
where it is precipitated in an inert form such as
calcium oxalate.
0007 In animals, calcium in mineral form is plentiful as
the carbonate (CaCO
3
) in shell and coral and as
hydroxyapatite (Ca
5
(PO
4
)
3
(OH)) in bone and tooth
enamel. Calcium in solution acts an important intra-
cellular signal mechanism in animals, essential for
muscle contraction, blood clotting and many other
functions. (See Calcium: Physiology.)
Overview of Occurrence in Foods
0008 Roughly 70% of the calcium present in the food
supply in the USA is in the form of milk and dairy
products, with about 10% in fruits and vegetables,
5% in grain products, and the remainder in all other
foods. Similar proportions have been reported from
other Westernized countries. The relative importance
of these food sources to people’s calcium intakes
depends upon the relative amounts consumed. Even
so, the main food sources of calcium for most people
consuming omnivorous diets in Western countries
are milk and dairy products, typically accounting
for over 50% of total calcium intake. Although the
concentration of calcium in cereal products and vege-
tables is typically much lower than in dairy products,
the amount of these foods eaten means that foods of
plant origin do make a significant contribution to
total calcium intakes for most people (20–25% from
cereals in the USA and the UK, with about 14% from
bread due to calcium fortification of white flour). It is
commonly estimated that the total contribution of
nondairy foods eaten over the course of a day pro-
vides 200–300 mg of calcium to those consuming a
mixed diet, or about the equivalent of one glass of
milk. Other foods that contain considerable amounts
of calcium include tinned fish such as sardines or
salmon (largely due to the calcium in the bones and
skin), tofu (especially if it has been processed
with calcium) and certain seeds and nuts, but typical
eating habits indicate that these do not contribute
much to total calcium intake for most people. Vegans,
or others who do not consume milk and dairy prod-
ucts, may need to take particular care to ensure
adequate calcium intake from their diet or may
require a supplemental source of calcium, particularly
during periods of rapid growth. Lactovegetarians,
who do not avoid milk and dairy products, generally
have little difficulty in attaining adequate calcium
intakes.
Factors Influencing Calcium
Bioavailability
0009Knowledge of the calcium content of a food must be
tempered to some extent by understanding the dietary
and physiological factors that can influence the bio-
availability of calcium. Calcium bioavailability can
be operationally defined as the amount of calcium
that is available to be absorbed and used for normal
metabolic functions in the body. Physiological factors
influencing calcium bioavailability include rate of
growth, calcium status and age (See Calcium: Physi-
ology). A number of dietary factors can influence the
bioavailability of calcium, including the level of cal-
cium intake and its chemical form, whether calcium is
taken with food or as a supplement on an empty
stomach, and the presence of inhibitory or enhancing
compounds coingested with the calcium. Calcium is
more bioavailable when taken with food than on an
empty stomach, due at least in part to the longer
passage time through the GI tract. Oxalate and phy-
tate are well-known inhibitors of calcium uptake
through the formation of insoluble complexes within
the gut. With the exception of vitamin D, which is
involved in the active transport uptake of calcium
from the gut (See Calcium: Physiology; Cholecalcif-
erol: Physiology), potential enhancers of dietary cal-
cium absorption are generally less well defined than
the inhibitors, and may have only limited influence on
the intestinal absorption of calcium ingested with a
meal. Absorption from the gastrointestinal tract is not
the only consideration in bioavailability; dietary
sodium and protein (particularly proteins high in
sulfur amino acid content due to the physiological
acidification effect) influence the amount of calcium
excreted in urine and consequently not available for
essential functions in the body.
Sources of Calcium Intake
0010As alluded to previously, calcium is quite unevenly
distributed in the diet of humans. Some foods are
rich sources of calcium, whereas others contain
relatively little. With developments in food
processing and fortification policies and practices,
the increasing availability of calcium-fortified foods
and dietary supplements containing calcium salts is
leading to a wider range of rich dietary sources of
calcium.
766 CALCIUM/Properties and Determination

Foods of Animal Origin
0011 Milk and many dairy products are good sources of
bioavailable calcium (Table 2). Removal of the milk
fat fraction from fluid milk leads to slight increases
in the calcium concentration as one proceeds from
whole milk to skim milk. Fluid milk with added
modified milk ingredients is a relatively new entry
into selected marketplaces, containing some 20–
25% more calcium than regular milk due to the add-
ition of whey calcium concentrates. Naturally, foods
containing milk or milk products, whether ‘hidden’ in
the recipe as in cakes or other baked goods or more
obviously added as with cream or cheese sauces, will
also provide a fair amount of calcium.
0012 Soft cheeses and cream cheese generally contain
less calcium on a wet-weight basis due to their higher
water content. The calcium content of cheeses also
depends on the process used in preparation and
whether the calcium precipitates with the milk solids
or remains in the whey. Cheeses precipitated with
lactic acid, such as cottage cheese or cream cheese,
contain lower calcium levels in the curd, as most
of the calcium remains soluble in the acid whey.
Rennin coagulation (e.g., in production of cheddar
or mozzarella cheese) yields cheeses with a higher
calcium content as the curd is formed before signifi-
cant acidification takes place.
0013 Meat and fish are not typically rich sources of
calcium (Table 2). Mechanically deboned meats can
contain much higher levels of calcium due to the
abrasion of bone during the deboning process. Tinned
salmon provides higher levels of calcium than fresh
salmon fillet (Table 2), because the bones and skin
tend to be consumed along with the flesh. Similar
reasoning applies to small fish consumed whole,
such as sardines and anchovies. Calcium is generally
somewhat higher in crustaceans than in fin fish.
Foods of Plant Origin
0014Foods of plant origin are not generally very rich
sources of calcium, and some contain significant
levels of calcium absorption inhibitors such as phy-
tate or oxalate. However, due to the large amounts
consumed, this class of foods typically makes a fairly
significant contribution to total calcium intake.
