Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

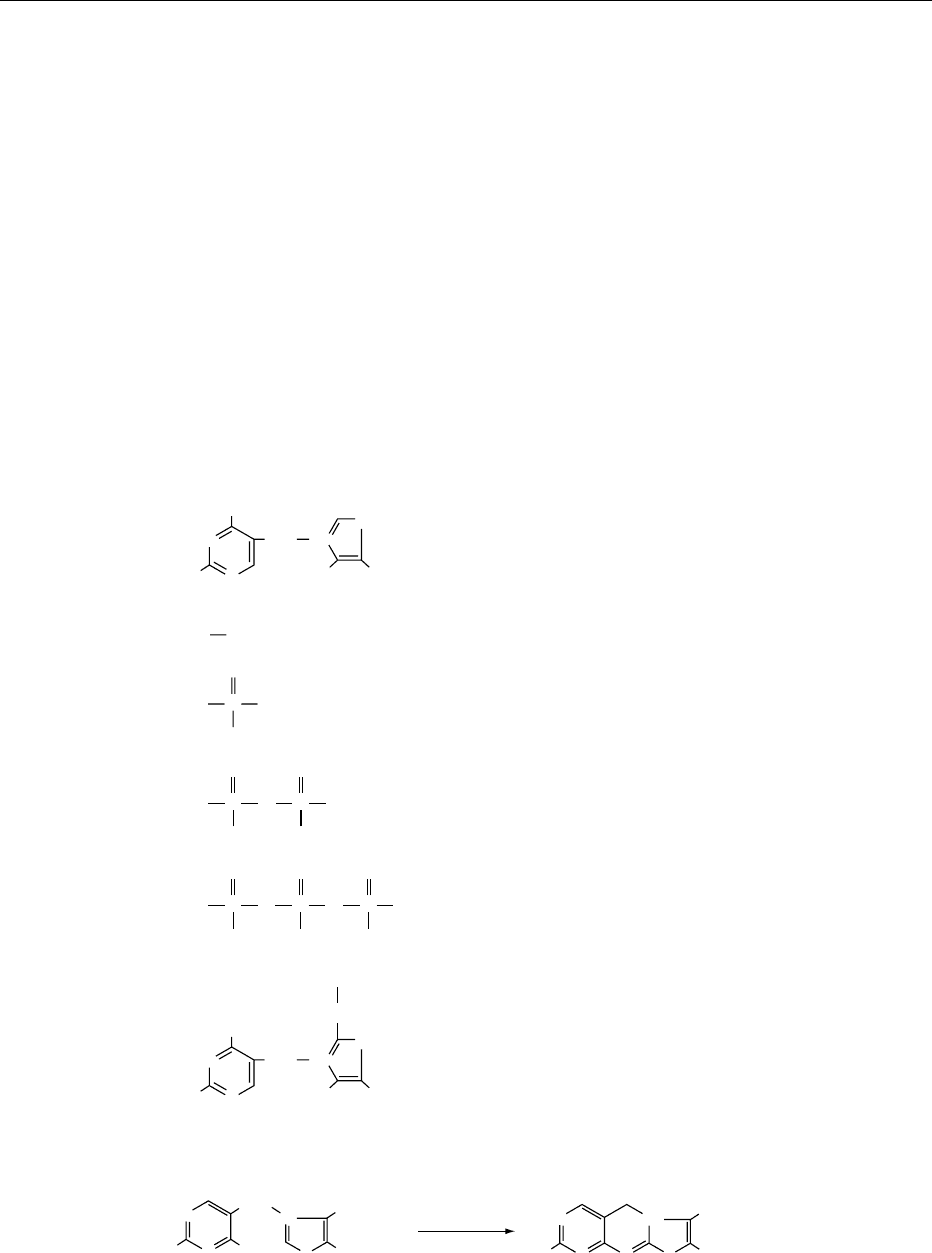
0006 Thiamin is readily split into the pyrimidine and
thiazole moieties by sulfite treatment at pH 6.0 or
above.
0007 In strong alkaline solution thiamin is oxidized by
oxidants (potassium ferricyanide, cyanogen bromide,
mercuric chloride, and others) to thiochrome (Figure
1). Thiochrome is a highly fluorescent compound
above pH 8, and thiamin phosphate esters are also
quantitatively converted to thiochrome phosphate
esters without affecting the phosphate bonds. All
these compounds have identical excitation maxima,
at 375 nm, and very similar fluorescence maxima, at
432–435 nm. This is the principle for the most
common and sensitive assay procedures for thiamin
as well as its phosphate esters.
