Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

or persons who are immunocompromised in some
way. The route of entry of the organism in these
cases is not well defined, although foods containing
the organism would be a likely source.
Treatment/Prevention
0026 As with epidemic V. cholerae, treatment of diarrhea
is dependent on adequate rehydration. Septicemia
requires aggressive antibiotic therapy and supportive
care. Prevention in the developed world is based on
the avoidance of raw or undercooked shellfish. In the
developing world prevention is similar to that for
other enteric pathogens, including avoidance of raw
or undercooked foods of all types.
See also: Contamination of Food; Diarrheal
(Diarrhoeal) Diseases; Shellfish: Contamination and
Spoilage of Molluscs and Crustaceans; Vibrios: Vibrio
parahaemolyticus; Vibrio vulnificus
Further Reading
Albert MJ, Neira M and Motarjemi Y (1997) The role
of food in the epidemiology of cholera. World Health
Statistics Quarterly 50: 111–118.
Blake PA, Allegra DT, Snyder JD et al. (1980) Cholera – a
possible endemic focus in the United States. New
England Journal Medicine 302: 305–309.
Franco AA, Fix AD, Prada A et al. (1997) Cholera in Lima,
Peru, correlates with prior isolation of Vibrio cholerae
from the environment. American Journal of Epidemi-
ology 146: 1067–1075.
Karaolis DKR, Somara S, Maneval DR Jr, Johnson JA and
Kaper JB (1999) A bacteriophage encoding a patho-
genicity island, a type-IV pilus and a phage receptor
in cholera bacteria. Nature 27: 375–379.
Morris JG, Jr (1990) Non-O group 1 Vibrio cholerae:a
look at the epidemiology of an occasional pathogen.
Epidemiology Review 12: 179–191.
Morris JG Jr, Takeda T, Tall BD, et al. (1990) Experimental
non-O group 1 Vibrio cholerae gastroenteritis in
humans. Journal of Clinical Investigation 85: 697–705.
Mujica OJ, Quick RE, Palacios AM et al. (1994) Epidemic
cholera in the Amazon: the role of produce in disease
risk and prevention. Journal of Infectious Diseases 169:
1381–1384.
Nair GB, Ramamurthy T, Bhattacharya SK et al. (1994)
Spread of Vibrio cholerae O139 Bengal in India. Journal
of Infectious Diseases 169: 1029–1034.
Pollitzer R (1959) Cholera. Geneva: World Health Organ-
ization.
St Louis ME, Porter JD, Helal A et al. (1990) Epidemic
cholera in West Africa: the role of food handling and
high-risk foods. American Journal of Epidemiology 131:
719–728.
Twedt RM, Madden JM, Hunt JM et al. (1981) Character-
ization of Vibrio cholerae isolated from oysters. Applied
Environmental Microbiology 41: 1475–1478.
Wachsmuth IK, Blake PA and Olsvik O (eds) (1994)
Vibrio cholerae and Cholera. Washington, DC: ASM
Press.
Waldor MK and Mekalanos JJ (1996) Lysogenic conversion
by a filamentous phage encoding cholera toxin. Science
272: 1910–1914.
Vibrio parahaemolyticus
R Sakazaki, Japan Institute of Biological Sciences,
Chiyodaku, Tokyo, Japan
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001Vibrio parahaemolyticus, the causative organism of
gastroenteritis associated with the consumption of
raw or undercooked seafish, shellfish, and other con-
taminated foods, is one of the best described of patho-
genic vibrios. Although several synonyms have been
suggested for it, the name V. parahaemolyticus has
been formally accepted.
Characteristics
0002V. parahaemolyticus is a short, slightly curved or
straight, Gram-negative, facultatively anaerobic,
motile rod. Sometimes it reveals polymorphism with
coccoid cells, especially in older strains. In broth cul-
tures, cells each exhibit a single polar, sheathed flagel-
lum, but on the surface of solid media young cultures
may also show unsheathed peritrichous flagella.
0003V. parahaemolyticus is a halophilic organism. It
can grow on/in ordinary media containing 1–8%
NaCl, but the most abundant growth occurs in the
presence of 2–4% salt. It fails to grow on/in media
lacking salt. It grows between 10
C and 44
C, but
the optimal temperature for growth is between 30
C
and 37
C. It fails to grow at 4
C and lower tempera-
tures not only arrest multiplication of the organism
but also cause a rapid decrease in viable cell numbers.
The vibrio can grow at a pH range from 5.6 to 9.6 but
grows best at pH 7.6–8.6. Under optimal conditions,
the generation time of the vibrio in the exponential
phase of growth is estimated at 9–13 min.
0004Cultures of V. parahaemolyticus produce moist,
circular colonies, opaque in appearance, attaining
a size of 2–3 mm on ordinary agar media containing
2–3% salt after incubation at 37
C for 24 h. Some-
times mucoid and viscid colonies may be seen.
After several subcultures, however, dissociated
translucent or rugose colony variants may also
occur. Cultures of this vibrio, especially those isolated
5988 VIBRIOS/
Vibrio parahaemolyticus

from environmental sources, often show swarming
on the surface of nutrient agar. In broth containing
2–4% salt, most strains produce heavy homologous
turbidity, but rugose variants form a pellicle on the
surface of the broth.
0005 With few exceptions, V. parahaemolyticus pro-
duces both lysine and ornithine decarboxylases as
well as indole. Arginine dihydrolase is not produced.
It gives a negative reaction in the Voges–Proskauer
test, and a positive reaction in the oxidase test. It
ferments glucose, maltose, mannose, trehalose, and
mannitol without gas production but fails to attack
lactose, sucrose, xylose, and sorbitol. The majority of
strains ferment arabinose, but occasional strains fail
to do so. Urea is not usually decomposed, but some
strains are able to hydrolyze it. It has been suggested
that the ability to hydrolyze urea may be a marker
for those Kanagawa-negative strains which are
associated with illness but which nevertheless pro-
duce a thermolabile TDH-related hemolysin (TRH)
related to, though different from, the direct thermo-
stable direct hemolysin (TDH) of Kanagawa-positive
strains.
0006 The vibrio is susceptible to most antibiotics except
penicillin, ampicillin, and polymyxin B, but resistant
mutants do occur. It is also susceptible to the vibrio-
static agent O/129 (2,4-diamino-6,7-diisopropyl pter-
idine) at a concentration of 150 mgml
1
but resistant
at 10 mgml
1
.