Whole grains and seeds generally provide more cal-
cium than most fruits or vegetables. Fortification of
refined flours and breakfast cereals can significantly
increase the contribution of these foods to total cal-
cium intake. Similarly, the high calcium content of
baking powder and the variety of calcium-containing
food additives used (e.g., as dough conditioners or
yeast nutrients – see below) adds to the amount of
calcium in baked goods.
0015While spinach appears to contain a reasonable
amount of calcium (129 mg per 95-g serving,
see Table 3), the high oxalate content of spinach
renders much of the calcium insoluble and much less
tbl0002 Table 2 Calcium content of selected animal-source foods
a
Foodname Servingsize Weight (g) Calcium (mg) Concentration
(mgg
1
as is)
Milk and dairy products
Milk, whole, 3.3% M.F.
b
250 ml 258 308 1.19
Milk, partly skimmed, 2% M.F. 250 ml 258 314 1.22
Milk, skim 250 ml 259 319 1.23
Milk, skim, with added milk solids 100 143 1.43
Milk, condensed, sweetened, canned 300 ml 388 1100 2.84
Milk, evaporated, partly skimmed, canned, undiluted, 2% M.F. 250 ml 268 739 2.76
Yogurt, fruit bottom, 1–2% M.F. 175 g 175 214 1.22
Cheese, cheddar 4 slices 52 378 7.27
Cheese, mozzarella, shredded, 22.5% M.F. 125 ml 60 321 5.35
Cheese, cottage, 2% M.F. 125 ml 119 82 0.69
Meat, fish, and egg
Egg, poached 1 large 50 24 0.48
Beef, ground, lean, medium broiled 1 patty 80 9 0.11
Beef, mechanically deboned, raw 100 485 4.85
Pork chop, centre cut, lean, pan-fried 1 chop 69 16 0.23
Chicken, broiler, breast, roasted ½ breast 98 5 0.05
Salmon, Sockeye, baked or broiled ½ fillet 155 11 0.07
Salmon, Sockeye, canned, solidsþboneþliquid 125 ml 79 181 2.29
Sardine, Atlantic, canned in oil, drained, with bone (7.5 cm long) 4 sardines 48 183 3.81
a
Data from Health Canada (1999) Nutrient Value of Some Common Foods. Ottawa: Health Protection Branch, Health Canada, Canadian Government
Publishing, except where no serving size is specified, which data are from the Canadian Nutrient File, Health Canada, 1997 revision.
b
Milk fat.
CALCIUM/Properties and Determination 767
bioavailable, so that it has been estimated that over
15 servings of spinach would be required to obtain
the same level of absorbable calcium as one glass of
milk (Table 4). Turnip greens contain a similar level
of calcium to that found in spinach, but without the
oxalate, so that about 2 servings of turnip greens
provide a similar level of absorbable calcium to one
glass of milk (Table 4). However, these types of food
calcium absorption values are often determined under
laboratory conditions while feeding only the single
food. Calcium bioavailability from a given food
source can be substantially modified by other foods
eaten at the same time. For example, milk calcium
absorption has been shown to decline from 33 to
27% when coingested with spinach, whereas spinach
calcium absorption showed an increase from 3%
when fed alone to 11% when coingested with milk.
In a Western mixed diet, the overall calcium absorp-
tion by adults typically averages about 25–30%.
Processing Effects on Food Calcium
0016There are numerous food additive uses of calcium
salts that may add appreciable amounts of calcium
to some foods. The Food Chemicals Codex, fourth
edition (1996) lists over 30 calcium compounds used
as food additives or processing aids. Some of the
common functions of calcium-containing food addi-
tives (and examples of the calcium compounds used)
include: dough conditioners such as calcium carbon-
ate, calcium iodate, calcium lactate, calcium oxide,
calcium phosphate or calcium sulfate; pH adjusters
such as calcium hydroxide or calcium phosphate; anti-
oxidants such as calcium ascorbate; preservatives
such as calcium propionate, calcium disodium EDTA
tbl0003 Table 3 Calcium content of selected plant-based foods
a
Foodname Serving size Weight (g) Calcium (mg) Concentration (mg g
1
as is)
Vegetables and fruit
Beans, snap, frozen, boiled, drained 125 ml 71 35 0.49
Carrots, raw 1 medium 80 22 0.28
Peas, green, frozen, boiled, drained 125 ml 85 20 0.24
Spinach, boiled, drained 125 ml 95 129 1.36
Apple, raw, with skin (7 cm diameter) 1 apple 138 10 0.07
Banana, raw 1 medium 115 7 0.06
Oranges, raw 1 fruit 131 52 0.40
Grain and cereal products, nuts, and seeds
Wheat, flour, all purpose 250 ml 132 20 0.15
Bread, white, commercial 1 slice 25 27 1.08
Corn meal 125 ml 73 4 0.05
Flax seeds 125 ml 73 196 2.68
Soy flour, defatted 250 ml 106 255 2.41
Tofu, firm, prepared with magnesium chloride 1 piece 80 164 2.05
Tofu, firm, prepared with calcium sulfate 100 683 6.83
Tofu, regular, prepared with calcium sulfate 100 350 3.50
Almonds, dry-roasted, salt added 125 ml 73 206 2.82
Peanuts, dry-roasted, without shell 125 ml 78 42 0.54
Hazelnuts, chopped, dried 125 ml 61 119 1.95
Sesame crisp 4 pieces 35 247 7.06
a
Data from Health Canada (1999) NutrientValueofSomeCommonFoods. Ottawa: Health Protection Branch, Health Canada, Canadian Government
Publishing, except where no serving size is specified, which data are from the Canadian Nutrient File, Health Canada, 1997 revision.
tbl0004 Table 4 Bioavailability of calcium from various food sources
a
Food Serving size (g) Ca content (mg) Fractionalabsorption (%) Servingsequivalent to 240 mlmilk
Milk 240 300 32.1 1
Soy milk (unfortified) 120 5 31.0 60.4
Almonds, dry-roasted 28 80 21.2 5.7
Sesame seeds, no hulls 28 37 20.8 12.2
Broccoli 71 35 52.6 5.0
Brussel sprouts 78 19 63.8 8.0
Spinach 90 122 5.1 15.5
Turnip greens 72 99 51.6 1.9
Tofu, calcium set 126 258 31.0 1.2
a
Selected data from Weaver CM and Plawecki KL (1994) Dietary calcium: adequacy of a vegetarian diet. American Journal of Clinical Nutrition 59
(supplement): 1238S–1241S.