0008 Hydroxyethylthiamin (Figure 1) exists in living
organisms in the form of hydroxyethyl-TDP. It is
converted to the corresponding thiochrome deri-
vative by alkaline ferricyanide oxidation but not
by cyanogen bromide oxidation. This difference
in the oxidation property is used for the assay of
hydroxyethylthiamin.
0009In aqueous solution below pH 5, thiamin is quite
stable to heat or even sterilization at 110
C. At a pH
of 5.5 or higher, it is rapidly destroyed by autoclaving,
and at pH 7 or higher by boiling or even storing at
room temperature.
0010In solution, TDP is unstable and partially decom-
poses to TMP and/or thiamin when stored for several
months at pH 5 and 37
C, but stable at pH 2–5 and
0
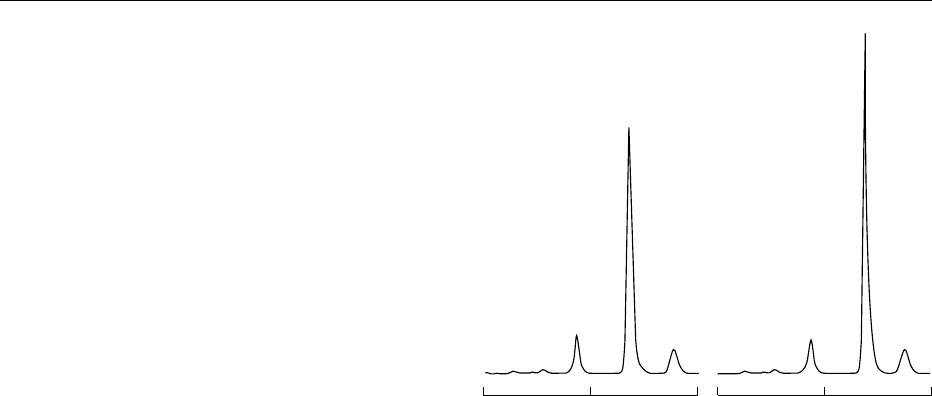
C. TTP in aqueous solution is stable for at least 6
months when stored at 80
C. In 0.1 mol l
1
hydro-
chloric acid, TTP is decomposed quantitatively to
TMP by boiling for 7 min at 100
C, indicating
N
N
H Thiamin
Thiamin
Alkali
oxidation
TMP
O
OHP
OH
R:
:
O
OP
OH
:
O
OHP
OH
CHOH
CH
3
O
OP
OH
O
OP
OH
:
O
OHP
OH
TPP
TTP
Hydroxyethylthiamin
S
NH
2
CH
2
CH
2
CH
2
OR
H
3
C
H
3
C
N
+
N
N
S
NH
2
CH
2
CH
2
CH
2
OH
H
3
C
H
3
C
N
+
S
CH
2
CH
2
OH
N
N
CH
2
NH
2
CH
3
S
Thiochrome
CH
2
CH
2
OH
CH
3
H
3
C
N
NN
N
H
3
C
N
+
fig0001 Figure 1 Structures of thiamin (vitamin B
1
) and its related compounds.
5768 THIAMIN/Properties and Determination
the nature of the high-energy bond of its g- and b-
phosphates.
0011 Thiamin-destroying or inactivating enzymes have
been discovered in a variety of foods. Two thiami-
nases are involved. Thiaminase I catalyzes a base
exchange between the thiazole moiety and another
base and is found in fresh fish, shellfish, ferns, and
some bacteria. Thiaminase II hydrolyzes thiamin to
its pyrimidine and thiazole moieties and is found in
certain bacteria.
0012 In addition to thiaminases, certain plants have been
shown to contain compounds which react with thia-
min in vitro to produce thiochrome-negative prod-
ucts. One such product is thiamin disulfide. Such
compounds in foods, which result in so-called thia-
min inactivation, include caffeic acid, tannic acid, a
variety of polyphenols, and some flavonoids (querce-
tin and rutin). The exact mechanisms of the inactiva-
tion reaction have not yet been elucidated. It appears
that oxidative processes are involved, since the pres-
ence of oxygen increases the amount of thiamin
inactivated.
0013 It has been discovered that the treatment of thiamin
with an extract of garlic containing allicin forms thia-
min allyl disulfide, which is thiochrome-negative, but
very active biologically. A variety of thiamin alkyl
disulfides has since been synthesized and studied,
and is now available commercially. These forms of
thiamin are more fat-soluble and are therefore more
rapidly absorbed than thiamin hydrochloride, and
result in higher blood thiamin levels when therapeut-
ically administered.
Occurrence in Foods
0014 In most animal tissues, over 90% of the thiamin
occurs in TMP, TDP, and TTP. The predominant
form (80–85%) is TDP, the active coenzyme form.
