Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

surface exposure, between the alcoholic liquid under-
going acetification and the ambient air. The passage
of oxygen from the gaseous to the liquid phase is
thereby accelerated and intensified and the acetifica-
tion time, which with traditional methods is rather
long, is reduced.
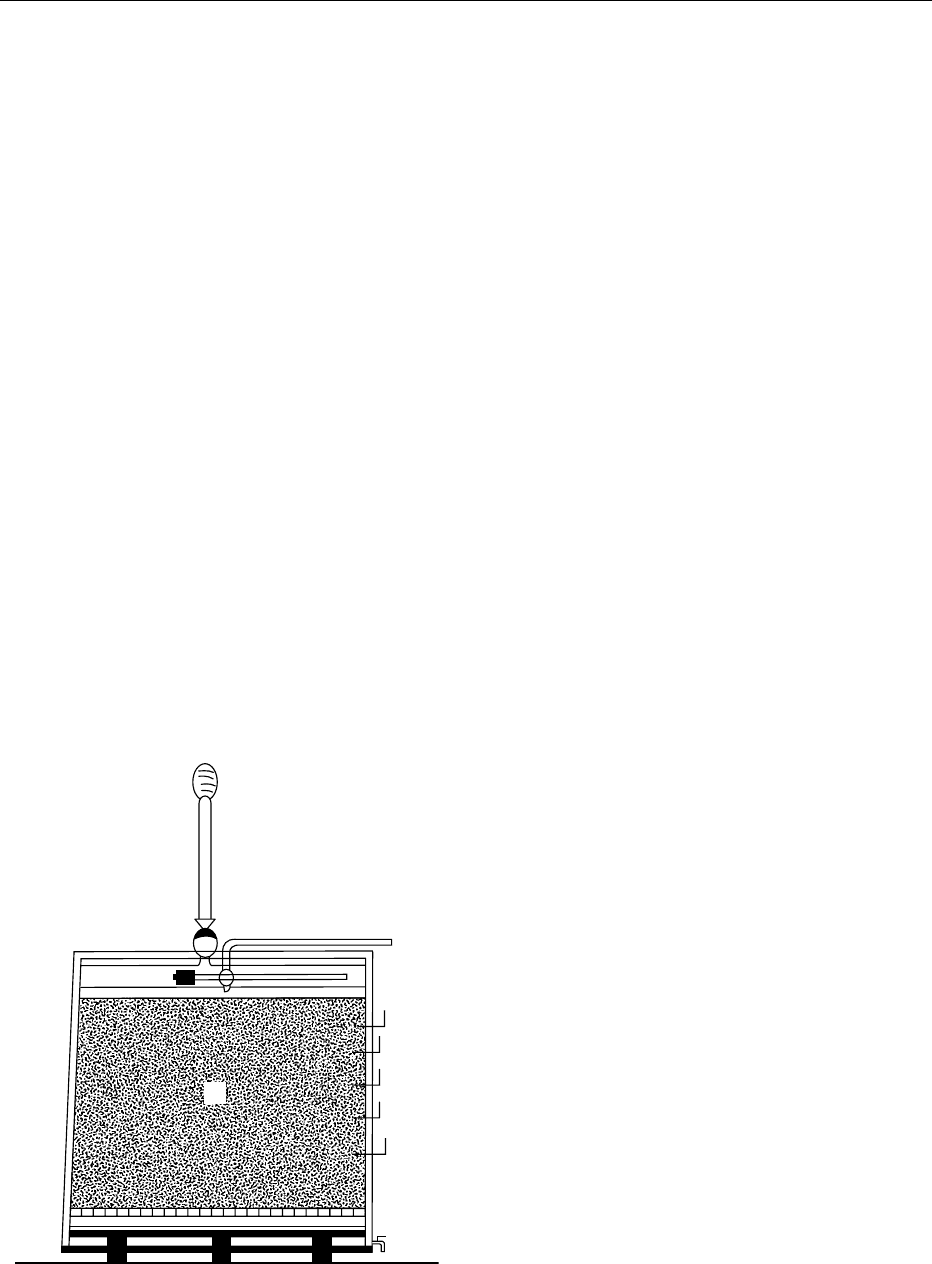
0016 Generator processes, first introduced in Germany,
have been in general use for more than 100 years. The
generator tanks are generally made of wood or some-
times steel, are equipped with cooling coils, and are
vented to allow the air to circulate; they have a per-
forated false bottom which serves to support the
wood shavings (preferably beech) or grape stalks
with which the tanks are filled. A spray mechanism
distributes the alcoholic liquid over the surface of the
packing (Figure 3). The liquid trickles over the wood
shavings covered with Acetobacters and then is
pumped back to the sprinkler header-tank and the
cycle is repeated. The process continues until acetifi-
cation is complete, which generally takes about a
week providing the temperature is kept in the optimal
range of 27–30
C. A measured volume of vinegar is
then withdrawn from the bottom of the tank and is
replaced with an equal volume of fresh liquid. The
generator process is a continuous process which gives
a fairly clear vinegar with good sensory characteris-
tics. However, it is slow and about 20% of yield is lost
through evaporation. Also, the wood shavings have
to be replaced at least once a year.
0017The third category involves the submerged pro-
cesses. These are the most up-to-date, benefitting as
they do from industrial experience with submerged
culture techniques employed in the field of antibiotics.
These processes do not use wood shavings, but air
forced into the liquid, and the mother of vinegar is
often replaced with selected cultures of Acetobacters.
The generator tank is made of stainless steel, or poly-
propylene reinforced with fiberglass; it is equipped
with devices to insure an adequate and continuous
flow of air, with cooling coils which maintain a steady
temperature of about 30
C in the fermenting body of
liquor, with thermometers for monitoring temperature
and, sometimes, with an automatic gage for measuring
the alcohol content of the fermenting liquid. Oxida-
tion of the alcohol is started slowly by means of aer-
ation, and fermentation is apparent after about 24 h.
Air is then introduced at regular hourly intervals; it
permeates the body of liquor in a uniform manner,
causing rapid and lively fermentation. Acetification
is deemed complete when the residual alcohol is
around 0.2–1.5% w/v (depending on the type of vin-
egar required). The process is extremely rapid. About
half of the resultant vinegar is then withdrawn; the
remaining vinegar acts as mother for the next batch.
0018This method was first developed in the USA and
Germany. It spread rapidly throughout the world and
displaced the older, slower processes. The yield is very
high (90–95%), since there is little loss through evap-
oration. However, the product is rather cloudy and less
aromatic than vinegars obtained by slower fermenta-
tion processes. This is due to the fact that, given the
brief period of contact, the esterases do not have time
to perform their function adequately and the charac-
teristic volatile substance content is low as a result.
0019The product is therefore filtered and, in some cases,
put into wooden casks to age; the result is a vinegar of
superior quality which is rich in biological principles,
generated by bacterial-cell autolysis, and perfectly
clear.
0020Malt vinegar Malt vinegar, which is the most
common variety in UK, is produced without inter-
mediate distillation from the double fermentation (al-
coholic and acetous) of malted barley with or without
the addition of other cereals, the starch of which
has been converted into fermentable sugars from
a-amylase of malted barley.
0021Malt vinegar manufacture involves mashing, fer-
mentation, and acetification. During mashing, the
malted barley, sometimes mixed with other cereals
such as maize and rice, is milled and mixed with hot
water in mash tuns, where the starch is converted
by a-amylase into maltose, dextrose, and dextrins.