768 CALCIUM/Properties and Determination

or calcium sorbate; anticaking agents such as calcium
stearate, calcium phosphate or calcium silicate;
and thickeners such as calcium gluconate or calcium
alginate. The percentage composition of calcium in
these compounds ranges considerably, e.g., calcium
gluconate is 9% calcium, calcium carbonate is 40%
calcium, and calcium oxide is 71% calcium by
weight. Solubility also differs considerably between
compounds, with some such as calcium carbonate
being relatively insoluble at neutral pH, whereas
others like calcium acetate or calcium chloride are
highly soluble.
0017 Tofu set with calcium sulphate contains consider-
ably more calcium than tofu set with magnesium
chloride (Table 3). Firm tofu contains a higher con-
centration of calcium than does regular tofu due to
the lower water content (and consequently higher
solids). Fermentation (including leavening of bread)
or germination of selected plant foodstuffs can
increase the availability of calcium due to breakdown
of complexing compounds such as phytic acid. The
calcium content of meat can be increased by process-
ing or cooking in acidic solutions due to the dissol-
ution of calcium from bone. For example, pork spare
ribs or chicken cooked with vinegar contain more
calcium than the uncooked flesh.
0018 Tap water typically contains calcium and many
other elements, and may make some contribution to
total calcium intake particularly in hard water areas.
Water hardness is measured as milligrams of calcium
carbonate equivalents per liter, and hard water may
be over 300 mg of CaCO
3
per liter. Calcium carbon-
ate is 40% elemental calcium by weight. Thus, a 250-
ml glass of water may provide 30 mg of elemental
calcium in some areas. The hardness of water added
in processing may also influence the calcium content
of foods to some extent. Conversely, losses through
soaking and/or boiling may amount to 5–25% of the
calcium content of a variety of vegetables that are
commonly prepared in this way.
0019 Foods may have nutrients added for a variety of
reasons including restoration of processing losses, or
to provide similar nutrient levels in substitute foods
(e.g., soy ‘milk’ and other plant based beverages,
which may be fortified with calcium in some coun-
tries, as they are used as a substitute for dairy prod-
ucts in the diet of their consumers), for fortification
(which, in this context, refers to addition of calcium
with the intent of raising the calcium content of the
food for the consumer, e.g., enriched flour or infant
cereals), or for special purpose foods such as meal
replacements or formulated liquid diets (See Legisla-
tion: Codex). Regulations and practices concerning
food fortification may vary considerably between
countries.
Supplements
0020Nutritional supplements and antacid medications can
make a significant contribution to calcium intakes for
some people. Calcium supplements are available in a
number of forms, including carbonate, phosphate,
lactate, or citrate salts of calcium, among others. Cal-
cium carbonate is found in many over-the-counter
antacid preparations. Multivitamin and multimineral
supplements may also contain calcium, though fre-
quently in smaller amounts. Natural source supple-
ments include oyster shell and dolomite, though it
should be noted that concerns have been raised with
the potential for lead contamination of these forms. It
has been estimated that, among supplement users in
the USA (who represent approximately 20% of the
population), an average of 300 mg of Ca per day is
consumed from supplements. In addition, calcium-
containing antacids, supplying 200–400 mg of Ca
per tablet, are consumed by about 18% of USA adults.
Analysis of Calcium in Foods and
Biological Samples
0021There is no satisfactory routine biochemical method
for assessing calcium nutritional status (See Nutri-
tional Assessment: Biochemical Tests for Vitamins
and Minerals). Serum calcium concentration varies
very little, even across a wide range of dietary calcium
intakes, because of adaptive alterations by which the
endocrine system regulates the level of this mineral in
blood (See Calcium: Physiology). Deviations of cal-
cium concentration from this narrow range are med-
ically significant, and in this setting, the measurement
of total plasma or serum calcium or specific measure-
ment of the ionized calcium fraction is important.
Total calcium in plasma or urine can be measured
by atomic absorption spectrophotometry, either dir-
ectly on the diluted sample or following sample min-
eralization (‘ashing’–see below), or by colorimetric
assay (e.g., using arsenazo III or orthocresolphthalein
complexone) on a clinical autoanalyzer. Ionized cal-
cium in plasma (the fraction that is not bound to
proteins or low-molecular-weight ligands, and conse-
quently is most physiologically active) represents
about 45% of plasma calcium under normal
conditions and can be assessed using an appropriate
ion-sensitive electrode. Assessment of intracellular
calcium levels in the research laboratory can be ad-
dressed using fluorescent probes such as FURA 2 or
QUIN 2, among others. Calcium nutritional status is
more often assessed indirectly through balance stud-
ies or by measurement of bone mineral concentration
or bone mineral density, reflecting the major struc-
tural role played by this mineral nutrient.
CALCIUM/Properties and Determination 769

Sample Preparation
0022 Most foods and biological tissue samples can be pre-
pared for analysis of their calcium content either by
dry ashing (in a muffle furnace) or wet ashing (acid
digestion) techniques or some combination of the
two. Dry ashing techniques typically involve com-
bustion at elevated temperature (e.g., 500–550
C)
until organic matter is fully destroyed, followed by
dissolution of the ash in a suitable acid for subsequent
analysis. Wet ashing techniques typically involve
destruction of organic matter by heating in concen-
trated nitric acid until a pale straw color is attained,
and may feature additional oxidation steps with
perchloric acid or hydrogen peroxide to clarify the
sample solution. In wet ashing procedures, particu-
larly for samples with a high calcium content such
as bone meal, sulfuric acid is generally to be avoided
due to the ready precipitation of calcium from
solution by sulfate, forming plaster of Paris (calcium
sulfate hemihydrate) or gypsum (calcium sulfate
dihydrate).