The exceptions are pig skeletal muscle and chicken
skeletal white muscle, in which TTP exists in 70–80%
of total thiamin (Figure 2). The most abundant form
in plant tissues is free thiamin.
0015 The content of thiamin in foods is relatively low.
Practically no thiamin is contained in high-fat prod-
ucts (e.g., vegetable oil) and refined products (e.g.,
sugar). It is also relatively low in green vegetables,
fruits, and seafoods. The thiamin content in a large
variety of natural and processed foods has been listed
in publications from the US Department of Agricul-
ture (USA), Medical Research Council (UK) and Re-
sources Council, Science and Technology Agency
(Japan).
0016 Table 2 lists the expected concentration in thiamin-
rich foods. Refer to individual foods.
0017In addition, average thiamin contents (m g per
100 g) in the following categorized groups of foods
are as follows: dried beans, 680; nuts, 560; whole-
grain cereals, 370; organ meats (liver, heart, kidney),
100; leaf and stem vegetables, 70; milk, 40; fruits, 30;
pork muscle, 600–800; common white fish (cod,
flounder, haddock, halibut), 50–90; hen’s whole egg,
170; egg yolk, 500. Interesting foods of high thiamin
content (mg per 100 g) are dried brewers’ yeast
(1800), wheat germ (2000), and tampala (a spinach-
like vegetable) leaves (1600).
Losses During Food Processing and
Storage
0018Factors affecting the survival of thiamin in the final
food products after harvesting, handling, and pro-
cessing the foods include pH, temperature, solubility,
oxidation, and radiation. Among these factors, the
effect of processing on the thiamin content has been
extensively reviewed by several authors. A brief sum-
mary of the findings is given here.
0019Thiamin losses in dehydrated products, such as
cereal grains, are very low if the materials are kept
dry, and thiamin undergoes only limited destruct-
ion in the commercial refrigeration of meats and
0
12
3
4
5
5
0
Retention time (min)
(a) (b)
5
fig0002Figure 2 A typical chromatogram of fresh pig skeletal muscle
extract by precolumn derivatization high-performance liquid
chromatography (HPLC). (a) Skeletal muscle extract; (b) as (a),
plus 1 pmol of authentic thiamin triphosphate (TTP) which was
converted into thiochrome triphosphate and then added to the
oxidized sample. The recovery rate of added TTP was 100%. 1,
Thiamin; 2, thiamin monophosphate (TMP); 3, thiamin diphos-
phate (TDP); 4, TTP; 5, unknown, but possibly thiamin tetra-
phosphate.
THIAMIN/Properties and Determination 5769

vegetables. Foods can be stored frozen for long
periods without significant loss of thiamin, but
losses are significant on thawing. Losses from frozen
muscle meats are 20–40% during storage for 2–8
months.
0020 On cooking and canning of meats, losses of thia-
min (25–85%) strongly depend on the processing
stresses encountered and type of meat product.
Cooking by microwaves leads to much lower losses.
Losses on cooking of vegetables vary from 0% to
60%. Vigorous boiling or other types of agitation
result in greater losses of thiamin than simmering at
the same temperature or pressure and steam cooking.
Losses are caused by degradation and by leaching.
(See Canning: Quality Changes During Canning;
Cooking: Domestic Techniques.)
0021Roasting of beef and pork involves thiamin losses
of 36–53%. Ionizing radiation used during steriliza-
tion destroys 53–88% of thiamin in meats, yet, in
certain poultry, fish, and vegetables, losses are only
5–37%. Baking of bread leads to losses of 5–35%. In
the processing of milk, losses of thiamin are 9–20%
by pasteurization, 30–50% by sterilization, 10–15%
by drying, and 40% by condensing and subsequent
canning. (See Irradiation of Foods: Applications.)
0022The milling of cereal grains, wheat flour extraction
of less than 85%, and the degerming of corn cause a
marked drop in thiamin. Parboiling of rice and drying
before milling conserve the majority of the thiamin.
(See Milling: Characteristics of Milled Products.)
Uses in Food Fortification
0023Thiamin hydrochloride is the most common additive.
(See Food Fortification.)
0024Thiamin (125 mg per 100 g) is added to polished
rice and (1.5 mg per 100 g) to pressed barley in Japan,
mostly in special cases, such as for use in school
lunches.
0025The recommended daily allowance of thiamin is
0.5 mg per 1000 kcal. The continuing need for thia-
min fortification of processed cereals has been pro-
posed in the UK (1986), in order to meet the adult
thiamin requirement, which has been determined bio-
chemically.