The sweet liquor drains off the mash through the
B
C
E
D
A
fig0003 Figure 3 Circulating generator: A, sprinkler for dispersal of the
liquidoverthewoodshavings;B,beechwoodshaving;C,perforated
false bottom; D, tap for withdrawal of the liquid; E, thermometers.
5998 VINEGAR

perforated false bottom of the tun and is collected in
vessels where it is fermented by the addition of yeasts,
which convert the fermentable sugars to ethanol and
carbon dioxide. When fermentation is practically
complete, the alcoholic liquor is separated from the
yeasts and acetified by inoculation with Acetobacter
cultures. The resultant alcohol is thus oxidized to
acetic acid in the presence of atmospheric oxygen.
The process lends itself to the different systems in
current use. The vinegars will be more or less aro-
matic, the superior varieties will be those produced by
the old and slow Orleans process.
0022 Malt vinegar is straw-colored and must in any case
contain 4% w/v of acetic acid. Distilled malt vinegar
is prepared by distilling malt vinegar. This product
contains only the volatile constituents of the original
vinegar and is colorless; it is generally used for the
preparation of pickled silverskin onions. (See Malt:
Chemistry of Malting.)
0023 Wine vinegar Wine vinegar, which is obtained from
acetous fermentation of wine, is largely produced in
continental Europe.
0024 The wines used for acetification are those with too
low an alcohol content (7–9% v/v) or those in which
volatile acidity is too high. Spoilt wines cannot be used.
If wines with a high alcohol content are to be used, they
need to be appropriately diluted with water, since a
high concentration of alcohol will inhibit the develop-
ment of the Acetobacters. For the same reason, the
wine must be free of sulfur dioxide and dregs.
0025 Both white and red or rose
´
wines can be used to
produce white or red vinegar, respectively. In the
domestic or small-scale production of wine vinegar,
the wine is poured into small wooden barrels,
together with the vinegar mother, which consists of
colonies of Acetobacters taken from barrels in which
vinegar has already been produced. The barrel must
contain air, so for this reason it is not filled com-
pletely. Acetification is slow and stops spontaneously
when acidity reaches 7–8%. The slow transformation
of wine into vinegar leads to the formation of many
substances which impart excellent organoleptic qual-
ities to the end product. The most important of these
are acetaldehyde and ethyl acetate – which in top-
grade vinegars are present in quantities far greater
than those of wine – volatile alcohols, particularly
3-methyl-1-butanol, and 3-hydroxy-2 butanone (or
acetylmethylcarbinol).
0026 The vinegar is then partly withdrawn for use and
replaced with fresh wine to be acetified. Vinegar pro-
duced in this way is bound to vary in composition and
character: it may be more or less cloudy and its
acidity may vary according to the degree of alcohol
in the wine and the nature of the fermentation.
0027However, most wine vinegar is produced by indus-
trial processes and, as such, is a standardized indus-
trial product; according to EC regulations, its total
acidity must not be less than 6 g of acetic acid per
100 ml and residual ethanol may be present in quan-
tities not exceeding 1.5% v/v. The color varies from
pale yellow to red, depending on the wine used; the
clear vinegars made from white wine are in great
demand for pickling. (See Wines:Types of Table Wine.)
0028Cider vinegar Cider vinegar is prepared from apple
wine that has undergone acetous fermentation and is
widely used as a table vinegar, above all in the USA
and UK. It is yellowish in color and may be darkened
with caramel. Its acidity is not very high and its
acidic, astringent flavor recalls the fruit of origin.
(See Cider (Cyder; Hard Cider): The Product and its
Manufacture; Chemistry and Microbiology of Cider-
making.)
0029Fruit vinegars Fermented juices from other fruits
such as peaches and berries are also used to produce
vinegar. Since these alcoholic liquids are not distilled,
they maintain the subtle flavors and aromas of the
raw ingredients; this confers on the vinegars much-
appreciated characteristics.
0030Rice vinegar In the Far East, where rice is the staple
cereal, vinegar is prepared from rice as such, from
sake (its fermentation product), or from the by-
products of sake manufacture. Traditional methods,
similar to the Orleans process, are still in use but have
been largely superseded by modern submersion tech-
niques. This vinegar has a fairly low acidity and a
high amino acid content. It is light in color and has a
clean, delicate flavor. It is therefore highly prized in
oriental cooking since it does not significantly alter
the taste of the food; it is excellent for flavoring with
herbs, spices, and fruits because of its mild flavor.
0031Molasses vinegar This vinegar is prepared from
sugar syrup or molasses by double fermentation, al-
coholic fermentation of sugars, and the subsequent
acetic bioxidation. It serves to make use of the by-
products of the sugar industry, but is not widely used.
0032Honey vinegar This vinegar is obtained from honey
which is added to the right quantity of water, sub-
jected to alcoholic fermentation so as to obtain a
drink containing ethanol (mead), which, at the right
temperature and in the presence of oxygen is subject
to the action of acetic bacteria so as to obtain acetic
acid. The vinegar is then clarified by bland filtration
or by decanting, so that all the qualities of honey
remain unaltered.
VINEGAR 5999
0033 Spirit vinegar Spirit vinegar, sometimes referred to
as white, distilled, or alcohol vinegar, is prepared
by acetous fermentation of an alcoholic distillate
obtained from the products of alcoholic fermentation
of natural sugar solutions. In countries where it is
permitted by law, wide use is made of synthetic etha-
nol, diluted to 10–14% v/v. Spirit vinegar is colorless
and is often colored with caramel; it is strongly acid
but not aromatic; the distillation of the alcoholic
liquids increases the concentration of ethanol, but
reduces flavor. This vinegar is less expensive and is
the most widespread in the world. When diluted to
4–5% acidity it is used for pickling.
0034 Balsamic vinegar A particular type of highly prized
vinegar has been produced for centuries in the prov-
inces of Modena and Reggio Emilia in Northern Italy.
0035 The raw material is grape must, preferably Treb-
biano. When alcoholic fermentation has just started,
about 24 h after pressing, the must is boiled gently
until it has reduced to about one-half or one-third of
its starting volume. The result is a liquor with a high
sugar concentration (about 30%) in which alcoholic
and acetous fermentation take place together ex-
tremely slowly; yeasts (Saccharomyces and Zygosac-
charomyces) and acetic bacteria (Acetobacter and
Gluconobacter) are needed for the formation of this
vinegar.