0007 Strains of V. parahaemolyticus may be typed with a
combination of both somatic O and capsular K anti-
gens, based on a serological scheme in which 13 O
and 75 K antigens were established. The number of K
antigens has since been extended to 84. Most strains
of the vibrio are inagglutinable with O antisera in the
living state, but after heating suspensions for 30 min
at 100
C they become O-agglutinable. H antigens are
not included in the antigenic scheme, since in all
strains they are regarded as serologically identical,
although antigenically the polar flagellum and the
peritrichous flagella are different.
Incidence in the Aquatic Environment
0008 V. parahaemolyticus occurs in estuarine environ-
ments throughout the world. It also occurs occasion-
ally in fresh-water sites, especially in brackish water
during the summer, but only in small numbers. The
halophilic nature of the vibrios is related to their
ability to survive in the aquatic environment. They
survive in estuarine water for more than 1 week at
15–20
C, whereas they rapidly become extinct in
fresh water under the same conditions as those of
estuarine waters. The fate of the vibrios in fresh
water is also partly concerned with osmotic pressure
which causes autolysis of the cells. In brackish water,
however, they may survive as long as in estuarine
water in the summer. They are not found in the estu-
arine water in winter, although the vibrio can usually
be isolated from sediment even when the water tem-
perature is less than 10
C. Indeed, it is likely that it
survives in sediment in estuarine areas during the
winter. The vibrio produces chitinase and the action
of this enzyme enables it to adhere to the chitin of
marine life. It thus colonizes zoo-plankton and the
surface of shellfish. It therefore occurs in the digestive
tracts of shellfish as a result of their ingestion of zoo-
plankton. V. parahaemolyticus probably survives in a
viable but nonculturable state in a cold environment,
as reported for V. cholerae and V. vulnificus.
Reservoirs
0009Gastroenteritis due to V. parahaemolyticus is usually
associated with seafoods. Coastal fish and shellfish
are usually contaminated with V. parahaemolyticus in
the summer. On the surface of market marine fish, the
vibrios do not proliferate if kept below 10
C, but at
20
C their numbers increase rapidly. About 10–100
cells of V. parahaemolyticus have been found on the
surface of coastal fish just after landing. If the fish
were kept at atmospheric temperature, especially in
summer, the number of vibrios increases to more
than 10
6
cells within a few hours. It has not been
found on market fish in winter.
0010Seafoods responsible for illness vary with local
eating habits in different countries. In Japan, the
high incidence of infection is undoubtedly due to
the national custom of eating the meat of raw fish,
shellfish, and other fish products. Any kind of seafish
served as sushi and sashimi can act as the vehicle
for the transmission of gastroenteritis. Although
the vibrio may be killed within several minutes in
0.5% acetic acid, raw fish or shellfish with vinegar,
which are widely eaten in Japan, nevertheless often
transmit this infection. Also, infection is sometimes
associated with consumption of cured vegetables
cross-contaminated with the vibrio in the kitchen.
The incidence of V. parahaemolyticus infection may
be less in other countries where fish are not usually
consumed uncooked. Nevertheless, cases of gastroen-
teritis caused by this organism have been reported in
East European countries, the UK, Africa, and the
USA. In the USA, for example, 40 outbreaks of infec-
tion were reported to the Centers for Disease Control
and Prevention between 1973 and 1998. In countries
other than Japan, although fish are usually cooked
shortly before consumption, crab meat and shrimp,
which are the seafoods most often implicated with
this infection, are usually extracted either by hand or
VIBRIOS/
Vibrio parahaemolyticus
5989

mechanically after cooking, becoming contaminated
from other sources. Oysters and clams which are
consumed raw are also significantly associated with
gastroenteritis caused by this vibrio. It is possible that
V. parahaemolyticus may cause diarrheal diseases in
developing countries more often than is currently
recognized. In such countries, waterborne infection
with this vibrio should also be considered.
Clinical Infection with
V. parahaemolyticus
0011 Although V. parahaemolyticus is associated with oc-
casional skin and soft-tissue infections as well as with
otitis media in fish handlers and bathers, gastroenter-
itis is the essential clinical manifestation of foodborne
infection. Symptoms usually occur about 12 h after
consumption of contaminated food. The outstanding
features are severe abdominal cramps and diarrhea,
together with nausea, vomiting, and fever. These
symptoms range from mild with a few loose stools
to very severe. In addition, dysentery-like stools with
blood and mucus may occur in some cases. Recovery
of patients and disappearance of the vibrio from
stools are usually complete within a few days. The
mortality rate is very low. Person-to-person transmis-
sion has not been recognized.
0012 Although the size of the infectious dose necessary
to produce clinical symptoms may vary with the strain
and individual, according to the data from human
volunteer experiments it, probably lies between
about 10
5
and 10
8
viable cells. However, based on
the low numbers of Kanagawa-positive cells usually
found in samples of the food implicated or in water-
borne infections encountered in developing countries,
it appears that the numbers of viable vibrios needed
to cause illness may be much smaller.
Virulence Factors
0013 It has been found that strains of V. parahaemolyticus
isolated from patients with gastroenteritis were
hemolytic, on a modified brain–heart infusion agar
containing human blood, whereas those from sea fish
and the marine environment were nonhemolytic. This
hemolysis is referred to as the Kanagawa reaction. It
was found that 96% of 2720 strains from patients
with gastroenteritis were Kanagawa-positive com-
pared with only 1% of 650 environmental strains.
Feeding tests with Kanagawa-negative strains in 15
adult volunteers failed to induce any clinical signs of
illness. The hemolysin responsible for the Kanagawa
reaction is known as TDH. Although V. parahaemo-
lyrticus is known to produce at least four hemolytic
substances – TDH, a thermolabile hemolysin, phos-
pholipase A, and lysophospholipase – it is probable
that only TDH plays a significant role in the patho-
genesis of gastroenteritis.
0014Occasional outbreaks of gastroenteritis may be
associated with Kanagawa-negative vibrios. These
strains produce a thermolabile TRH but not the
thermostable TDH.
0015TDH is a 21-kDa protein not affected by heating at
100
C for 15 min at pH 6.0. It is a pore-forming
toxin which expresses hemolytic activity, cytotoxi-
city, and cardiotoxicity. In hemolytic tests, TDH is
strongly active against erythrocytes of dogs, rats,
mice, and humans, weakly active against those of
rabbits and sheep, and inactive against horse erythro-
cytes. Although it is clear that TDH is associated with
the pathogenesis of infection, it is not clear how this
toxin causes diarrhea. TDH, when inoculated at
doses of 100 mg into ligated ileal loops in rabbits,
failed to produce fluid accumulation but caused ero-
sive lesions and necrosis of the intestinal mucosa.