0026Human thiamin deficiency, called beriberi, has
been largely eliminated in those countries where for-
tification of staple foods (rice, cereal, dairy products)
is practiced, but it is still prevalent in those countries
where unfortified foods are used. However, even in
western countries it is becoming evident that subclin-
ical thiamin deficiency is more widespread than pre-
viously realized, especially among infants and the
elderly, and other groups who consume snacks and
highly refined, processed foods. Thiamin fortification
of rice, dairy products and cereals is also recom-
mended for these ’at-risk’ groups and for athletes
whose metabolic demands are greater.
Analytical Procedures
0027Alkaline oxidation of thiamin by either ferricyanide
or cyanogen bromide gives thiochrome, an intense
blue fluorescence compound. This principle is the
basis for the official standard method of the Associ-
ation of Official Analytical Chemists (AOAC). How-
ever, high-performance liquid chromatography
(HPLC) is most frequently used at the present time
for the assay of thiamin in foods. Microbiological
tbl0002 Table 2 Top sources of thiamin
To p s o u rc e s T hiami n
(mg100 g
1
)
Yeast, brewers’, debittered 15.61
Yeast, torula 14.01
Yeast, brewers’ 12.12
Sunflower seed, flour, partially defatted 3.60
Yeast, bakers’, dry 2.33
Rice bran 2.26
Wheat–soy blend (WSB)/straight grade,
wheat flour
2.02
Wheat germ 2.02
Sunflower seed kernels, dry, hulled 1.96
Rice polish 1.84
Wheat germ, toasted 1.65
Wheat–soy blend (WSB)/bulgur, flour 1.49
Pinenuts, pin
˜
on 1.28
Coriander leaf, dried 1.25
Cottonseed flour 1.21
Cornflakes, with added nutrients 1.20
Peanuts, raw with skins 1.14
Pork, fresh, loin, lean, boiled 1.13
Safflower seed, flour, partially defatted 1.12
Soybean flour, defatted 1.09
Alfalfa seeds 1.08
Sesame seed 0.98
Bacon, Canadian, broiled or fried, drained 0.92
Sausage, links or bulk, cooked 0.79
Wheat bran 0.72
Kidneys, beef, braised 0.67
Wheat flour, all-purpose, enriched 0.64
Rye flour, dark 0.61
Nuts, mixed, shelled 0.59
Wheat flour, whole (from hard wheats) 0.55
Pork, cured, canned, ham 0.53
Cornmeal, degermed, enriched 0.44
Rice, white, enriched, raw 0.44
Soybean sprouts, cooked 0.42
Bread, white, enriched 0.40
Peas, green, immature, boiled, drained 0.28
Turkey, hamloaf 0.27
Beef liver 0.25
Luncheon meat, salami, cooked 0.25
From Ensminger AH (ed.) (1994) Foods and Nutrition Encyclopedia, 2nd edn,
pp. 2102–2108. Boca Raton: CRC Press.
5770 THIAMIN/Properties and Determination

assays are still also widely used for both foods
and animal feeds. (See Chromatography: High-
performance Liquid Chromatography.)
0028 An outline of the manual fluorimetric AOAC
method follows. Thiamin in foods is protein-bound
in both animal and plant tissues, although it occurs
principally as the diphosphate in the former and as
the free form in the latter. It is therefore necessary to
extract thiamin and its phosphates from food pro-
teins.
0029 This is carried out by boiling the finely ground or
homogenized sample in 0.1 mol l
1
hydrochloric acid
or 0.1 mol l
1
sulfuric acid. The extract is then neu-
tralized to pH 4–4.5 and treated with an enzyme
preparation exhibiting phosphatase activity (Taka-
diastase, Mylase P, or Clarase). After filtration or
centrifugation, free thiamin thus obtained is purified
through a column of cation exchange-type silica (Per-
mutit T, Decalso F, or Zepolite S/E). After washing the
column several times with almost boiling water,
the thiamin is eluted with almost boiling acid potas-
sium chloride solution.
0030 An aliquot of the above eluate is oxidized by alka-
line ferricyanide or alkaline cyanogen bromide solu-
tion and another aliquot is mixed with an alkali
solution (blank). Thiochrome thus obtained is ex-
tracted into isobutanol. Thiochrome fluorescence in-
tensity is measured spectrofluormetrically at an
excitation wavelength of 375 nm and an emission
wavelength of 435 nm. The amount of thiamin ex-
tracted is calculated from the standard curve obtained
with thiamin standard solution treated in the same
manner as above. Fortified foods and pharmaceut-
icals can be analysed for thiamine with reduced
sample preparation.