0036 Traditional balsamic vinegar takes many years to
produce. During the process of fermentation, matur-
ing, and aging, the product becomes highly concen-
trated and the sugars, alcohols, aldehydes, and
organic acids undergo extremely gradual chemical
transformation. The vinegar battery consists of a
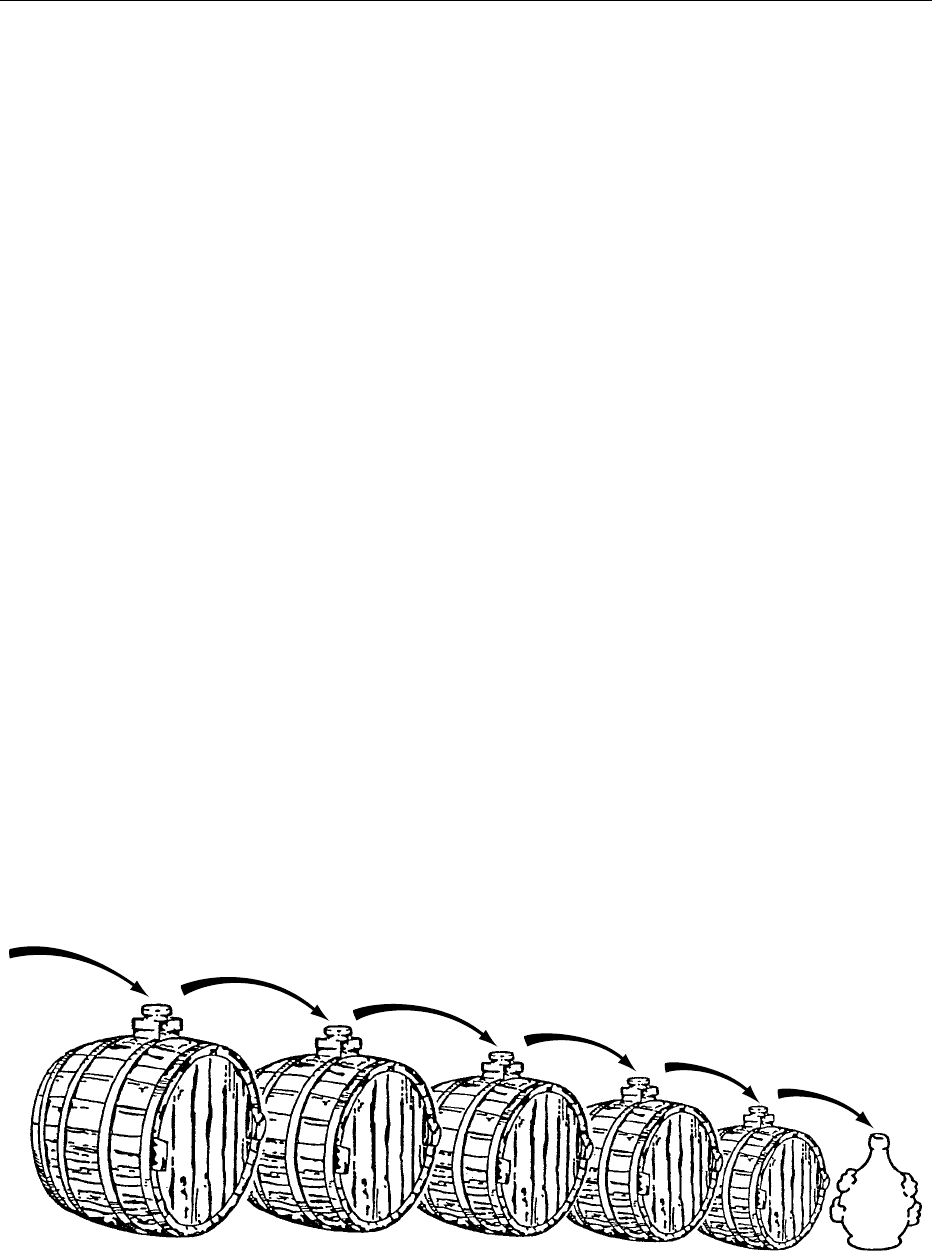
variable number of barrels (between five and 12 or
even more) of different woods and decreasing in size.
They are set up in well-ventilated areas which are hot
and dry in summer and cold in winter. According to
the traditional method, part of the contents of the
smallest barrel is withdrawn annually for consump-
tion, and is replaced with an equal volume from the
next largest barrel in the battery. This is in turn re-
plenished from its neighbor, and so on up the line.
Finally, the largest barrel is topped up with the
season’s boiled must (Figure 4).
0037The process takes at least 12 years, though it is not
uncommon to find vinegars that are 50 or more years
old, and the yield is very low (no more than 1 l of
vinegar from 100 kg of fresh must). The end product,
however, is of exceptionally high quality, dark brown
in color, of a syrupy consistency, sweet and sour to the
taste, and with a characteristically pleasant aromatic
smell. In traditional balsamic vinegar the total solids
are very high (20–70%), acidity varies between 6 and
18% w/v acetic acid and there are large amounts of
sugars, essentially glucose and fructose, as well as
numerous aromatic substances that have gradually
been formed over the years.
Chemical Synthesis
0038Acetic acid is usually synthesized by catalytic oxida-
tion from acetaldehyde. Acetaldehyde is in turn
obtained by catalytic hydration of acetylene or by
catalytic dehydrogenation of ethanol (Figure 5).
Formic acid and formaldehyde, byproducts of acetic
acid synthesis, are removed by distillation.
0039The acetic acid is subsequently purified and diluted
with water to 60–80% by volume to obtain the
6th
operation
Oak
60 l
5th
operation
Chestnut
50 l
4th
operation
Cherry
40 l
3rd
operation
Ash
30 l
2nd
operation
Mulberry
20 l
1st
operation
fig0004 Figure 4 Battery of barrels for manufacture of the ‘traditional balsamic vinegar of Modern’.
6000 VINEGAR
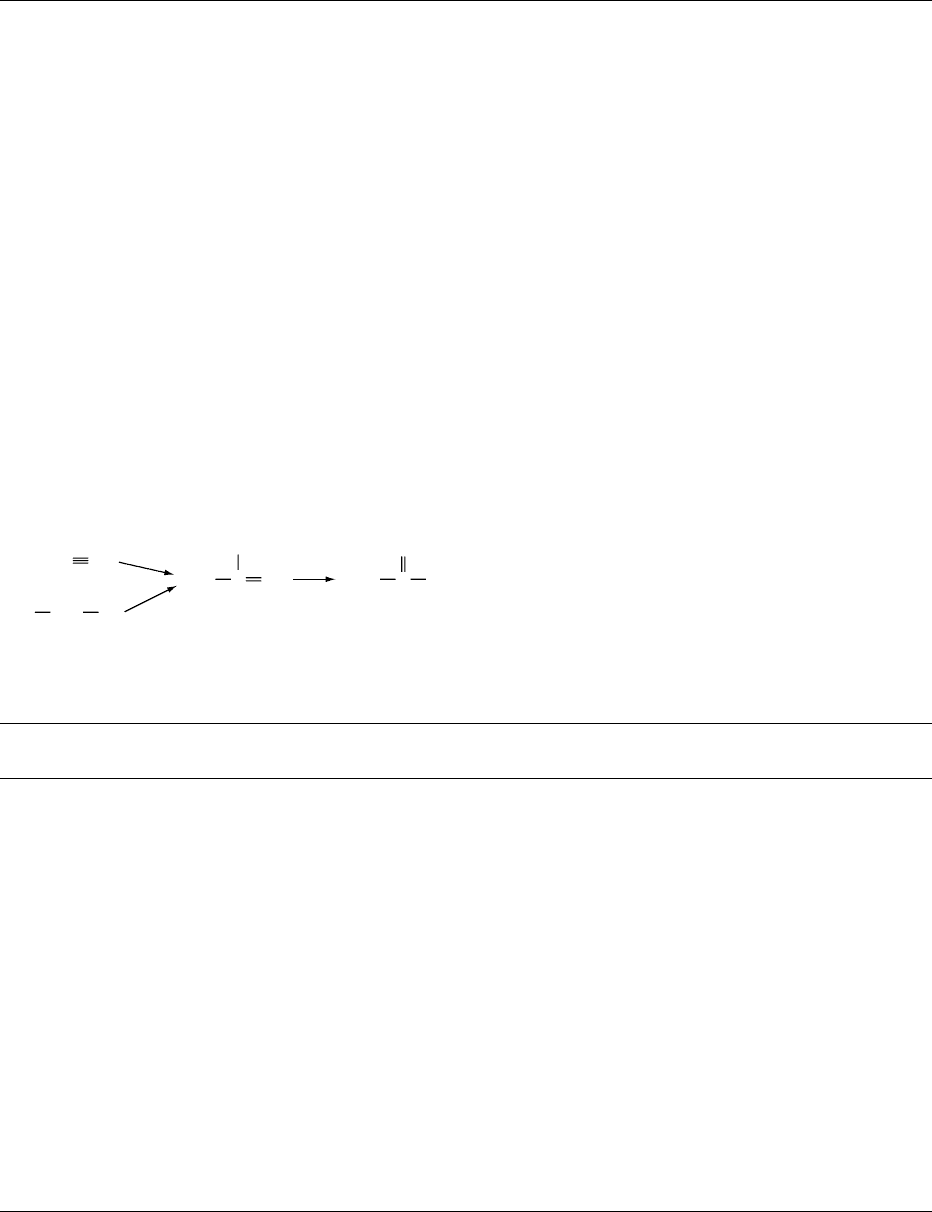
‘vinegar essence.’ In order to produce a food-grade
vinegar, this ‘vinegar essence,’ which is a concentrated
solution of acetic acid and highly corrosive, is further
diluted until it contains 4–5% w/v of acetic acid. This
liquid is used in the preparation of synthetic vinegar.