Such histological changes do not occur when whole
bacteria are tested in ileal loops.
0016It has been reported that a TDH-positive parent
vibrio strain caused fluid accumulation in ligated
ileal loops in rabbits, whereas a TDH-negative
mutant did not cause fluid accumulation. Similar
results were obtained with culture supernatants of
TDH-positive strains tested on rabbit ileal tissues
mounted in an Ussing chamber, which represents a
more sensitive measure of secretory activity. In these
assays, the ability of TDH to alter iron transport in
the intestinal tract was demonstrated at nanogram
levels, with no histological changes. TDH induces
intestinal chloride ion secretion and trisialoganglio-
side G
T1b
appears to be the cellular receptor. How-
ever, recent work suggests that other unknown
receptor(s) may be present. TDH uses Ca
2þ
as an
intracellular second messenger, and is thus the first
bacterial enterotoxin for which a linkage between
changes in intracellular calcium and secretory activity
has been established.
0017TDH is encoded by two copies, tdh1 and tdh2,of
the tdh gene in V. parahaemolyticus. The two gene
copies are not identical and the predicted protein
products differ in seven amino acid residues, although
the proteins themselves are immunologically indistin-
guishable. The level of TDH production may be
under the control of a regulator similar to that of
ToxR in V. cholerae.
0018Although TRH and TDH are similar in their
biological, immunological, and physicochemical prop-
erties, TRH is thermolabile and differs from TDH in its
activity on erythrocytes. TRH is encoded by the trh
gene which shares 69% identitiy with the tdh2 gene.
TRH is linked epidemiologically to gastroenteritis, but
the secretory mechanism of this toxin is still uncertain.
5990 VIBRIOS/
Vibrio parahaemolyticus

0019 Several adhesive factors, including fimbriae, peri-
trichous flagella, outer membrane proteins, and a
mannose-resistant, cell-associated hemagglutinin,
have also been proposed, but no substantial studies
have yet implicated any of the candidate adhesins in
the pathogenicity of the organism.
Isolation and Identification
0020 Several selective agar media have been devised, but
thiosulfate citrate bile salts sucrose (TCBS) agar is
recommended for the isolation of V. parahaemolyti-
cus. In clinical microbiology laboratories, MacCon-
key agar containing 0.5% additional NaCl is also
convenient for routine culture of diarrheal stools.
0021 Enrichment culture is used for the detection of the
vibrio from food and marine samples. Polymyxin salt
broth, containing 2% NaCl and 50 mgml
1
of poly-
myxin B (pH 7.6) may be used for the selective
growth of V. parahaemolyticus. It should be noted
that some factor(s) in shellfish may inhibit the growth
of vibrios. It is therefore recommended that shellfish
are cut into small pieces, but not homogenized. After
shaking the enrichment broth vigorously, the pieces of
shellfish are removed with forceps. However, enrich-
ment culture of seafoods incriminated in outbreaks of
infection may be unrewarding, because most yield
Kanagawa-negative isolates, in contrast to those
from patients.
0022 The colonial appearance of V. parahaemolyticus on
TCBS agar is so typical that provisional identification
of the isolates from stool specimens may be made
directly from the plates. However, isolates from
marine sources must be further examined in order to
differentiate them from related organisms. The add-
ition of 1% NaCl to medium for biochemical tests is
essential to obtain valid reactions.
0023 The Kanagawa reaction is a reliable test for the
recognition of virulent strains. For determination,
isolates should if possible be tested using Wagatsuma
agar, which contains 0.5% yeast extract, 1% pep-
tone, 0.5% mannitol, 0.05% K
2
HPO
4
, 7% NaCl,
0.0001% crystal violet, 1.5% agar, and washed
human red blood cells. However, as adequate sources
of human blood, or of blood from other suitable ani-
mals, are not always readily available, other suitable
media may be considered. Thus, an enzyme-linked
immunosorbent assay has been described for the
detection of TDH-producing vibrios.
0024 Several molecular approaches for the detection
of Kanagawa-positive vibrios have also been de-
veloped. DNA and oligonucleotide probes specific
for the genes tdh and trh have been described. How-
ever, the probes also hybridize with tdh genes in some
strains of non-O1 V. cholerae, V. hollisae, and
V. mimicus. A polymerase chain reaction technique
has been reported using oligonucleotide primers
derived from the nucleotide sequence of the tdh gene.
0025Serotyping of isolates of V. parahaemolyticus may
be performed by slide agglutination tests using O and
K antisera. In outbreaks of V. parahaemolyticus infec-
tion, however, the same serovar as that identified in
patients is seldom detected in incriminated seafoods.
Serotyping of isolates from seafoods and marine
sources is thus not usually significant unless they are
Kanagawa-positive.
See also: Fish: Spoilage of Seafood; Shellfish:
Contamination and Spoilage of Molluscs and
Crustaceans; Vibrios: Vibrio cholerae
Further Reading
Asakawa Y, Akahane S and Noguchi M (1974) Quantita-
tive studies on pollution with Vibrio parahaemolyticus
during distribution of fish. In: Fujino T et al. (eds) Inter-
national Symposium on Vibrio parahaemolyticus pp.
97–103. Tokyo: Saikon Publishing Co.
Daniels NA, MacKinnon L, Bishop R et al. (2000) Vibrio
parahaemolyticus infections in the United tates, 1973–
1998. J Infect Dis 181: 1661–1666.
Honda T, Ni Y, Yoh M et al. (1989) Production of mono-
clonal antibodies against thermostable direct hemolysin
of Vibrio parahaemolyticus and application of the anti-
bodies for enzyme-linked immunosorbent assay. Med
Microbiol Immunol 178: 245–253.
Kato T, Obara Y, Ichinoe H et al. (1965) Grouping of Vibrio
parahaemolyticus (biotype 1) by hemolytic reaction.
Shokuhin Eisei Kenkyu 15: 83–86 (in Japanese).
Lee C and Pan S-F (1993) Rapid and specific detection of
the thermostable direct hemolysin gene in Vibrio para-
haemolyticus by polymerase chain reaction. J Gen
Microbiol 139: 3225–3231.
Nishibuchi M, Ishibashi M, Takeda Y et al. (1985) Detec-
tion of the thermostable direct hemolysin gene and
related DNA sequences in DNA colony hybrid-ization
test. Infect. Immun 49: 481–486.