Chromatographic Methods
0031 Recent advances in HPLC techniques allow the deter-
mination of thiamin content in foods more rapidly,
accurately, sensitively, and reproducibly than the
standard AOAC method. The purification step of
the extracts can usually be omitted in the HPLC
procedure. In addition, thiamin and its phosphate
esters can be determined separately by the HPLC
method when the appropriate extraction procedure
is employed. Furthermore, thiamin, riboflavin, pyrid-
oxine, or niacin in foods can be simultaneously
assayed when appropriate detection systems are
used. This procedure has been successfully used for
quantifying these vitamins in a wide range of foods,
including cereal products, raw meat, processed meats,
fruits, and vegetables.
0032 Samples are treated, for example, by the AOAC
method and then subjected to enzymatic hydrolysis.
The filtrate or the supernatant after centrifugation
can be directly analyzed by a reversed-phase column
with ion-pairing chromatography. A wide range of
mobile phases has been employed, but those based
on octane sulfonate (as ion pair) in aqueous methanol
or acetonitrile have proved to be generally successful.
Detection is performed spectrophotometrically at
254 nm, which gives a detection limit on injection of
30 ng (90 pmol) of thiamin. This procedure is suitable
for food containing a relatively large amount of
thiamin, especially for fortified rice or cereals. (See
Spectroscopy: Overview.)
0033An HPLC system equipped with fluorometric de-
tection is suitable to quantify much smaller amounts
of thiamin in foods with a detection limit of 5–
50 fmol thiamin. Both precolumn derivatization and
postcolumn derivatization procedures of thiamin into
thiochrome are used.
0034When the extraction procedure is carried out
with cold trichloroacetic acid or perchloric acid,
thiamin and its phosphate esters are extracted intact
and can be individually measured. Both straight-
phase and reversed-phase columns are used in the
HPLC method. In the former system, thiamin,
TMP, TDP, and TTP are eluted and detected in
that order, and in the latter system the elution
order is reversed. Total thiamin is then calculated
as a sum of thiamin and its phosphates. This pro-
cedure can avoid the enzymatic hydrolysis step.
This HPLC method of determining thiamin and its
phosphates has been successfully used to quantify
the thiamin level not only in animal tissues, but also
in human blood or serum to assess thiamin status in
humans.
0035The chromatographic profile of thiamin and its
phosphates in fresh pig skeletal muscle, obtained by
the precolumn derivatization method, is shown in
Figure 2. The analytical system consisted of LiChro-
sorb NH
2
(150 4.6 mm inner diameter (ID), 5 mm
particle size) as the stationary phase, and acetonitrile–
90 mmol l
1
potassium-phosphate buffer (pH 8.4)
(60:40, v/v) as the mobile phase. Detection was
carried out fluorometrically (excitation, 375 nm;
emission, 430 nm) at room temperature.
Microbiological Methods
0036Microbiological methods are based on the nutritional
requirement of a particular microorganism for the
vitamin in question, in this case thiamin. A nutrient
medium which is complete in all respects except for
thiamin is prepared for the microorganism. The
growth response for standard vitamin solutions is
then compared with that achieved with extracts
of the food sample, and hence the concentration of
THIAMIN/Properties and Determination 5771
thiamin in the sample is calculated. In most instances
it is necessary to extract thiamin from foods under
hydrolytic conditions and it may also be necessary to
release bound forms with an enzymatic digestion, as
in the standard thiochrome method.
0037 Over the years, many microorganisms have been
used in the assay of thiamin. Assays based on the use
of Lactobacillus fermentum and L. viridescens are
now widely employed as these microorganisms re-
spond only to intact thiamin. Measurement of rate
of growth is usually followed by nephelometry or
measurement of turbidity, although production of
acidity may also be monitored.
0038 Microbiological methods are slowly being replaced
by more rapid instrumental techniques, e.g., HPLC.
However, they do provide simple, sensitive analyses,
especially where a high degree of precision is not
required.
See also: Canning: Quality Changes During Canning;
Chromatography: High-performance Liquid
Chromatography; Coenzymes; Cooking: Domestic
Techniques; Food Fortification; Irradiation of Foods:
Applications; Milling: Characteristics of Milled Products;
Spectroscopy: Overview
Further Reading
Budavari S (ed.) (1996) The Merck Index. An Encyclopedia
of Chemicals, Drugs, and Biologicals, 12th edn. Rah-
way. NJ: Merck.
Ensminger AH (ed.) (1994) Foods and Nutrition Encyclo-
pedia, 2nd edn, pp. 2102–2108. Boca Raton: CRC Press.
Fayol V (1997) High-performance liquid chromatography
determination of total thiamin in biological and food
products. In: McCormik DB, Suttie JW and Wagner C
(eds) Methods in Enzymology, vol. 279, pp. 57–66. San
Diego: Academic Press.