0040 Synthetic Vinegar A number of countries allow a
nonfermented vinegar to be used for alimentary pur-
poses which may be produced using synthetic acetic
acid that is diluted, aromatized, and colored. The
synthetic vinegar thus obtained needs to be colored
artificially, and for this purpose caramel is commonly
used. It is then aromatized with the addition of
sugars, chemical seasoning, and salt or with the
addition of natural vinegar. The end product must
contain at least 4% w/v of acetic acid, as in the case
of fermented vinegars. However, the name ‘vinegar’ is
not always accepted for this product; in the UK for
example, it must be labeled ‘nonbrewed condiment.’
Characterization and Analysis of Vinegars
0041The relative density of vinegars ranges from 1.007 for
synthetic vinegars to 1.360 for balsamic vinegars.
Total solids and ash vary according to type. They
are very high in the ‘traditional balsamic vinegar of
Modena.’ They are more abundant in malt, wine, and
cider vinegars than they are in spirit vinegars and
synthetic vinegars.
0042Brewed vinegars, being a natural product, contain
a large number of components that may derive from
the raw materials, be the result of primary or second-
ary fermentation, or derive from the interaction of
components from these two sources (Table 1).
0043The most abundant volatile constituent is acetic
acid, which is largely responsible for the acidity of
the vinegar. It ranges from 4–5% for rice vinegars to
10–12.5% for distilled vinegars.
0044The presence of acetaldehyde, a byproduct of acet-
ous fermentation, is typical. Numerous esters, major
contributors to aroma, are present, notably ethyl
acetate, the major portion of which was formed
during storage, and also methyl and isopentyl acetate;
various isomeric butanols and pentanols, present in
the raw material, occur in vinegars.
0045The formation of 3-hydroxy-2-butanone and 2,3-
butanedione are typical, deriving from 2,3-butandiol,
HC CH
H
3
CCH
2
OH
+H
2
O
H
3
C
H
3
C
−H
2
H
C
O
O x
C
O
OH
fig0005 Figure 5 Chemical syntheses of acetic acid.
tbl0001 Table 1 A comparison of some typical figures for the chemical composition of various vinegars
Malt
vinegar
a
Wine
vinegar
b
Cider
vinegar
a
Spirit
vinegar (conc.)
a
Rice
vinegar
c
Balsamic
vinegar
d
Synthetic
vinegar
a
Relative density (20
) 1.013–1.022 1.013–1.020 1.013–1.024 1.015–1.020 1.042–1.361 1.007–1.022
Total solids (g l
1
) 3.00–28.40 8.71–24.88 19.00–35.00 1.50–6.00 336.70–873.90 1.00–4.50
Total ash (g l
1
) 0.60–7.60 1.47–3.10 2.00–4.50 0.20–0.50 0.50–7.60 4.00–18.80 0.20–0.50
Alkalinity of ash (mmol l
1
) 9.60–31.60 26–152
Total acidity as acetic acid (%) 4.30–5.90 5.94–9.20 3.90–9.00 11.50–12.20 4.00–5.24 6.25–14.88 4,10–5.30
Volatile acidity as acetic acid (%) 5.55–7.95 3.79–5.16 3.90–13.60
Fixed acidity as acetic acid (%) 0.20–0.40 0.02–0.55 0.10–0.20 0.05–0.66 1.58–2.27 Negligible
Alcohol (% v/v) 0–0.14 0–0.81 0.03 0.15 0–0.68 0.04–0.08
Total nitrogen (g l
1
) 0.40–1.40 1.15–1.86 0.03–0.30 0.80–1.29 1.02–2.16 0–2.00
Sugars (g l
1
) 0–6.20 1.50–7.00 0–91.00 351.00–689.70
Glycerol (%)
e
0.37 0.28 0.94–1.7
Gluconic acid (%)
e
0.02 0.02 0.68–1.14
Tartaric acid (%)
e
0.11 0.46–0.52
Acetic acid (%)
e
7.2 5.5 6.38–7.64
Citric acid (g l
1
) 0.26–0.39 1.66–3.47
Malic acid (g l
1
) 0.47–0.80 0.70–1.60 8.00–37.40
Acetaldehyde (mg l
1
) 18.9–114.4 84.9–374.7
2,3 Butanediol (mg l
1
) 470–2890
2,3 Butanedione (mg l
1
) 17–37
3 Hydroxy-2 butanone (mg l
1
) 122.0–1257.0 249.1–1597.8
Ethylacetate (mg l
1
) 206.0–590.0
a
Data from Egan H, Kirk RS and Sawyer R (1987) Pearson’s Chemical Analysis of Foods, 8th edn. Harlow: Longman Scientific and Technical.
b
Data from Stella C (1976) La composizione degli aceti Vini d’Italia 18: 269.
c
Data from Mizumoto H, Minamisone H, Mori K and Azumo K (1975) Chemical composition of commerical rice vinegars. Kagoshima-ken Kogjo Shikenjo
Nempo 22: 67.
d
Data from Plessi M and Coppini D (1984) L’aceto balsamico tradizionale di Modena. Atti della Societ
a
`
dei Naturalisti e Matematici 115: 39–46.
e
Data from Giudici P et al. (1994) Origine ed evoluzione degli acidi organici durante l’invecchiamento dell’aceto balsamico. Industria delle bevande
23: 569–574.
VINEGAR 6001

a product of the condensation of acetaldehyde by
microorganisms, which are always, and only, found
in brewed vinegars, albeit in variable quantities
(Table 2).
0046 Organic acids, other than acetic acid, are also pre-
sent: tartaric and citric are largely found in wine
vinegars, while formic and malic acid are those most
commonly found in cider vinegars. During the
fermentation process small quantities of other acids,
such as succinic acid, lactic acid, and fumaric acid,
are formed.
0047 Of the many analyses that vinegars lend themselves
to, the most useful from the point of view of general
characterization are the following:
.