Determination of Calcium Content of Foods and
Tissues
0023 Determination of calcium content of foods and
tissues is commonly done by atomic absorption
spectrophotometry, though a number of alternative
methods exist including titrimetric methods using
EDTA or KMnO
4
, neutron activation analysis, or
inductively coupled plasma emission spectrometry.
Details of sample preparation and analytical proced-
ures for a variety of food sample types are published
by the Association of Official Analytical Chemists.
Atomic Absorption Spectrophotometry
0024 For atomic absorption spectrometric analysis of cal-
cium, 0.1–1.0% (w/v) lanthanum is included in the
analytical working solution as a matrix modifier to
reduce anion interferences due to phosphate or sul-
fate, which otherwise can form refractory complexes
and depress the absorption of light by atomic calcium.
Absorbance is measured at the 422.7-nm calcium
spectral line, following atomization in a reducing
air–acetylene flame, and compared with certified
analytical standard calibration solutions. A reducing
flame gives a higher sensitivity, though an oxidizing
flame may give a higher precision where this is crit-
ical. Typical analytical working ranges are obtained
up to 5 mg l
1
in the analytical working solution
when using a standard nebulizer assembly, and may
be approximately doubled with the use of a high
sensitivity nebulizer. (See Spectroscopy: Atomic Emis-
sion and Absorption.)
KMnO
4
Titrimetric Method for Calcium in Wheat
Flour (Method from the Association of Official
Analytical Chemists)
0025After dry ashing and dilution of the ash to a suitable
volume with demineralized water, bromocresol green
indicator is added along with enough 20% sodium
acetate solution to change the pH to 4.8–5.0 (blue).
The sample solution is covered and heated to boiling.
The calcium is precipitated by slow addition (1 drop
every 3–5 s) of 3% oxalic acid solution (w/v) until pH
is 4.4–4.6, as indicated by a distinct green shade. The
solution is then boiled for 1–2 min and allowed to
settle overnight. The supernate is filtered through
quantitative paper, Gooch, or fine fritted glass filter,
and the beaker and precipitate are washed with small
portions of ammonium hydroxide (1 þ50). A mix-
ture of 125 ml of water and 5 ml of sulfuric acid is
added to the precipitate with heating to 80–90
C.
The solution is finally titrated at 70–90
C with
0.01 M KMnO
4
to a slight pink endpoint, 1 ml of
permanganate solution equating to 1 mg of calcium.
See also: Calcium: Physiology; Cholecalciferol:
Physiology; Nutritional Assessment: Biochemical Tests
for Vitamins and Minerals; Spectroscopy: Atomic
Emission and Absorption
Further Reading
Bender AE (1978) Food Processing and Nutrition. London:
Academic Press.
Flynn A and Cashman K (1999) Calcium. In: Hurrell R (ed.)
The Mineral Fortification of Foods, pp. 18–53. Leather-
head, UK: Leatherhead International.
Fraser D, Jones G, Kooh SW and Radde IC (1986) Calcium
and phosphate metabolism. In: Tietz NW (ed.) Textbook
of Clinical Chemistry, pp. 1317–1372. Philadelphia, PA:
W.B. Saunders Company.
Gue
´
guen L and Pointillart A (2000) The bioavailability of
dietary calcium. Journal of the American College of
Nutrition 19(2): 119S–136S.
Health Canada (1999) Nutrient Values of Some Common
Foods. Ottawa: Health Protection Branch in cooper-
ation with Health Promotion and Programs Branch,
Health Canada. Canadian Government Publishing.
Hibbins SG (1992) Calcium and calcium alloys. In: Kirk–
Othmer Encyclopedia of Chemical Technology, 4th edn,
vol. 4, pp. 777–786. New York: John Wiley.
Horowitz W (ed.) AOAC (2000) Official Methods of An-
alysis of AOAC International, 17th edn. Gaithersburg,
MD: The Association of Official Analytical Chemists.
Institute of Medicine (1996) Food Chemicals Codex, 4th
edn. Washington, DC: Committee on Food Chemicals
Codex, Food and Nutrition Board, Institute of Medi-
cine, US National Academy of Sciences. National
Academy Press.
770 CALCIUM/Properties and Determination

Institute of Medicine (1997) Dietary Reference Intakes for
Calcium, Phosphorus, Magnesium, Vitamin D, and
Fluoride. Washington, DC: Standing Committee on the
Scientific Evaluation of Dietary Reference Intakes, Food
and Nutrition Board, Institute of Medicine, US National
Academy of Sciences. National Academy Press.
Karmas E and Harris RS (eds) (1988) Nutritional Evalu-
ation of Food Processing, 3rd edn. New York: Van
Nostrand Reinhold Company.
Petersen RL and Freilich MB (1992) Calcium compounds
(survey). In: Kirk–Othmer Encyclopedia of Chemical
Technology, 4th edn, vol. 4, pp. 787–796. New York:
John Wiley.
Weaver CM and Plawecki KL (1994) Dietary calcium: ad-
equacy of a vegetarian diet. American Journal of Clinical
Nutrition 59(supplement): 1238S–1241S.