Gregory JF III (1996) Vitamins. In: Fennema OR (ed.) Food
Chemistry, 3rd edn, pp. 531–616. New York: Marcel
Dekker.
Helrich K (1990) Official Methods of Analysis of the Asso-
ciation of Official Analytical Chemists, 15th edn,
pp. 1049–1052. Washington: Association of Official
Analytical Chemists.
Kamas E and Harris RS (1988) Nutritional Evaluation of
Food Processing, 3rd edn. New York: Van Nostrand
Reinhold.
Kawasaki T and Egi Y (2000) Vitamin B
1
: thiamin. In:
DeLeenheer A, Lambert W and Van Bocxlaer J (eds)
Modern Chromatographic Analysis of the Vitamins,
3rd edn, pp. 365–389. New York: Marcel Dekker.
Machlin LJ (ed.) (1991) Handbook of Vitamins, 2nd edn.
New York: Marcel Dekker.
Paul AA, Southgate DAT and Buss DH (1986) McCance
and Widdowson’s The Composition of Foods: Supple-
mentary Information. London: Her Majesty’s Stationery
Office.
Resources Council. Science and Technology Agency. Japan
(1998) Standard Tables of Food Composition in Japan,
5th edn. Tokyo: Ministry of Finance Printing Office.
US Department of Agriculture (1995) Composition of
Foods. US Department of Agriculture. Human Nutrition
Information Services, Agriculture Handbook 8. Wash-
ington, DC: US Government Printing Office.
Physiology
R Bitsch, Friedrich-Schiller-University, Jena, Germany
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001The discovery of thiamin or vitamin B
1
as an essential
nutrient has opened a new area in the field of nutri-
tion research in two respects. First, the nomenclature
’vitamin,’ used for the whole class of the later on
discovered essential micronutrients of organic origin,
was derived from a functional group (NH
2
group) of
the molecule. Second, thiamine was the first nutri-
tional factor that could be identified as a curative
agent against a deficiency disease in animals.
0002At the end of the nineteenth century, the Dutch
medical officer Ch. Eijkman discovered the beneficial
effect of rice bran or unpolished rice, respectively, for
the treatment of polyneuritis in chickens resembling
beriberi in man, which had been previously induced
by feeding polished rice. In 1911, the German-Polish
chemist C. Funk isolated a crystalline substance from
rice bran and regarded the amino function as the
essential principle of the molecule. The exact chem-
ical structure of the thiamin molecule was elucidated
by R. Williams and A. Windaus. Because of the sulfur
content of the molecule or its thiazol moiety com-
bined with the amine function, the compound was
designated as thiamin instead of the former name
aneurin. Nevertheless, the classical form of thiamin
deficiency, beriberi, mainly affects human beings and
has been well known in the rice-eating countries of
East Asia for centuries. Even 25 years after the ingeni-
ous work of Eijkman, who clarified the cause of this
deleterious disease, the mortality rate from beriberi in
industrialized countries such as Japan was still more
than 25 000 per year.
Physiological Function
0003Like other water-soluble B-vitamins, thiamin is in its
primary function coenzymatically active. The thia-