0048 Sensory examination: The sensory characteristics
to be taken into account are color, odor, and taste,
which must conform to those attributed to the type
of vinegar being analyzed. If the vinegar is cloudy,
it must be examined under strong magnification for
vinegar eels (Anguillula aceti), a nematode that is a
typical parasite of vinegar.
.
0049 Relative density: This is determined at 20
C using
a Westphal balance or pyknometer.
.
0050 Total acidity: This is determined by titrating the
diluted product with a standard alkaline solution.
It is expressed as grams of acetic acid per 100 ml of
vinegar.
.
0051 Fixed acidity: A 10-ml aliquot of vinegar is evapor-
ated to dryness and reconstituted with water, the
process being repeated in order to eliminate acetic
acid. The final residue is dissolved in water and
titrated with an alkaline solution. It is expressed
as grams of tartaric acid per 100 ml of vinegar.
.
0052 Volatile acidity: This is calculated from the differ-
ence between total and fixed acidity, both expressed
as grams of acetic acid per 100 ml of vinegar.
.
0053Total solids: A 50-ml aliquot of vinegar is evapor-
ated in a platinum capsule with water in order to
eliminate the acetic acid. It is then dried to constant
weight in an oven at 100
C.
.
0054Ash: The total solids are ignited at 500–550
C. If a
high ash figure is obtained, salt should also be
determined.
.
0055Ash alkalinity: This is chiefly due to potassium
carbonate formed during calcination and is deter-
mined by dissolving the ash in an exact quantity
of standard sulfuric acid, of which the excess is
titrated with alkali. It is expressed in mmol of alkali
per liter.
.
0056Ethanol: A 100-ml aliquot of vinegar is neutralized
with sodium hydroxide and double-distilled. The
density of the solution is measured and, conse-
quently, the quantity of ethanol is calculated from
tables.
.
0057Total nitrogen: This is determined by the Kjeldahl
method on 25 ml of vinegar.
Methods of Differentiating Between Various Types
of Vinegar
0058Apart from color and odor, the nitrogen, phos-
phates, ash, and their alkalinity also serve to differen-
tiate between the various types of vinegar. In a
malt vinegar, nitrogen and phosphate figures are
low, while in a rice vinegar the nitrogen figure
is higher and distilled vinegar has a very low ash
content.
0059However, useful methods of routine differentiation
are based on those values that depend on the volatile
substances present, namely:
.
0060Oxidation value: The number of ml of 0.002
mol l
1
KMnO
4
discolored by 100 ml of sample in
30 min under standard conditions. Alcohol and
tbl0002 Table 2 A comparison of major volatile components of various vinegars
Malt vinegar Wine vinegar Cider vinegar Distilled vinegar Balsamic vinegar
Ethyl acetate Acetaldehyde Acetaldehyde Acetaldehyde Ethyl acetate
Ethyl formate Ethyl acetate Ethyl acetate Ethyl acetate Ethanol
3-Methyl-1-butanol Ethyl formate Ethyl formate Ethyl formate 3-Hydroxy-2-Butanone
2-Methyl-1-propanol 2-Methyl-1-propanol 3-Methyl-1-butanol Ethanol 2,3-Butanediol
2-Methyl-1-butanol 3-Hydroxy-2-butanone 2-Pentanol 2-Pentanone 2,3-Butanedione
Acetaldehyde 2,3-Butanediol 2-Methyl-1-butanol 3-Pentanol Methyl acetate
Ethanol 3-Methyl-1-butanol 2-Methyl-1-propanol 2-Pentanol Acetaldehyde
Isobutyl acetate Isopentyl acetate Ethanol Methyl formate Furfural
Isopentyl formate 2-Methyl-1-butanol 2-Pentanone Propionaldehyde 2,4,5-Trimethyl-1,3-dioxolane
Isopentyl acetate Ethanol 3-Pentanol Methanol Acetone
Benzaldehyde Isovaleraldehyde Benzaldehyde Acetal 3-Methyl-1-butanol
Acetone Isobutyl acetate Propionaldehyde 2-Methyl-1-butanol Ethyl formate
Isobutyl formate g-Butyrolactone Ethyl lactate 3-Methyl-1-butanol Isobutanal
2-Pentanone Acetone Phenylacetaldehyde 2-Methyl butanal
2-Methyl-3-buten-2-ol Isobutyl formate Diethyl succinate 2-Butanone
2-Pentanone Furan
6002 VINEGAR

acetylmethylcarbinol are mostly responsible for the
oxidation value.
.
0061 Iodine value: The number of ml of 0.01 mol l
1
iodine absorbed by 100 ml of sample under stand-
ard conditions. This value is mostly influenced by
acetylmethylcarbinol and diacetyl.
.
0062 Ester value: The number of ml of 0.01 mol l
1
KOH required to saponify the esters contained in
100 ml of sample under standard conditions.
0063 Brewed vinegars give comparatively high values,
but those for artificial products are low as the latter
are almost devoid of volatile reducing substances.
0064 Vinegars can be more specifically identified by
means of gas chromatography. This technique can
distinguish brewed vinegar from synthetic vinegar
and also brewed vinegars from different origins by
analyzing the accompanying compounds (metabolic
byproducts of Acetobacter strains and substances
derived from the raw material).
0065 In addition, the blending or adulteration of brewed
vinegar with synthetic acid can be detected by mass
spectrometric determination of the
13
C/
12
C-isotope
ratio; brewed vinegar has 5% more
13
C isotope than
acetic acid synthesized petrochemically.
Food Uses
0066 Vinegar has long been used worldwide as a basic
seasoning in the preparation and cooking of certain
foods, because its sharp taste makes it so useful and
versatile.
0067 A considerable quantity of vinegar is marketed as
such for domestic use. In the UK and USA the table
vinegar most widely used is cider vinegar, while in
Ireland it is malt vinegar and, in grape-growing coun-
tries, such as Italy, France, and Spain, wine vinegar.
In the Far East, in addition to the traditional rice
vinegar, synthetic vinegar is very common.
0068 Vinegar adds flavor to vegetable and meat prod-
ucts. It is one of the ingredients of salad dressings,
sauces, such as tabasco, and tomato products, such as
ketchups, mustard, and aspics. Mixed with oil and
salt it makes the classic vinaigrette, and it can be used
as a condiment for salad and as a sauce for cold,
cooked vegetables, meat, and fish.
0069 For condiment uses, vinegar can be aromatized
with herbs and spices like tarragon, basil, garlic, shal-
lot, and elder. They are steeped in the vinegar when
acetification is complete; in certain types of vinegar
sugars are added as well. These vinegars add special
and unusual tastes to food.
0070 The aroma and sweet and sour flavor which bal-
samic vinegar confers on foods to which it has been
added is decidedly particular; it is suitable for all
types of food, such as sauces, green salads, and meat
and sprinkled on strawberries, peaches, or melon
adds a pleasant flavor and aroma.
0071Finally, because of its acetic acid content and low
pH, vinegar is used as a preservative for both domes-
tic use and in the food industry. It is in fact used for
the preservation, or pickling, of a wide variety of
foods such as vegetables, meat, fish products, and
spiced fruits. For this purpose, the food-processing
industry uses mainly distilled vinegar and, where the
law permits, synthetic vinegar.
See also: Acids: Properties and Determination; Natural
Acids and Acidulants; Preservatives: Classifications and
Properties; Spices and Flavoring (Flavouring) Crops:
Use of Spices in the Food Industry
Further Reading
Adams MR (1985) Vinegar. In: Wood BJB (ed.) Microbiol-
ogy of Fermented Foods, pp. 1–47, vol. 1. Essex: Elsevier
Applied Science.