Nishibuchi M, Hill WE, Zon G et al. (1986) Synthetic
oligodeoxyribonucleotide probes to detect Kanagawa
phenomenon-positive Vibrio parahaemo-lyticus. J Clin
Microbiol 23: 1091–1093.
Nishibuchi M, Taniguchi T, Misawa T et al. (1989) Cloning
and nucleotide sequence of the gene (trh) encoding the
hemolysin related to the thermostable direct hemolysin
of Vibrio parahaemolyticus. Infect. Immun. 57: 2691–
2697.
Osawa R, Okitsu T, Morozumi H et al. (1996) Occurrence of
urease-positive Vibrio parahaemo-lyticus in Kanagawa,
Japan, with special reference to presence of thermostable
direct hemolysin (TDH) and the TDH-related hemolysin
genes. Appl Environ Microbiol 62: 725–727.
Sakazaki R, Iwanami S and Fukumi H (1963) Studies on
the enteropathogenic, facultatively halophilic bacteria,
VIBRIOS/
Vibrio parahaemolyticus
5991

Vibrio parahaemolyticus I. Morphological, cultural and
biochemical properties and its taxonomical position. Jpn
J Med Sci Biol 16: 161–188.
Sakazaki R, Iwanami S and Tamura K (1968a) Studies on
the enteropathogenic, facultatively halophilic bacteria,
Vibrio parahaemolyticus. II. Serological characteristics.
Jpn J Med Sci Biol 21: 313–324.
Shinoda S, Miwatani T, Honda T et al. (1974) Antigenicity
of flagella of Vibrio parahaemolyticus. In: Fujino T et al.
(ed.) International Symposium on Vibrio parahaemo-
lyticus pp. 193–197. Tokyo: Saikon Publishing Co.,
Tokeo.
Sanyal SC and Sen PC (1974) Human volunteer study on
the pathogenicity of Vibrio parahaemolyticus In: Fujino
T et al. (eds) International Symposium of Vibrio para-
haemo-lyticus pp. 227–235. Tokyo: Saikon Publishing
Co.
Takikawa I (1958) Studies on pathogenic halophilic bac-
teria. Yokohama Med Bull 2: 313–322.
Yamamoto K, Honda T and Miwatani T (1992) Enzyme-
labeled oligonucleotide probes for detection of the genes
for thermostable direct hemolysin (TDH) and TDH-
related hemolysin (TRH) of Vibrio parahaemolyticus.
Canad J Microbiol 38: 410–416.
Vibrio vulnificus
A C Wright, University of Florida, Gainesville, FL, USA
J G Morris, Jr., University of Maryland School of
Medicine, Baltimore, MD, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Vibrio vulnificus, first recognized as a distinct species
in the late 1970s, is a Gram-negative bacterium that is
indigenous to the estuarine environment. Virtually all
oysters harvested in the USA during warmer, summer
months harbor this organism, and it has been
reported in association with shellfish and fish in a
number of locations in South America, Asia, and
Europe. For persons in ‘high risk’ groups (i.e., persons
with liver disease, iron overload states, or immuno-
suppression), ingestion of V. vulnificus in raw or
undercooked seafood can result in a syndrome of
‘primary septicemia,’ characterized by intractable
shock and a mortality rate of greater than 50%.
These infections are the leading cause of death asso-
ciated with consumption of shellfish in the USA. The
microorganism can also cause serious wound infec-
tions and may be a cause of gastroenteritis. In popu-
lation-based studies in US coastal areas, the incidence
of V. vulnificus infections is approximately 0.5/
100 000 persons per year. Case series from Korea
and Taiwan suggest that V. vulnificus infections are
even more common in these latter areas.
0002Virulence of V. vulnificus is most closely linked
with the presence of a polysaccharide capsule and is
greatly influenced by availability of iron in the host
during infection. V. vulnificus is also known to pro-
duce a variety of extracellular factors, including a
cytolysin, siderophores, pili, lipopolysaccharide, pro-
teases, and other exoenzymes; however, the role of
these factors in virulence remains unclear. Animal
studies of the lethality of this organism have indicated
that mutants deficient in cytolysin and protease do
not exhibit reduced virulence. Mutants deficient in
siderophores or pili expression are somewhat less
virulent than wild-type strains, but these results are
complicated by the pleiotropic nature of these muta-
tions. However, excess iron in the host and expression
of capsular polysaccharide (CPS) by V. vulnificus
clearly increase the lethality of this disease. For mice
with normal iron status, the LD50 ranges from 10
5
to
10
7
, depending on the strain and the ability to pro-
duce CPS. The LD50 for unencapsulated mutants in
iron-overloaded mice is also about 10
5
; however, en-
capsulated strains in iron-overloaded mice may be
lethal at a dose of one bacterium.
0003There is tremendous diversity in capsular types
among strains, and no one type (or group of types)
predominates among clinical or environmental isol-
ates. Three biotypes (or two biotypes and one distinct
serovar) of V. vulnificus are currently recognized. The
majority of clinical and environmental V. vulnificus
isolates reported to date are in biotype 1. Strains
initially classified as biotype 2 are responsible for
sepsis in eels; they do not cause human disease.
More recently, it has been recognized that the biotyp-
ing characteristics described for these strains lacked
specificity; however, the eel pathogens are homoge-
neous in their lipopolysaccharide-based serogroup,
leading to their reclassification as serovar E. Biotype
3 strains have been described in association with
wound infections related to handling of live fish
(tilapia) from fish farms in Israel.
Occurrence in the Environment
0004As noted above, V. vulnificus is commonly isolated
from water and organisms, particularly shellfish, col-
lected from estuarine environments. The highest
numbers are found in areas with intermediate sali-
nities (5–25 p.p.t.) and warmer temperatures (opti-
mally, > 20
C). Isolation has been reported from
the US Atlantic, Gulf, and Pacific coasts, as well
as the Atlantic coast of Europe, the Mediterranean,
the Indian Ocean, Malaysia, and the Pacific Coast
of Asia. It is likely that the organism is present in
5992 VIBRIOS/
Vibrio vulnificus
virtually all estuarine areas with appropriate salinity
and temperature ranges. Using a DNA probe, V. vul-
nificus has been identified in 80% of Chesapeake Bay
water samples collected during months in which
water temperatures exceeded 8
C: concentrations
ranged from 3.0 10
1
to 2.1 10
2
CFU
1
ml
1
, rep-
resenting approximately 8% of the total culturable
heterotrophic bacteria. However, none of the samples
collected during February and March, when tem-
peratures were less than 8
C, were positive for this
organism.