min-dependent enzymes are involved in carbohydrate
and energy metabolism (Figure 1). Key enzymes in
5772 THIAMIN/Physiology
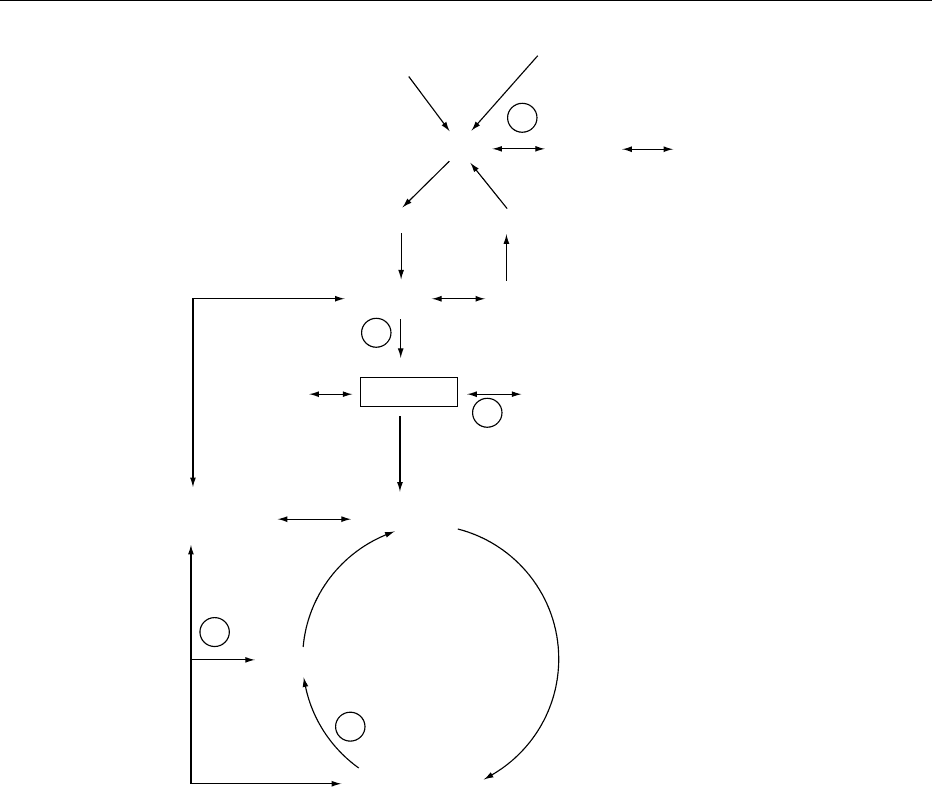
these metabolic pathways are mitochondrial pyruvate
dehydrogenase (EC 1.2.4.1) linking glycolysis with
the citric acid cycle, a-ketoglutarate dehydrogenase
(KDH) (EC 1.2.4.2) within the citric acid cycle, and
the cytosolic transketolase (EC 2.2.1.1) in the pentose
phosphate shunt. Hence, it follows that the energy
turnover of the organism is related to thiamin
demand. As dietary carbohydrates are the predomin-
ant fuel for the rapid metabolizable energy of the
organism, the thiamin requirement is often related
merely to the carbohydrates ingested. KDH was iden-
tified as the rate-limiting enzyme of the cerebral glu-
cose utilization. A low enzyme activity caused by a
reduced intracellular TDP concentration results in
lactacidosis, cerebral dysfunction of the energy me-
tabolism, and neuronal cell degeneration. Additional
thiamin-dependent enzymes are involved in the
metabolism of branched-chain amino acids, e.g., leu-
cin, isoleucin, and vallin. All enzyme reactions are
characterized by transfer of an ’active aldehyde’
from a-keto acids formed after previous decarboxyla-
tion to coenzyme A or an aldopentose.
0004The coenzymatically active form is thiamin diphos-
phate (TDP), also called thiamin pyrophosphate
(TPP) or cocarboxylase, which is formed in the muco-
sal cell of the intestinal barrier with the aid of thiamin
pyrophosphokinase (EC 2.7.6.2) in the presence of
ATP. The latter enzyme is also found in mammalian
liver and other tissues such as brain, nerves, and heart
of mammals and birds. Further phosphorylated forms
of thiamin, which are found in varying quantities in
tissue, are thiamin monophosphate (TMP) and thia-
min triphosphate (TTP). These derivatives are synthe-
sized and metabolized according to the scheme in
Figure 2.
0005Thiamin phosphorylation represents a partitioning
process in a deeper compartment. Cell membranes
are impermeable for the intracellularly active
Dietary
carbohydrates
Glycogen
Glucose
Pentoses Nucleic acids
B
1
B
1
B
1
B
1
B
1
Glycolysis
Gluconeogenesis
Pyruvate Lactate
Amino acids
(ketogenic)
Acetyl-CoA
Fatty acids
Amino acids
(glucogenic)
Oxalacetate
Succinyl-CoA
α-ketoglutarate
fig0001 Figure 1 Metabolic pathways involving thiamin.
THIAMIN/Physiology 5773
coenzyme TDP, which is released only after hydroly-
sis via TMP to thiamin. The total thiamin concen-
tration of the blood cells as well as of the
cerebrospinal fluid (CSF) has a nonlinear relation to
the corresponding plasma concentrations. A linear
increase in blood cells and CSF has been observed
only with thiamin plasma levels above 10 nM
(3 mgl
1
). Otherwise, with extremely low plasma
and CSF concentrations, a disproportionately high
TDP content of erythrocytes and TMP content of
the CSF is maintained for a longer period. Thus, the
intracellular phosphorylation should be seen as a pro-
tecting mechanism, inhibiting rapid thiamin deple-
tion by decreasing the membrane permeability.