AA VV (1996) L’aceto Balsamico di Modena. Modena:
Consorzio Tutela Aceto Balsamico di Modena.
Carnacini A and Gerbi V (1992) L’aceto di vino, un pro-
dotto da valorizzare e tutelare. Industrie delle Bevande,
19: 465–483.
Conner HA and Allgeier RJ (1976) Vinegar: its history and
development. Advances in Applied Microbiology 20:
81–133.
Coppini D, Plessi M and Monzani A (1978) Ulteriore
contributo allo studio dell’aceto Balsamico. Rivista di
Viticoltura ed Enologia 31: 351–357.
Egan H, Kirk RS and Sawyer R (1987) Pearson’s Chemical
Analysis of Foods, 8th edn. Harlow: Longman Scientific
and Technical.
Garcı
´a-Parrilla
MC, Gonza
´
les GA, Heredia FJ and Trocoso
AM (1997) Differentiation of wine vinegars based on
phenolic composition. Journal of Agriculture and Food
Chemistry 45: 3487–3492.
Garoglio PG (1973) Enciclopedia Vitivinicola Mondiale.
Milano: Edizioni Scientifiche UIV.
Masai H (1980) Recent technical developments on vinegar
manufacture in Japan. Proceedings of the Oriental
Fermented Foods 192–198.
Mizumoto H, Minamisone H, Mori K and Azumo K (1975)
Chemical composition of commercial rice vinegars.
Kagoshima-ken Kogyo Shikenjo Nempo 22: 67–68.
Nieto J, Gonza
´
-Vin
˜
as MA, Barba P et al. (1993) Recent
progress in wine vinegar R & D and some indicator for
the future. In: Charalambous G (ed.) Food Flavours,
Ingredients and Composition, pp. 469–500. Amster-
dam: Elsevier Science.
Plessi M and Coppini D (1984) L’aceto balsamico tradizio-
nale di Modena. Atti della Societa
`
dei Naturalisti e
Matematici 115: 39–46.
Plessi M, Monzani A and Coppini D (1987) Determination
of the monosaccharide and alcohol content of balsamic
VINEGAR 6003

and other vinegars by enzymatic methods. Agricultural
and Biological Chemistry 52: 25–30.
Plessi M, Monzani A and Coppini D (1989) Quantitative
determination of acids and derivatives in balsamic and
other vinegars. Sciences des Aliments 9: 179–183.
Saccani F and Ferrari Amorotti V (1999) Il Balsamico della
Tradizione Secolare. Modena: Consorteria dell’Aceto
balsamico tradizionale di Modena.
Sacchetti M (1970) Sull’aceto Balsamico Modenese.
Bologna: Edagricole.
Stella C (1976). La composizione degli aceti. Vini d’Italia
18: 269–274.
Tesfuge W, Morales ML, Garcia-Parrilla MC and Troncoso
AM (2002). Wine vinegar authenticity and quality
evaluation. Trends in Food Science and Technology 13:
2–33.
VIRUSES
H Appleton, PHLS Central Public Health Laboratory,
London, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Viruses are usually transmitted directly from person
to person, but it is clearly recognized that, on occa-
sions, some viruses may also be transmitted by foods
and water. Unlike bacteria, viruses do not multiply or
produce toxins in food: food items merely act as
vehicles for their passive transfer. Any virus that is
infectious via the gastrointestinal tract could be con-
sidered a potential foodborne pathogen, but in prac-
tice, the most commonly reported foodborne viral
infections are gastroenteritis and, less frequently,
hepatitis. Although the clinical characteristics of
these two illnesses differ, their food-related mode
of transmission is essentially the same. Foodborne
spread of both these infections has been clearly de-
fined, but the extent of the problem is unknown. For
various reasons, the incidence of both is almost
certainly under-reported.
Viral Gastroenteritis
0002 Gastroenteritis is the most common foodborne viral
infection. Discovery of the viruses responsible for
causing gastroenteritis began with the identification
of the Norwalk virus in 1972 and then the human
rotavirus in 1973. These viruses are usually transmit-
ted directly from person to person via the fecal–oral
route. In 1977, however, there was a report that a
virus had been responsible for a community-wide
outbreak in England associated with shellfish. At
least 800 people were affected, and it is now clearly
established that food may also play an important role
in transmission.
0003 Prior to the discovery of gastroenteritis viruses and
recognition of their foodborne mode of transmission,
there were many reports of ‘food poisoning outbreaks
of unknown etiology,’ which were assumed to be
bacterial but in retrospect have the characteristics of
viral gastroenteritis. Such reports still occur when
appropriate viral investigations are not undertaken.
Viral gastroenteritis tends to mimic bacterial food
poisoning, and invariably, outbreaks are initially in-
vestigated for traditional food poisoning bacteria.
However, there are characteristic features that should
alert investigators to the possibility of a viral cause.
The incubation period is somewhat longer and more
variable than for most bacteria, with a peak of
around 30–36 h (range 12–60 h, which may be dose-
dependent). Symptoms are variable and may include
both vomiting and diarrhea as well as the usual range
of associated complaints such as abdominal pain,
general malaise, and nausea.
0004Secondary person-to-person spread is a character-
istic feature. Illness is usually regarded as mild and
self-limiting with recovery within 24–48 h. However,
people commonly feel debilitated for 2–3 weeks, and
this can have considerable impact socially and eco-
nomically in impaired performance. Outbreaks may
be very disruptive and have led to closure of hospitals
and schools. Some outbreaks have been very large,
involving several hundred people. Treatment is not
normally required unless people are severely affected
and become dangerously dehydrated. This is a more
common problem in children suffering diarrheal dis-
eases in underdeveloped countries. Oral rehydration
therapy with simple balanced salt solutions is an
effective treatment.
0005The overall incidence viral gastroenteritis is un-
known. A community-based study of infectious intes-
tinal disease, from all causes in England from 1993 to
1996, confirmed that the vast majority of cases go
unreported. The study found that viruses were re-
sponsible for a far greater proportion of gastrointest-
inal illness than national surveillance figures had
previously indicated. The study also concluded that
infections resulting from viruses are less likely to be
6004 VIRUSES
reported than bacterial infections. It was estimated
that only one in 35 infections with rotavirus and one
in 1562 infections caused by Norwalk-like viruses
occurring in the community are reported to national
surveillance, compared with one in three cases of
salmonellosis.
0006 The proportion of these viral gastrointestinal infec-
tions that is foodborne is similarly unknown. Viruses
account for 6% of foodborne outbreaks and 5%
of waterborne outbreaks occurring in England and
Wales and reported to national surveillance, but this
is likely to be a gross underestimate of the true inci-
dence. The mild nature of the illness means that per-
sons do not tend to consult a medical practitioner.
However, the short incubation period means that
when an illness is investigated, possible association
with a food source is more likely to be recognized
than for hepatitis A, for which there is a long incu-
bation period. The highly infectious nature of the
viruses and the frequent occurrence of secondary
person-to-person spread also add to the difficulties
of identifying a food- or waterborne outbreak.