0005 V. vulnificus is generally not recovered from the
environment during colder months, and several lines
of evidence suggest that it may enter a viable but
nonculturable state, where recovery on typical solid
media is not possible. Other studies have suggested
that the majority of V. vulnificus cells die off with the
stress of cold temperature or starvation, and only a
small resistant subpopulation remains viable. How-
ever, both experimental and epidemiological data
strongly indicate that nonrecoverable forms probably
are not virulent, as little or no disease is observed in
colder months. Thus, the relevance of this issue to the
seafood industry may be negligible. However, further
study may shed light on how the species is maintained
in the environment and help elucidate survival mech-
anisms that could be targets for control measures.
Entry to the Food Chain
0006 Epidemiologically, there is a very strong association
between V. vulnificus infection and eating raw
oysters, particularly those harvested from warmer
waters. It should be noted that most, if not all, serious
disease is associated with the raw, freshly shucked
product; proper cooking eliminates the disease risk.
In Chesapeake Bay studies, V. vulnificus was found in
virtually all oysters collected during warmer months
(water temperature 7.6
C or above), with the
numbers of this species approximately two orders of
magnitude above those found in the surrounding
water. In studies conducted by the US Food and
Drug Administration, V. vulnificus was detected in
virtually all oysters from the US Gulf Coast. The
numbers varied based on temperature and salinity;
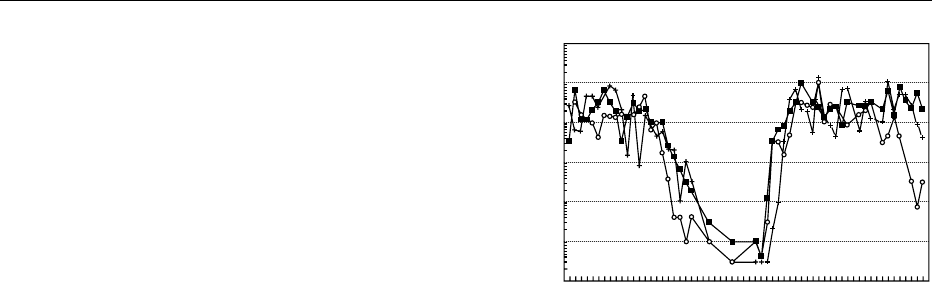
representative data from this study are shown in
Figure 1. The prevalence of this organism in the en-
vironment correlates well with disease incidence, as
approximately 90% of the infections occur between
April and October.
0007 Unlike other pathogens associated with seafood
consumption, several studies have shown no correl-
ation between the presence of V. vulnificus and
fecal coliforms. V. vulnificus is clearly an indigenous
estuarine species with an environmental reservoir;
however, many aspects of this reservoir are still un-
clear. For example, does this organism exist and rep-
licate as a free-living species or in association with
algae, shellfish, or fish? While it may be assumed that
concentrations of V. vulnificus in shellfish, in part,
reflect filtering of the organism from surrounding
water, there are data suggesting that V. vulnificus
can assume a symbiotic relationship with oysters. At
a practical level, this relationship implies specific at-
tachment or internalization, which may account for
the difficulties in clearing the organism from oysters
by using traditional depuration techniques.
0008The condition and overall health of the oyster may
also influence the numbers of V. vulnificus in oyster
tissue. Numerous variables such as heavy metal con-
centrations, water turbidity, availability and types of
food sources, spawning status, etc. may influence the
ability of oysters to clear or control endogenous bac-
terial populations. Bacterial growth also may be
enhanced by coinfection with parasites such as Per-
kinsus marinus, the presence of which may depress
innate defense mechanisms.
Fate on Processing/Storage and Control
Measures
0009V. vulnificus can persist in oyster shellstock for
extended periods of time. In oysters held at 18
C
for 30 h, or at ambient temperatures for 12 h, there
are significant increases in V. vulnificus counts,
highlighting the importance of refrigeration after
harvest and during storage and transport. Currently
in the USA, Hazard Analysis Critical Control Point
0.1
1994 1995Months
..A....S...O...N....D...J....F...M...A...M....J...J...A...S....
1
10
100
V. vulnificus (MPN per g)
1000
10 000
100 000
fig0001Figure 1 Weekly densities of V. vulnificus in oysters from Gulf
Coast sites in Alabama (+), Florida (), and Louisiana (
&
) during
a 14-month period. Each point represents the geometric mean
(n ¼2) of the MPN of bacteria per gram of oyster meat. From
Motes ML, DePaola A, Cook DW et al. (1998) Influence of water
temperature and salinity on Vibrio vulnificus in northern Gulf and
Atlantic Coast oysters. Applied and Environmental Microbiology 64:
1459–1465, with permission.
VIBRIOS/
Vibrio vulnificus
5993

recommendations are being implemented that pro-
vide more rapid postharvest refrigeration of shellfish
to limit increases in the numbers of V. vulnificus
resulting from temperature abuse.
0010 A variety of approaches have been proposed to
reduce or eliminate V. vulnificus from oyster shell-
stock. Traditionally, depuration or the incubation of
oysters in recirculating, sanitized fresh water has been
used to reduce bacterial contamination of fecal origin
in shellstock; however, several studies found this treat-
ment to be ineffective for V. vulnificus control. In one
study, V. vulnificus was cleared from the digestive
organs but persisted in other tissues following depur-
ation treatment. Recently, alternative treatments such
as freezing, cold or hot water immersion, relaying to
high salinity waters, high pressure, and irradiation
have shown promising results for the reduction and
possible elimination of this species from oysters.
Unfortunately, many of these treatments are lethal to
oysters as well, and their application to the ‘oyster on
the half shell’ market may be limited. Some suppliers
are currently using these methods to produce a prod-
uct that greatly resembles a freshly shucked oyster but
will not be sold as live product.
Detection in Food, Water, and
Physiological Samples
0011 V. vulnificus has traditionally been evaluated in food
and water samples using most probable number
(MPN) calculations and standard microbiologic tech-
niques. In this procedure, oysters are homogenized in
buffer and serially diluted to MPN enrichment tubes,
which are subsequently screened for vibrios by
growth on selective/differential media. Unfortunately,
owing to the diversity and abundance of vibrios in the
environment, this method lacks reliability, and fur-
ther confirmatory methods such as species-specific
monoclonal antibody are generally required for
identification.