Physiology
MRL’Abbe
´
, Nutrition Research Division, Ottawa,
Ontario, Canada
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Calcium (Ca, element number 20, atomic weight
40.08, oxidation state þ2) is the most abundant cation
in the human body and is a nutritionally essential min-
eral. More than 99% of body calcium is in the skeleton,
accounting for 1.5–2.0% of body weight (approxi-
mately 1.5 kg). Calcium has two main types of func-
tions: structural and metabolic. It is responsible for the
mechanical and structural functions of bone and teeth
as well as many metabolic functions involving numer-
ous regulatory enzymes that require calcium. The
physiological functions of calcium are so vital that
total serum calcium is maintained in a very narrow
range through a highly integrated system of hormones
(parathyroid hormone, calcitonin, and vitamin D). If
dietary sources of calcium are inadequate, bone re-
serves are mobilized; inadequate dietary calcium has
long been associated with osteoporosis – a disease
characterized by low bone mineral density.
Calcium Metabolism
Absorption
0002 Intestinal absorption of calcium proceeds by two
independent routes, a saturable, active absorption
pathway regulated almost exclusively by vitamin D
and a nonsaturable passive pathway that depends on
the concentration of calcium in the intestinal lumen.
Most calcium absorption occurs in the duodenum
and jejunum, but minor amounts can also be
absorbed in the colon. The active absorption pathway
is located primarily in the duodenum and upper
jejunum. Absorption by this pathway varies with
age, calcium and vitamin D status, and total calcium
intake. It constitutes the mechanism whereby the
body increases relative absorption during periods of
low dietary calcium intakes or low calcium status.
Most calcium is absorbed by the nonsaturable passive
route in adults with a mature skeleton, and active
transport is of relatively minor importance to the
total calcium absorbed. Thus, the major factor that
determines the amount of calcium absorbed is the
quantity ingested. Although the efficiency of calcium
absorption declines with increasing calcium dose,
there is evidence that the total amount absorbed
may continue to rise.
0003The absorption of calcium from food can vary
from 5 to 60%. It is influenced by calcium status,
vitamin D metabolites, pH of the gut lumen, and
dietary factors such as protein, phosphorus, sodium,
phytate, and oxalate (see sections on Bioavailability
Dietary Factors Affecting Calcium Balance, for more
details).
Bioavailability
0004Foods vary in both calcium content and bioavailabil-
ity. Together, these two factors determine how much
calcium a particular food source will provide per
serving. Information on the content and bioavailabil-
ity of calcium in various food products is given in
Table 1. Milk and milk products have a high calcium
content and bioavailability, and thus are major food
sources of calcium in Western diets. Many vegetables
have a higher fractional absorption (bioavailability)
of calcium but a lower content of calcium than is
found in milk and milk products. In addition, some
vegetables and plant products contain inhibitors of
calcium absorption, such as oxalate (e.g., spinach), or
phytate (e.g., wheat bran, legumes, nuts, oats, soy
beans, maize), which reduce the bioavailability of
calcium.
Balance
0005Calcium balance is controlled through an integrated
response to calcium-regulating hormones that affect
calcium transport at three primary sites of regulation:
intestine, bone, and kidney. The most important cal-
cium-regulating hormones are parathyroid hormone
(PTH), calcitonin and vitamin D, and the effects of
these are briefly described below. Glucocorticoids,
thyroid hormones, growth hormone, insulin, estro-
gen, and testosterone also affect bone turnover and
calcium metabolism.
CALCIUM/Physiology 771
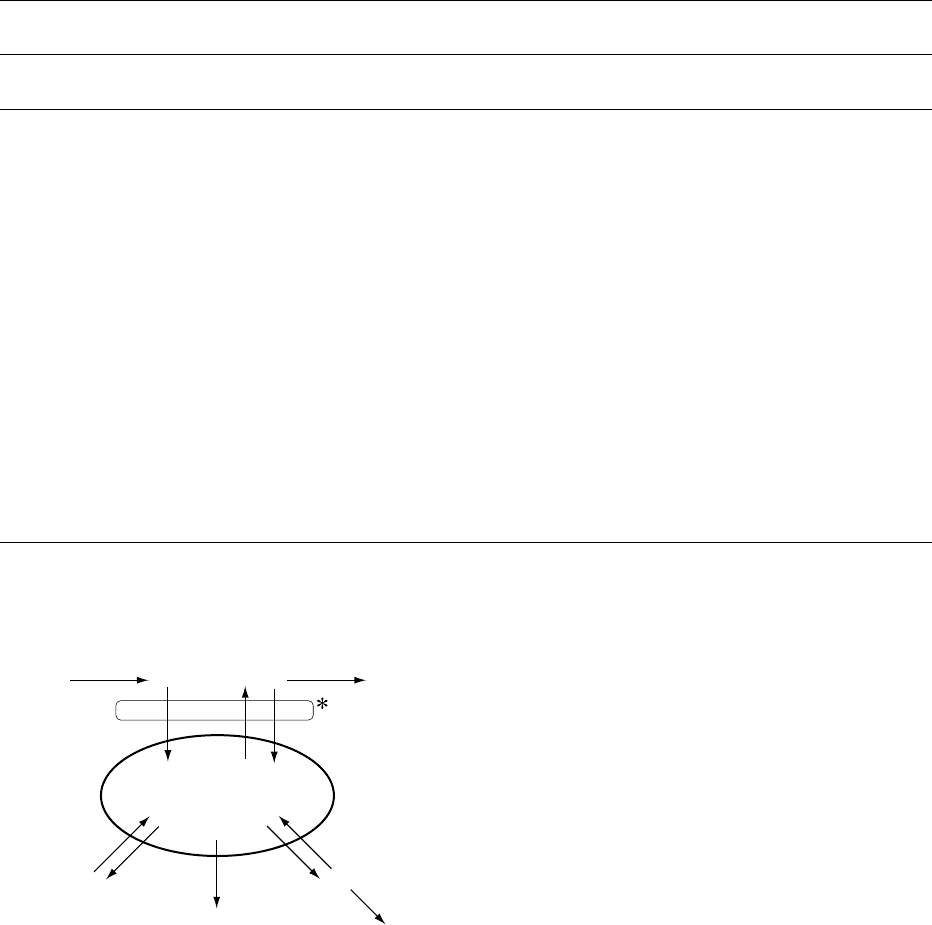
0006 At equilibrium, calcium balance is maintained
through regulation of plasma calcium levels, with
the calcium influx/efflux occurring at several sites,
as illustrated in Figure 1. If dietary calcium intakes
are insufficient, or in the case of malabsorption
syndromes, calcium is mobilized from bone to main-
tain blood concentration.