The Gastroenteritis Viruses
0007 Several different viruses cause gastroenteritis
(Table 1), but it is the viruses of the Norwalk group
that have been particularly linked to foodborne trans-
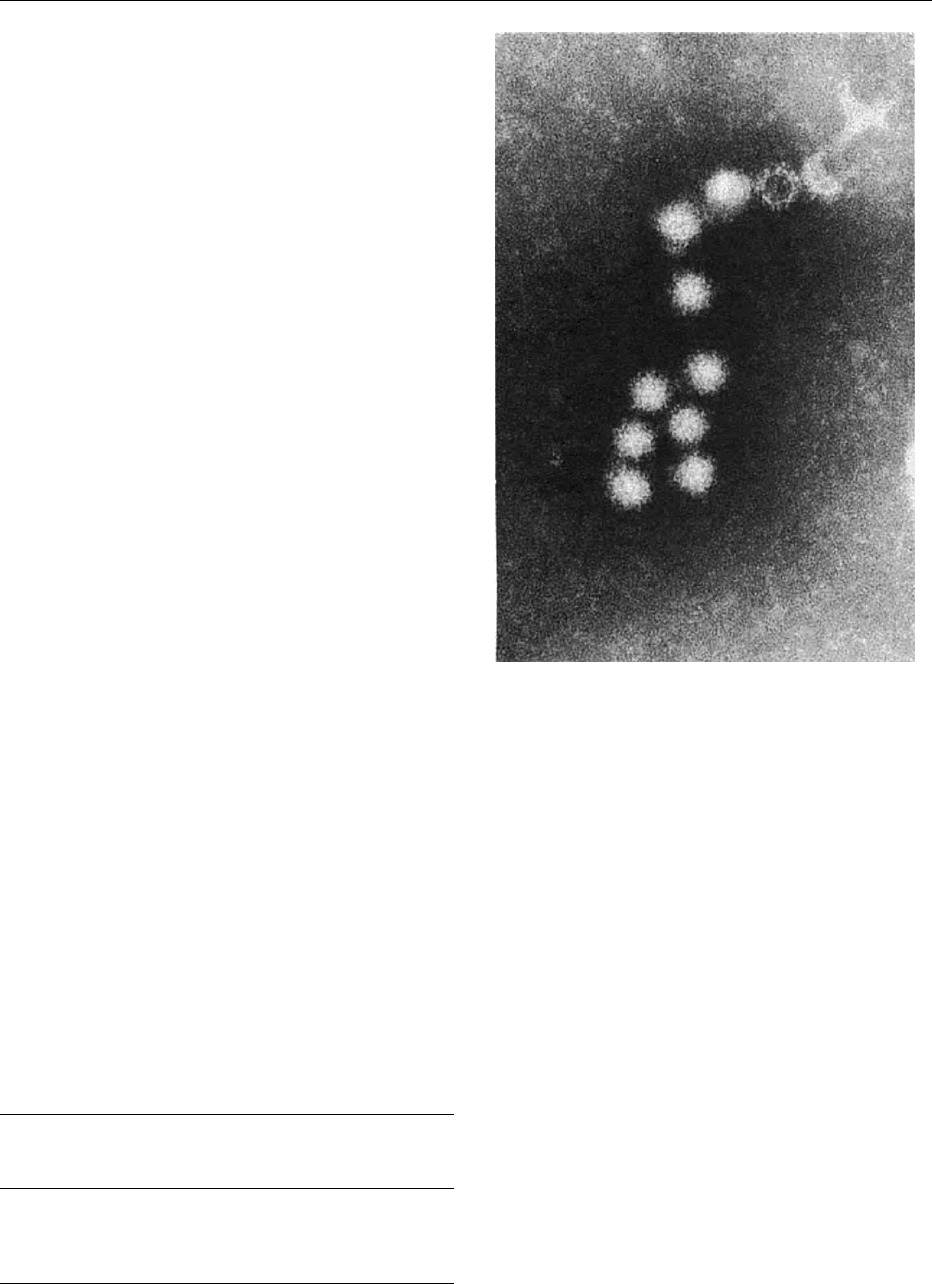
mission. They form a complex group, and the nomen-
clature is confusing. The viruses have been known
variously as Norwalk-like viruses (NLV), and as
small round structured viruses (SRSV) because of
their appearance when viewed in the electron micro-
scope (Figure 1). The virus particle measures
30–35 nm in diameter and has an amorphous surface
and a ragged outline. They are classified as members
of the Caliciviridae family and hence are sometimes
referred to as human caliciviruses.
0008 Norwalk-like Viruses The Norwalk virus, which
originated from a school outbreak of nonbacterial
gastroenteritis at Norwalk, Ohio, in the USA, was
the first virus of the group to be discovered. Viruses
of this group are frequently the cause of both commu-
nity and institutional outbreaks of nonbacterial gas-
troenteritis, whether resulting from person-to-person
spread or a food or water source. Outbreaks in hos-
pitals, residential homes, schools, hotels, restaurants,
and cruise ships have been reported frequently. The
rapid spread of infection can cause major disruption,
leading to closure of schools and hospital wards.
There are also considerable economic costs in terms
of working days lost. They are probably the most
important cause of sporadic gastroenteritis in the
community, although because of the relatively minor
nature of illness, such incidents are not usually inves-
tigated. In the UK, NLVs have been implicated in
more than 90% of foodborne outbreaks where a
virus has been detected, and it is clear that this virus
group is the main cause of foodborne viral gastroenter-
itis in developed countries.
0009Several different serotypes have been identified.
Immunity to NLVs is poorly understood and may be
short-lived. Volunteer studies have shown that some
tbl0001 Table 1 Viruses commonly causing gastroenteritis
Virus Person-to-person
spread
Food- or
waterborne
spread
Norwalk-like virus
a
Yes Yes
Rotavirus Yes Infrequent
b
Astrovirus Yes Infrequent
Adenovirus (types 40 and 41) Yes Not reported
a
Also known as small round structured viruses.
b
Food- and waterborne transmission is probably more significant in
underdeveloped countries.
fig0001Figure 1 Electron micrograph of a group of Norwalk-like vir-
uses. The virus particles have an amorphous surface and ragged
outline. Most particles are complete, but an empty stain-
penetrated particle can be seen at the top of the group. Virus
particles negatively stained with phosphotungstic acid. Magnifi-
cation: 200 000.
VIRUSES 6005

people may be infected on more than one occasion by
the same virus. Thus, people who experience several
incidents of illness may not only be infected with
different antigenic types of NLV, but may also
develop illness on more than one occasion from the
same virus.
0010 The development of polymerase chain-reaction
(PCR) assays has allowed the molecular characteriza-
tion of virus strains from many outbreaks. The human
gastroenteritis viruses in the Caliciviridae family form
three broad genogroups. Two groups have the morph-
ology of NLVs (Figure 1) and broadly similar genomic
features. Members of the third group are known as
Sapporro-like viruses (SLVs). The genomic arrange-
ment of this third group is distinct from the other two
groups. In addition, SLVs have the classical morph-
ology more usually associated with the calicivirus
group, with hollow cup-like depressions on the sur-
face, which are not usually seen in NLVs. (The name
calicivirus derives from the Latin calix meaning cup.)
The different serotypes of the human caliciviruses
broadly correspond with the genotypic groups. The
majority of reported infections caused by viruses with
classical calicivirus morphology have occurred in
young children. Outbreaks in nursery groups in-
volving person-to-person transmission have been
recorded. The virus has also observed in older per-
sons, and some reported foodborne outbreaks have
been attributed to calicivirus.