0012 More recently, there has been increasing use of
molecular approaches, including DNA probes and
PCR techniques. Several of the probes and PCR
primers are based upon the DNA sequence for the
V. vulnificus cytolysin gene, vvh. This gene appears to
be common to most, if not all, strains and is highly
species-specific. Applications include a colony hy-
bridization protocol that can be combined with total
viable counts on nonselective medium to provide
more direct enumeration. Multiplex PCR analyses
have also been described that simultaneously detect
V. vulnificus and other Vibrio spp. Unfortunately,
these probes, as well as the standard MPN methods
described, do not discriminate between potentially
virulent and avirulent strains.
0013For patient samples, V. vulnificus will grow with-
out difficulty in standard blood culture media or on
nonselective media (such as blood agar) routinely
used for wound cultures; identification and speciation
of the organism are possible through any of the stand-
ard, commercially available microbiology identifica-
tion systems. As with all Vibrio species, isolation of
the organism from stool generally requires the use of
a specific selective culture media (thiosulfate citrate
bile-salts sucrose).
Symptoms and Characteristics
0014V. vulnificus has been linked with three different clin-
ical syndromes: (1) ‘primary septicemia’ (presence of
the organism in the blood, without an obvious source
such as a wound infection), (2) wound infections,
and, possibly, (3) gastroenteritis. Serious V. vulnificus
infections occur almost exclusively in persons who
have underlying liver disease (including alcoholic
liver disease), are immunosuppressed, or have chronic
diseases such as diabetes or renal failure. Risk of
infection is also substantively increased in persons
with increased iron stores, as found in patients with
hemochromatosis.
Primary Septicemia
0015Approximately one-third of patients with primary
V. vulnificus septicemia present in shock or become
hypotensive within 12 h of hospital admission. Three-
fourths of patients have distinctive bullous skin
lesions. Thrombocytopenia is common, and there is
often evidence of disseminated intravascular coagula-
tion. Complications such as gastrointestinal bleeding
are not infrequent. The mortality rate for persons
with primary V. vulnificus septicemia is more than
50% overall and more than 90% in persons who are
hypotensive. Although the actual infection may be
cleared rapidly, patients often require prolonged
hospitalization in intensive care units because of the
associated shock syndrome and resultant multiorgan
system failure.
Wound Infections
0016V. vulnificus may contaminate wounds exposed to
estuarine waters or shellfish. In persons in the risk
groups noted above, the infection may spread rapidly,
producing severe myositis and fasciitis reminiscent of
gas gangrene and requiring amputation in extreme
cases.
Gastroenteritis
0017V. vulnificus has also been associated with gastro-
enteritis. However, an etiologic role is difficult to
5994 VIBRIOS/
Vibrio vulnificus

establish, as isolation from stool samples is of uncer-
tain significance, given the ubiquitous presence of the
organism in both water and shellfish.
Treatment and Prevention
0018 In V. vulnificus septicemia, the sooner antibiotic ther-
apy is initiated, the greater the chance that the patient
will survive. Based upon clinical observations and
in vitro susceptibilities, tetracycline and quinolone
antibiotics have been recommended for management
of V. vulnificus infections. Recent in vitro and animal
studies from Taiwan indicate that there is synergism
between minocycline and cefotaxime in treatment of
serious V. vulnificus infections, leading to the recom-
mendation that these latter two drugs be used as ‘first
line’ therapy. Patients with serious infections need to
be aggressively managed in an intensive care unit
setting to minimize the possible consequences of
hypotension, septic shock, and multiorgan system
failure.
0019 Given the high mortality associated with V. vulni-
ficus infections, persons in the high-risk groups noted
above should avoid eating raw or undercooked shell-
fish, particularly oysters. By law, restaurants in many
states are now required to post warnings about this
risk. Persons at high risk for infection should also
avoid situations in which estuarine-associated
wounds are likely to occur.
See also: Fish: Spoilage of Seafood; Shellfish:
Contamination and Spoilage of Molluscs and Crustaceans
Further Reading
Bisharat N, Agmon V, Finkelstein R et al. (1999) Clinical,
epidemiological, and microbiological features of Vibrio
vulnificus biogroup 3 causing outbreaks of wound infec-
tion and bacteremia in Israel. Lancet 354: 1421–1424.
Blake PA, Merson MH, Weaver RE, Hollis DG and
Heublein PC (1979) Disease caused by a marine Vibrio:
clinical characteristics and epidemiology. New England
Journal of Medicine 300: 1–5.
Brasher CS, Depaola A, Jones DD and Bej AK (1998)
Detection of microbial pathogens in shellfish with multi-
plex PCR. Current Microbiology 37: 101–107.
Bush CA, Patel P, Gunawardena S et al. (1997) Classifica-
tion of Vibrio vulnificus strains by the carbohydrate
composition of their capsular polysaccharides. Analyt-
ical Biochemistry 250: 186–195.
Chuang YC, Ko WC, Wang ST et al. (1998) Minocycline
and cefotaxime in the treatment of experimental murine
Vibrio vulnificus infection. Antimicrobial Agents and
Chemotherapy 42: 1319–1322.
Cook DW (1997) Refrigeration of oyster shellstock: condi-
tions which minimize the outgrowth of Vibrio vulnifi-
cus. Journal of Food Protection 60: 349–352.
Dixon DW and Rodrick GE (1998) Effect of gamma radi-
ation on shellstock oysters: extension of shelf-life and
reduction in bacterial numbers, with particular reference
to Vibrio vulnificus. In: Combination Processes for Food
Irradiation, pp. 97–110. Vienna: IAEA.
Elliot EL, Kaysner CA, Jackson L and Tamplin ML (1998)
Vibrio cholerae, V. parahaemolyticus, V. vulnificus and
other Vibrio spp. In: U.S. Food and Drug Administra-
tion Bacteriological Analytical Manual, 8th edn.
Gaithersburg, MD: AOAC International.
ISSC (2000) Gulf Oyster Industry Council, FL. Dept of
Agriculture, and FDA Office of Seafood Processing con-
trols for vibrios in raw oysters. Online abstracts of the
25th Annual Meeting of Seafood Science and Technol-
ogy Society, Longboat Key, FL http://www.seafood.uc-
davis.edu/organize/SSTSA.htm
Motes ML and DePaola A (1996) Offshore suspension
relaying to reduce levels of Vibrio vulnificus in oysters
(Crassostrea virginica). Applied and Environmental
Microbiology 62: 3875–3877.