Hormonal Regulation
0007Vitamin D is an important nutrient for regulating
plasma calcium concentration and bone metabolism.
The two most physiologically active forms are vita-
min D
2
(ergocalciferol), found mainly in yeast and
plant foods, and vitamin D
3
(cholecalciferol), synthe-
sized by skin and the most common form added to
foods. Vitamin D is hydroxylated first in the liver at
the 25-carbon position to form the 25-hydroxyvita-
min D (25(OH)D), which enters the circulation. Cir-
culating (25OH)D is a good measure of vitamin D
status. In the kidney, the 25(OH)D is further hydro-
xylated to form the dihydroxy compound (1,25
(OH)
2
D), which is the most active metabolite. The
production of 1,25(OH)
2
D in the kidney is tightly
regulated via PTH (see below). The 1,25(OH)
2
D
increases calcium absorption in the intestine and is
absolutely critical during growth as well as during
times of low calcium intake. In calcium deficiency,
1,25(OH)
2
D production is increased, causing en-
hanced intestinal absorption and renal reabsorption
of calcium, and increased bone resorption.
0008PTH is released in response to a decrease in extra-
cellular fluid calcium concentration. PTH directly
stimulates bone and renal tubular calcium resorption
and indirectly increases intestinal absorption through
tbl0001 Table 1 Content and bioavailability of calcium in various food products
a
Serving
size (g)
Calcium
b
content (mg)
Fractional
absorption (%)
Estimatedabsorbable
Ca/serving (mg)
Servings needed
to ¼1cupofmilk
Milk (1 cup) 240 300 32.1 96.3 1.0
Almonds, dry roasted (1 oz) 28 80 21.2 17.0 5.7
Beans, pinto 86 4.7 17.0 7.6 12.7
Beans, red 172 40.5 17.0 6.9 14.0
Beans, white 110 113 17.0 19.2 5.0
Broccoli 71 35 52.6 18.4 5.2
Brussel sprouts 78 19 63.8 12.1 8.0
Cabbage, Chinese (bok choi) 85 79 53.8 42.5 2.3
Cabbage, green 75 25 64.9 16.2 5.9
Cauliflower 62 17 68.6 11.7 8.2
Kale 65 47 58.8 27.6 3.5
Kohlrabi 82 20 67.0 13.4 7.2
Mustard green 72 64 57.8 37 2.6
Radish 50 14 74.4 10.4 9.2
Rutabaga 85 36 61.4 22.1 4.4
Sesame seeds no hulls (1 oz) 28 37 20.8 7.7 12.2
Soy beverage, not fortified 120 5 31.0 1.6 60.4
Spinach 90 122 5.1 6.2 15.5
Tofu, calcium set 126 258 31.0 80.0 1.2
Turnip greens 72 99 51.6 51.1 1.9
Watercress 17 20 67.0 13.4 7.2
a
Selected data from Weaver CM and Plawecki KL (1994) Dietary calcium: adequacy of a vegetarian diet. American Journal of Clinical Nutrition 59(5):
1238S–1241S.
b
Based on a half-cup serving size unless otherwise noted.
Skin
Plasma
10 mg/100 mL
(350 mg)
Gut
Feces
Kidneys
Urine
Bone
500
500 8460
180
8640
15
Diet
300 150
8051000
45
fig0001 Figure 1 Outline of calcium balance (mg per day) under equi-
librium conditions (input ¼output) in adult humans. *Assuming
equilibrium conditions and 30% absorption, 300 mg of the daily
dietary intake of 1000 mg are absorbed from the gut. Endogenous
calcium secretion returns 150 mg per day to the gut lumen. Using
the same assumption of 30% absorption, 45 mg per day of the
endogenously secreted calcium are reabsorbed. Net calcium
uptake is therefore 195 mg per day under these conditions,
which offsets the net loss of 195 mg per day through the skin
and in urine.
772 CALCIUM/Physiology

enhanced formation of 1,25(OH)
2
D in the kidney
from the monohydroxy form, 25(OH)D.
0009 Calcitonin is synthesized by the C cells of the thy-
roid in response to a rise in serum calcium; it inhibits
bone resorption and agents that have resorptive
effects on bone (PTH, vitamin D, prostaglandins,
and vitamin A).
Excretion
0010 The kidneys filter 8–10 g of Ca per day, with almost
all of it reabsorbed; only about 100–200 mg per day
is excreted in urine. Tubular reabsorption therefore
plays an important part in the conservation of cal-
cium, and a reduction in the number of functional
nephrons or in their capacity to reabsorb normally
can result in hypocalcemia.
0011 Under ideal conditions (i.e., in calcium balance),
excretion via the urine (and, to a lesser degree, via
sweat and feces) is matched by the influx into the
extracellular fluid compartment, usually through
intestinal absorption.
Dietary Factors Affecting Calcium
Balance
Vitamin D
0012 As mentioned above (see section Hormonal Regula-
tion), adequate vitamin D is critical to the absorption
of calcium D; yet, there is little or no natural vitamin
D in most foods that humans normally eat, except
some fish. Vitamin D is present in the flesh of
fatty fish such as sardines and herring, and in the
liver oils of other fish such as cod and tuna.
Meat such as poultry, pork, and beef contain small
amounts of vitamin D. In Canada and in many
other countries, vitamin D fortification of milk and/
or margarine provide the major sources of dietary
vitamin D.