0011 Rotavirus Rotaviruses mainly infect young children
and are the most frequently reported of the gastroen-
teritis viruses. It is estimated that they may cause a
million deaths a year in children under 5 years of
age, mostly in developing countries. In developed
countries, deaths are relatively rare, but rotavirus
gastroenteritis is the most frequent reason for admis-
sion of young children to hospital. Foodborne
and particularly waterborne spread are probably
significant modes of transmission in developing
countries, but in developed countries, reports are
rare. Although rotavirus has been detected in shell-
fish, so far, there have been no reports of illness from
this source.
0012 Astrovirus Astroviruses form a morphologically
distinct group of viruses, and are named from the
five- or six-point star seen by electron microscopy
on the surface of some particles. Astroviruses are
normally associated with gastroenteritis in young
children, often under 1 year of age. There have been
occasional reports of astrovirus infection in some
adults following consumption of shellfish or contam-
inated water, but these incidents appear compara-
tively rare.
0013Adenovirus Adenovirus serotypes 40 and 41 have
been specifically linked to gastroenteritis, and infec-
tions in young children are frequently reported.
Reports in older patients are rare. There have been
no reports of food- or water-associated adenovirus
infection.
0014Parvovirus Parvoviruses are small DNA-containing
viruses. Their role as causative agents of gastroenter-
itis in humans is uncertain, since, unlike other gastro-
enteritis viruses, there is a prolonged carrier state in
some persons. However, parvoviruses are an import-
ant cause of gastroenteritis in some animal species.
Parvoviruses have been observed in school outbreaks
of winter vomiting disease and in a number of shell-
fish associated outbreaks of gastroenteritis. Parvo-
virus-like particles have been detected occasionally
in shellfish that have been implicated in illness and
where a similar virus has been found in patients.
Hepatitis
0015Hepatitis is an inflammation of the liver and is usually
recognized by the occurrence of jaundice. Descrip-
tions of hepatitis can be traced back to the time of
Hippocrates, but it was not until the eighth century
that the infectious nature of the disease was first
mentioned in letters from Pope Zacharias to Saint
Boniface, Archbishop of Mainz. The viral etiology
was first postulated by MacDonald in 1918 and was
later confirmed by volunteer studies in the 1940s. It is
now known that several different viruses may cause
hepatitis, but it is the enterically transmitted viruses,
hepatitis A and hepatitis E, that are of importance in
foodborne and waterborne transmission.
Types of Hepatitis
0016Hepatitis A Hepatitis A is transmitted by the fecal–
oral route and is prevalent in conditions of over-
crowding and poor hygiene. Although it has long
been recognized that hepatitis A may be transmitted
by food and water, the incubation period of 3–6
weeks makes it difficult to link infection to a food
source. Hence, reports of foodborne hepatitis are
infrequent and are unlikely to indicate the true extent
of the problem. Symptoms in addition to, or instead
of, jaundice are nausea and general malaise. Patients
may feel unwell for several weeks, but recovery is
complete; fatalities are rare. There is no specific treat-
ment, but a vaccine is available. Illness tends to be less
severe in children, and in some, infection is symptom-
less. Once infected, a person is immune from further
infection. As hygienic conditions improve, infection
becomes less common in children, and a larger pro-
portion of the adult population becomes susceptible.
6006 VIRUSES

Serological surveys in the UK indicate that only
10–15% of young adults have antibody to hepatitis
A virus (HAV).
0017 HAV is a small, compact icosohedral virus measur-
ing 25–30 nm in diameter. It is classified as a picorna-
virus (from the Latin pico (small) þ rna as it has an
RNA genome). Within the Picornaviridae family, it is
assigned to the hepatovirus genus. There is only one
serotype.
0018 Hepatitis E More recently, another enterically
transmitted form of hepatitis (designated hepatitis
E) has been described. This virus has been associated
with large waterborne outbreaks in some developing
countries, notably in Asia, Africa, and Central Amer-
ica. Illness appears more severe than hepatitis A, par-
ticularly in pregnant women for whom a mortality
rate of 17–34% has been reported. Secondary person-
to-person transmission is estimated at only 0.7–8%.
The primary source of infection appears to be con-
taminated water rather than person-to-person spread.
Cases in the UK are reported infrequently and are
mainly imported from endemic areas.
0019 Hepatitis E is classified as a calicivirus but is ser-
ologically unrelated to the viruses that cause gastro-
enteritis. Like all caliciviruses, the virus particles have
a diameter of 30–35 nm. When viewed in the electron
microscope, the characteristic cup-like depressions of
members of the calicivirus group can be seen on the
surface of the virus particles.
0020 Other Hepatitis Viruses There are other hepatitis
viruses that are not transmitted by the fecal–oral
route. Hence, they are not considered to be associated
with foodborne transmission. The commonest, hepa-
titis B and hepatitis C, are usually transmitted paren-
terally, e.g., by blood transfusions, from injections of
contaminated blood products such as factor VIII, or
from contaminated needles used by drug addicts.
They may also be transmitted by sexual contact.
Other Viruses
Enteroviruses
0021 Enteroviruses form a distinct genus within the Picor-
naviridae family and include the polioviruses, echo-
viruses, and Coxsackie viruses. Most infections are
asymptomatic, but enteroviruses can cause a variety
of symptoms, including paralysis, meningitis, cardio-
myopathy, and less severe conditions such as colds
and fever. Most enteroviruses grow readily in cell
cultures of human and primate origin. An outbreak
of poliomyelitis associated with raw milk in 1914 is
believed to be the first recorded foodborne outbreak
of viral illness. Raw milk was implicated in a further
10 outbreaks in the UK and USA by 1949. Milkborne
outbreaks of poliomyelitis then appeared to cease in
developed countries, even before the introduction of
polio vaccine. This could largely be attributed to the
widespread use of pasteurization. Various entero-
viruses have been isolated from foods such as meat
and shellfish, but there is no clear epidemiological
evidence to suggest that foodborne infection is
occurring.
Tick-borne Encephalitis
0022A further mode of foodborne transmission of viruses
is by zoonotic infection. Cases of tick-borne enceph-
alitis arising from consumption of raw milk from
infected goats, sheep, and cattle or products made
from unpasteurized milk have occurred in Eastern
Europe. The incidence is fortunately rare, owing to
the limited distribution of the appropriate species of
ticks.
Entry of Viruses to the Food Chain
0023Foods may become contaminated with viruses in two
main ways. Food may be contaminated at source,
usually by coming into contact with sewage-polluted
water: this is known as primary contamination. Add-
itionally, food may be contaminated during prepar-
ation and serving by infected food handlers, in just
the same way as some types of foodborne bacterial
contamination can arise. This is known as secondary
contamination.
Contamination at Source
0024Shellfish The most clearly implicated foods in the
transmission of viruses are bivalve molluscs (oysters,
clams, cockles, and mussels). Indeed, most illness
associated with this type of shellfish is viral. These
shellfish are harvested from shallow estuarine and
coastal waters, which are frequently polluted with
sewage. They feed by filtering particulate matter
from the large volumes of water passing over their
gills, and this can include potentially pathogenic
microorganisms. Although human viruses do not rep-
licate in shellfish, they can be concentrated within
molluscs to higher concentrations than occur within
the surrounding water up to 100-fold. Bivalve mol-
luscs are not usually thoroughly cooked before being
eaten, and oysters are frequently consumed raw.
0025Fruit and Vegetables There is the potential for
fruits, vegetables, and salad items to become contam-
inated during irrigation with polluted water and by
fertilization with untreated or inadequately treated
sewage sludge. Many vegetables are cooked before
VIRUSES 6007