Motes ML, DePaola A, Cook DW et al. (1998) Influence of
water temperature and salinity on Vibrio vulnificus in
northern Gulf and Atlantic Coast oysters. Applied and
Environmental Microbiology 64: 1459–1465.
Park SD, Shon HS and Joh NJ (1991) Vibrio vulnificus
septicemia in Korea: Clincal and epidemiologic findings
in seventy patients. Journal of the American Academy of
Dermatology 24: 397–403.
Strom MS and Paranjpye RN (2000) Epidemiology and
pathogenesis of Vibrio vulnificus. Microbes and Infec-
tion 2: 177–188.
Tamplin ML and Capers GM (1991) Persistence of Vibrio
vulnificus in tissues of Gulf coast oysters (Crassostrea
virginica) exposed to seawater disinfected with UV
light. Applied and Environmental Microbiology 58:
1506–1510.
Wright AC, Hill RT, Johnson JA, Roghman M-C, Colwell
RR and Morris JG Jr. (1996) Distribution of Vibrio
vulnificus in the Chesapeake Bay. Applied and Environ-
mental Microbiology 62: 717–724.
VIBRIOS/
Vibrio vulnificus
5995

VINEGAR
M Plessi, Dipartimento di Scienze Farmaceutiche,
Universita
`
degli Studi di Modena, Modena, Italy
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Summary and Background
0001 Vinegar is traditionally the product of the acetous
fermentation of dilute alcoholic solutions. At the pre-
sent time it is produced microbiologically from nat-
ural alcoholic solutions or by dilution of acetic acid.
Vinegar and its characterization are presented in this
article.
0002 Vinegar has been known since ancient times, for
more than 10 000 years, and has been used for its
typically acid flavor. It uses were many and various
for centuries, not only for food but also for medicinal
and ritual uses. It is traditionally the product of the
acetic fermentation of dilute alcoholic liquors and
it was wine that was the first alcoholic liquid used,
thus the derivation of its name (from the French vin
aigre ¼ sour wine).
History
0003 During Babylonian times vinegar was used as a con-
diment and for food preservation. Hippocrates recog-
nized its medicinal properties and it is also mentioned
in the Bible as a remedy on account of its sedative
and curative properties. Pliny reported that vinegar,
diluted with water (known as posca), was given to
the Roman legionnaires during their long marches.
0004 The medicinal use of vinegar was widespread
during the Middle Ages and the Renaissance, for
both internal and topical use. It was in fact used as a
digestive, a prophylactic against liver disorders, an
anthelmintic, for sore throats, and to rub on the
wrists against fever, but also against hair loss and
tinea.
0005 Vinegar production as an industry began during the
period of the Communes; those who swore not to
reveal the secret of its fabrication were admitted to
the corporation of manufacturers. Although widely
used, little was known about the real nature of vin-
egar and its fermentation until the eighteenth century.
In 1723, Stahl obtained acetic acid from vinegar, but
nothing was known of the causes which determined
the formation of acetic acid. The first breakthrough
came with Berzelius, who, at the beginning of the
nineteenth century, explained how acetic acid was
formed from alcohol by a process of oxidation.
Persoon (1822) and Ku
`
tzing (1837) were the first
to suggest the presence of microorganisms in the
process. Finally, in 1864, Pasteur brilliantly demon-
strated the biological origin of the acetic fermenta-
tion, indicating Mycoderma aceti as the fermentation
agent. Later studies clarified the nature of the micro-
organisms, which turned out to belong to several
species.
0006Vinegar is now used above all to give foodstuffs a
pleasant acidic flavor; it is also used as a preserver of
foodstuffs and is recognized to have certain pharma-
cological qualities.
Production
0007At the present time, vinegar is still produced micro-
biologically from a mostly natural alcoholic solution,
but can also be produced by dilution of acetic acid.
Microbiological Production
0008Vinegar is the product of the acetic fermentation
of slightly alcoholic liquids (less than 10–12% by
volume of ethyl alcohol); transformation of alcoholic
liquids into vinegar is not really fermentation, but
oxidation.
0009Raw materials may be wine, cider, beer, and other
liquors deriving from the alcoholic fermentation of
cereals, fruit and potatoes, sugar solutions, such as
molasses, honey, and whey, and also diluted pure
ethanol with the addition of nutrients. These are the
so-called brewed vinegars, derived from the oxidation
activity of aerobic microorganisms. The microorgan-
isms belong to the genus Acetobacter (initially called
Mycoderma), and the most widespread species are A.
aceti, A. pasteurianus,andA. hansenii. These are the
ones that oxidize ethyl alcohol into acetic acid. The
oxygen used for the bioxidation is that of air.
0010These microorganisms, in the shape of aspori-
genous rods or cocci, form membranes of different
consistencies (vinegar mother). The optimum tem-
perature for their multiplication is between 18 and
34
C, depending on the species.
0011Ethanol is dehydrogenated to acetic acid and the
reduced cosubstrates are oxidized via the respiratory
chain (Figure 1). Similarly, but less abundantly and in
an anaerobic atmosphere, acetic acid is formed by
dismutation of 2 molecules of acetaldehyde (derived
in their turn from alcohol by oxidation). In theory, 1 g
of alcohol yields 1.3 g of acetic acid; in practice,
however, the yield is 15–20%, lower mainly because
alcohol, acetaldehyde, and acetic acid tend to volatil-
ize. The theoretical amount of air required for 1 l of
vinegar containing 6% of acetic acid is about 120 l,
5996 VINEGAR
whereas, in practice, given the slow rate of liquid–gas
exchange, the amount required is much greater.
0012 Acetous fermentation is accompanied by secondary
fermentation which combines to produce the flavor
and typical aroma; small quantities of volatile sub-
stances are formed (e.g., ethane, acetaldehyde, ethyl
formate, ethyl acetate, isopentyl acetate, butanol,
methylbutanol, 3-hydroxi-2 butanone or acetyl-
methylcarbinol), which vary from vinegar to vinegar
depending on the starting material and which,
because of their individual characteristics, produce
vinegars with a variety of odor, taste, color, and
other properties. The fermentation is usually stopped
at a minimum residual ethanol level to avoid
overoxidation, as oxidation of acetic acid to
water and CO
2
.(See Fermented Foods: Origins and
Applications.)
0013 Methods of manufacture The manufacture of
vinegar is an ancient craft, known and practiced all
over the world from time immemorial. A certain
amount of vinegar is still manufactured following
the centuries-old empirical methods of the small
producer, but since the last century a flourishing
vinegar-manufacturing industry has developed.