0013 The other major source of vitamin D for humans is
synthesis by skin exposed to ultraviolet or sunlight
irradiation, and most people could obtain sufficient
vitamin D from this source. However, this vital
photosynthetic process can be affected by anything
that limits the amount of solar ultraviolet B photons
to reach the skin’s surface and penetrate into the
viable epidermis. In northern latitudes, the photosyn-
thetic production of vitamin D is negligible during
the winter months (e.g., from November to March,
or virtually half the year in much of Canada). The
elderly also have a reduced capacity for vitamin D
synthesis, and deficiency is most prevalent among
the elderly, particularly among institutionalized or
house-bound individuals. Factors such as pollution
in the air and melanin of the skin can affect the
cutaneous synthesis of vitamin D. In addition,
sunscreens block the absorption of the sunlight spec-
trum that helps the cutaneous synthesis of vitamin D,
and there has been a marked increase in the use of
sunblocks in recent years in many countries. Thus,
providing a reliable, safe, and adequate source of
vitamin D in the diet is essential for optimum calcium
absorption.
Phosphorus
0014There is some controversy concerning the effects of
high phosphorus on calcium balance. In the past,
considerable emphasis was placed on the Ca:P ratio
of the diet, particularly in infant nutrition. In the most
recent review of calcium and phosphorus require-
ments done by the National Academy of Sciences,
this concept was considered to have no demonstrable
relevance in adults, although the report stated: ‘The
concept still has some utility under conditions of
rapid growth (in which a large share of the ingested
nutrients is converted into tissue mass).’ The ratio of
calcium to phosphorus by itself does not take into
account the differing bioavailabilities of the two
nutrients, but estimates of optimal Ca:P ratios have
frequently been based on the calcium and phosphorus
needs of bone building. It was estimated that an
absorbed Ca:P ratio sufficient to support the sum of
bony and soft tissue growth would be approximately
1.3:1, and correcting for the relative net absorptions,
this would be met by Ca:P intakes in the range of
about 2:1.
0015Currently, in the USA, the intake of phosphorus
is high relative to calcium. Furthermore, an individ-
ual’s actual phosphorus intake may be higher than
estimated due to inaccurate nutrient composition
tables for phosphorus values, coupled with underre-
porting of processed foods. In recent years, the
usage of phosphate salts as food additives has in-
creased by nearly 20%. Currently, there are more
than 45 phosphorus-containing compounds ap-
proved for use in food processing as nutrients, dietary
supplements, or for functional purposes such as to
preserve moisture or color, and as emulsifiers. Cola
beverages and root beer beverages can be a significant
source of phosphorus, containing about 70 mg of
phosphorus per serving. One study has suggested
that high intakes of phosphoric acid-containing cola
beverages were associated with slight reductions in
serum calcium in Mexican children, but it is unclear
to what extent the effect was due to the acid load of
the cola beverages, low calcium intakes, or the phos-
phorus itself.
0016Because phosphorus absorption is not strictly regu-
lated, a high-phosphorus diet will directly increase
the level of serum phosphate, which in turn lowers
CALCIUM/Physiology 773

serum calcium, a signal for PTH release. Current
research describing diets in terms of the Ca:P ratio
has suggested that diets with low ratios (i.e., high
phosphate intakes where Ca:P equals 0.25:1) cause
serum calcium to drop, thereby increasing PTH secre-
tion, and inducing secondary hyperparathyroidism.
This increase in PTH is often thought to be harmful
to bone. Other researchers, however, have shown that
calcium and phosphorus have no effect on each other
in the healthy adult population. For example, balance
studies in human adults with Ca:P ratios ranging
from 0.08:1 to 2.40:1 had no effect on either calcium
balance or calcium absorption.
Protein
0017 North Americans consume considerably more cal-
cium than people in developing countries; yet, the
incidence of osteoporosis is much higher in North
America. Recommended intakes for calcium in de-
veloped countries is much higher than for countries
that consume a plant-based diet. One possible reason
for this is the high amount of processed foods and
animal protein found in Western diets that are not
present in diets from primarily cereal-based food
economies. With regard to processed foods, phos-
phorus has been discussed above, and salt and
caffeine will be discussed below. Animal proteins
contain a high proportion of sulfur amino acids, and
the oxidation of these sulfur amino acids to inorganic
sulfate generates endogenous acid, which in turn
increases urinary excretion of acid and calcium. In-
creasing urinary calcium excretion will result in bone
loss when there is insufficient capacity to replace the
loss by absorbing more dietary calcium. High-pro-
tein-induced hypercalciuria is a misnomer, in that
simply comparing high protein with low protein
does not show hypercalciuria unless phosphorus is
controlled or the amount of protein added is
exceedingly high, or there is a change in the type of
protein from one low in sulfur amino acids to a
protein that is high in sulfur amino acids. In general,
for every gram of total protein consumed, urinary
calcium increases by approximately 1 mg.
Salt
0018 High intakes of sodium chloride (NaCl) result in
increased renal calcium loss, which promotes nega-
tive calcium balance. Urine calcium level increases by
approximately 26 mg for every gram of salt ingested.
Habitual excessive sodium chloride intakes have been
suggested as a factor in promoting bone loss. How-
ever, given the small amount of calcium loss for every
gram of salt consumed, the overall impact from diet-
ary salt on calcium metabolism is likely minimal
unless excessive amounts are consumed.
Caffeine
0019Oral doses of caffeine increase the urinary excretion
of calcium for at least 3 h after consumption. Calcium
balance studies have been performed in which
caffeine was shown to have no effect when dietary
calcium levels were adequate. The amount of caffeine
in a small cup of coffee could raise urinary calcium
excretion by approximately 4 mg, an effect that is less
than that for salt. However, for persons with a low
calcium intake, caffeine ingestion can be a significant
predictor of low bone mineral density. Caffeine is
found in only a small number of food products (e.g.,
colas, coffee, tea); its consumption would be unlikely
to have a negative impact on calcium status, as urin-
ary losses would be more than compensated for by a
teaspoon of milk.