0014Industrial vinegar-manufacturing processes fall
into three main categories. The slow processes,
which are the oldest commercial procedure and re-
semble home-brewing techniques, are no longer used.
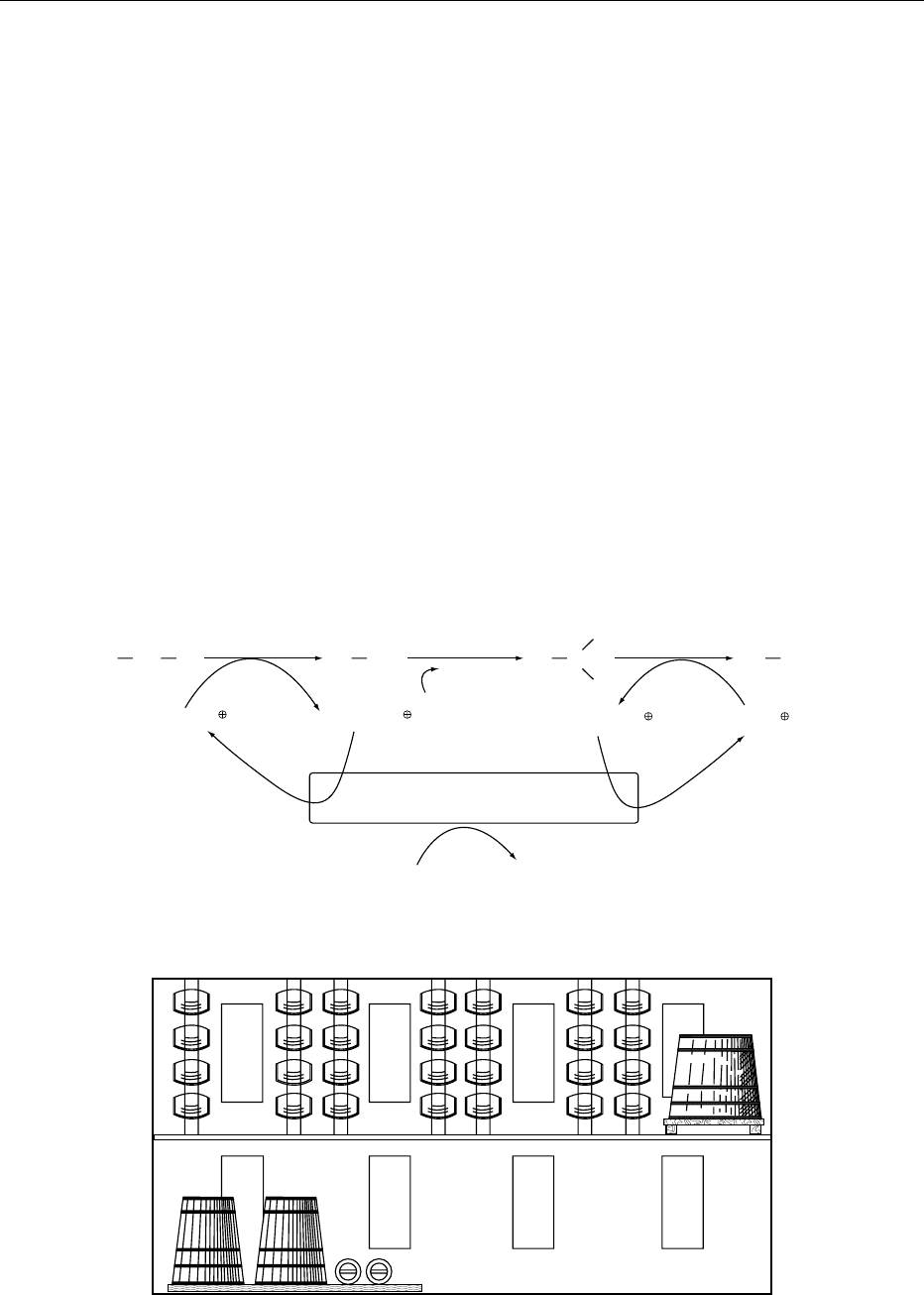
The Orleans process is still in use for the production
of high-quality vinegar; the procedure has remained
unchanged through the years but is very slow and
requires a great deal of space (Figure 2). The starting
liquor is placed in a large cask, containing wood
shavings or grape stalks, where the acetification pro-
cess gets underway. After about 8 days, the liquid is
withdrawn and transferred into barrels, which are left
only one-half or two-thirds full and where the liquid
remains until acidity reaches its peak (about 3
months). From now on, two-thirds or three-quarters
of the vinegar is withdrawn from the bottom of the
barrel every week and an equal volume of liquid from
the generator cask is added from the top. (See Barrels:
Wines, Spirits, and Other Beverages.)
0015The processes of the second and third categories
aim at a closer contact, with maximum possible
CH
3
CH
2
CH
3
CH
3
CHOOH
Alcohol-DH
NAD(P) NAD(P)H + H
H
2
O
CH
OH
OH
Aldehyde-DH
CH
3
COOH
NAD(P)NAD(P) + H
Respiratory chain
O
2
2 H
2
O + 6 ATP
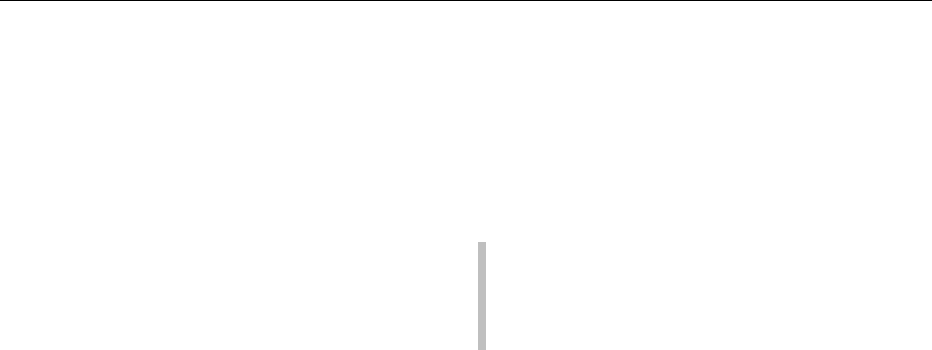
fig0001 Figure 1 Ethanol oxidation to acetic acid by Acetobacter spp.
B
C
A
fig0002 Figure 2 Vinegar making Orleans method: A, starting vat; B, acetifier casks; C, vats of clarification.
VINEGAR 5997