Methods for Assessment of Calcium
Status
0020Serum concentration of calcium varies very little,
even across a wide range of dietary calcium intakes,
because of the adaptive alterations by which the
endocrine system regulates the level of this mineral
in blood. Therefore, there is no satisfactory routine
biochemical method for assessing nutritional calcium
status. If serum calcium levels are found to be outside
the normal range, it is usually due to underlying
disease conditions rather than nutritional problems.
Calcium is found in several forms in serum: ionized,
protein bound, and other minor blood components,
with the ionized form of calcium being the function-
ally regulated form. Numerous biological markers or
techniques have been used to assess calcium status.
These can be classified into several groups: serum
calcium or ionic calcium, urinary excretion of cal-
cium, bone mineral density measurements or other
techniques to measure the degree of bone loss, quan-
tification of markers of bone resorption or bone re-
modelling, and finally calcium balance or absorption
studies. Information on each of these measures is
given in the following section.
Serum Calcium
0021Serum calcium concentration cannot be used as an
index of calcium status, as it is homoeostatically con-
trolled. Calcium concentration is serum varies very
little, despite large changes in dietary calcium,
because of the adaptive alterations by which the
endocrine system regulates this mineral (see section
Hormonal Regulation). Calcium in serum is distrib-
uted in the following forms: ionized 47.5%, protein
bound 46%, Ca citrate 1.7%, CaHPO
4
1.6%, and
unidentified complexes 3.2%.
774 CALCIUM/Physiology

Serum-ionized Ca Concentration
0022 This test measures ‘physiologically active’ calcium
and thus better reflects calcium metabolism than
total calcium values. It is useful in determining cal-
cium changes in patients with altered proteins (e.g.,
chronic renal failure, nephrotic syndrome, hyperpara-
thyroidism, malabsorption, multiple myeloma) but is
not well correlated with dietary intakes of calcium.
Urinary Calcium Excretion
0023 Urinary excretion of calcium can be elevated
following high intakes of dietary calcium, although
urinary excretion of calcium accounts for only 0.2%
of the calcium filtered by the kidneys, because 99.8%
of filtered calcium is reabsorbed. Many conditions
can be associated with hypercalciuria, including
metabolic disturbances such as hyperparathyroidism,
bone metastases, osteoporosis (especially after
immobilization), Cushings syndrome, and vitamin D
intoxication. High dietary sodium or high dietary
protein can also promote increased excretion of
calcium. Hypercalciuria usually occurs prior to
hypercalcemia.
Bone Mineral Density
0024 Bone mineral density (BMD) is used as an index of
body calcium stores, and techniques such as radiog-
raphy, single or dual energy X-ray absorptiometry,
and computerized tomography have been developed
to measure BMD. The standard technique for diag-
nosing the presence or absence and severity of osteo-
porosis is based on BMD and a comparison with
reference ranges established for normal BMD in
young adults (see section Osteoporosis).
Markers of Bone Resorption
0025 Blood biochemical markers of bone resorption rates
tend to be less sensitive and specific than those for
bone remodeling and include: urinary hydroxy-
proline, a measure of total collagen turnover, and
pyridinoline and deoxypyridinoline, which are 3-
OH-pyridinium derivative cross-links more specific
to bone collagen. These measures indicate the degree
by which bone reserves of calcium are being depleted.
Markers of Bone Formation
0026 Markers of bone formation include serum osteocalcin
(bone gla-protein), bone alkaline phosphatase, and
urinary nephrogenous cyclic AMP.
Calcium Balance
0027 Calcium status has been assessed indirectly by assess-
ing calcium balance data, as increased retention is
usually seen when status is poor. Techniques used
include stable isotope or radiolabeled absorption
and calcium retention measures to estimate calcium
balance.
Function
0028Calcium has two main types of functions: structural
and metabolic. It is responsible for the mechanical
and structural functions of bone and teeth as well as
many metabolic functions involving numerous soft
tissue regulatory enzymes that require calcium. These
include such processes as neuromuscular transmis-
sion of chemical and electrical stimuli, enzyme acti-
vation, membrane transport of inorganic ions, muscle
contraction, hormone and cellular secretions, signal
transduction, blood clotting, and reproductive
functions such as sperm motility and fertilization of
the ovum. Calcium regulates release and storage of
neurotransmitters and hormones, the uptake and
binding of amino acids, absorption of vitamin B
12
,
and gastrin secretion. The activities of many enzymes
are affected by calcium, either acting directly (e.g.,
glyceraldehyde phosphate dehydrogenase, pyruvate
dehydrogenase, a-ketoglutarate dehydrogenase) or
indirectly through its activation of calmodulin (e.g.,
adenylate cyclase, cyclic nucleotide phosphodiester-
ase, Ca, Mg-ATPase, myosin light chain kinase,
phosphorylase b kinase, NAD kinase, ornithine dec-
arboxylase). Calcium therefore plays a role in normal
cardiac function, renal function, respiration, blood
coagulation, cell and membrane capillary permeabil-
ity, cyclic nucleotide metabolism, glycogen metabol-
ism, microtubule and microfilament function, and
cell division.
0029The physiological functions of calcium are so vital
to survival that total serum calcium is maintained in
a very narrow range (2.2–2.5 mmol l
1
). Calcium in
bone is in constant exchange with calcium in plasma.
When calcium absorbed from the diet is insufficient
to balance obligatory fecal and urinary losses, it is
drawn from bone to maintain plasma levels of ionic
calcium within this tightly controlled range.
Calcium Requirements
0030Recommended intakes for calcium for North Amer-
ica were recently released by the Standing Committee
on the Scientific Evaluation of Dietary Reference
Intakes (DRI), US National Academy of Sciences,
Food and Nutrition Board, Institute of Medicine,
under a program jointly commissioned by federal
government departments in the USA and Canada.
These revised standards encompass both recom-
mended dietary allowances (RDA) and tolerable
upper intake levels for nutrients. It should be noted
CALCIUM/Physiology 775