0006 After TDP, TTP is the next most phosphorylated
metabolite, albeit the steady state concentrations in
tissues are rather low. No coenzymatic function has
been found for this metabolite. Several investigators
have demonstrated in a variety of animals (rats,
rabbits, cows, frogs, pigs) that TTP, together with
a distinct part of TDP, is neurophysiologically active
in the central and peripheral nervous system inde-
pendent of the coenzyme function of the latter. After
electrical stimulation of nerves or treatment with
neuroactive drugs, e.g., acetylcholine, tetrodotoxin,
or LSD, a release of the phosphorylated esters TDP
and TTP concomitant with a shift to TMP and free
thiamin has been observed. Furthermore, in patients
with the deleterious ’subacute necrotizing encephalo-
pathia,’ or Leigh’s disease, the TTP level in the brain
was low and tended toward zero. It has been known
for a long time that severe thiamin depletion of the
mammalian central nervous system leads to encepha-
lopathias, which are accompanied by regionally se-
lective changes in neurotransmitter function. Though
the exact function of TTP is not yet known, it plays an
essential role in nerve excitation and transmission,
possibly in gating the ion transport. From recent in-
vestigations, it has been argued that TDP might also
be involved in the inhibition of nonenzymatic glyco-
sylation processes. It is suggested that the emerging
advanced glycosylation end products (AGE) are the
reason for the segmental demyelinization observed
in neurodegenerative diseases. Studies on cultivated
cells have demonstrated that thiamin and TDP could
prevent the AGE formation. In this regard, TDP is
more effective than the standard inhibitor aminogua-
nidine that is normally used.
Absorption, Storage, and Excretion
0007Thiamin is absorbed from the gastrointestinal tract
by a dual system, as has been shown for humans
and rats. At low concentrations, it follows energy-
dependent active transport with a saturation kinetics
up to a maximal concentration of 2 mM. At higher
concentrations, a simple diffusion process is predom-
inant. However, the relative absorption decreases
with increasing vitamin doses, so that with gram
doses, only about 5% are incorporated.
0008A close association was assumed between thiamin
transport and phosphorylation as well as Na
þ
de-
pendency. However, the coupling of thiamin trans-
port with phosphorylation is now doubtful, and
there is much evidence of a specific protein carrier
being involved in the absorption, but the following
intracellular phosphorylation seems to be a rate-
limiting step in the active transport of thiamin across
the epithelial cells of intestine. In contrast, in the
guinea-pig intestine, thiamin seems to cross the
brush-border membrane by simple diffusion through-
out the concentration range from 0.06 to 10 mM.
0009The jejunum and ileum have been found to be the
main sites of thiamin absorption in rats with a max-
imal rate in the proximal 22 cm of the small intestine.
This specificity seems to resemble that of other
animals as well as humans.
0010After uptake from the intestinal tract, thiamin
is distributed rapidly into the organs and tissues
according to the requirements. In humans and
animals, the heart contains the highest thiamin
concentration (3–8 mgg
1
fresh weight), followed by
the kidney (2.4–6 mgg
1
fresh weight) and liver (2–
7.5 mgg
1
fresh weight), and the brain (1.4–4.4
mgg
1
fresh weight). Lesser amounts are found in
other tissues. Whole blood contains 0.05–0.12 mg
of thiamin per milliliter, about 90% of this being
found in the corpuscular constituents. The total
storage capacity of man is estimated to be 25–30 mg
of thiamin, 40% of this being in muscles. Inter-
estingly, pig muscle has a relatively high thiamin con-
tent (8–12 mgg
1
) because of feeding conditions,
higher than that in muscles of other mammals
(1–2 mgg
1
) and pig liver (2–4 mgg
1
). The thiamin
contents of several plant and animal foods are given
in Table 1.
ATP AMP ATP ADP
TDP
TTP
TMP
Thiamin (T)
P
P
P
fig0002 Figure 2 Interconversion of thiamin phosphates (according to
Rindi, modified).
5774 THIAMIN/Physiology
0011 As with other water-solublevitamins, thiaminexcre-
tion is dependent on intake. Within the physiological
range, most of it is excreted by urine. Besides free
thiamin and small amounts of TMP, numerous metab-
olites can be detected as minor excretion products such
as thiazol, pyramin, and methylthiazol acetic acid.
0012 After ingestion of therapeutic dosages, the amount
of thiamin excreted via bile increases proportionally,
as does the unabsorbed part in feces.
Lipid-soluble Thiamin Derivatives
0013Under relatively mild conditions (e.g., in the gastro-
intestinal lumen) thiamin reacts with allicin, the
active principle of garlic and onions, to form a lipid-
soluble compound, called allithiamin, that is able to
penetrate through membranes more easily than the
water-soluble salts (Figure 3). Additional S-alkyl de-
rivatives have been identified in cruciferous plants,
and several lipid-soluble derivatives have been
synthesized with a better stability than that of the
spontaneously formed allithiamine (S-benzoyl-, S-
tetrahydrofurfuryl analogs being the most important).
0014Oral administration of these lipid-soluble analogs
results in higher thiamin blood levels than with equi-
molar thiamin doses, because the allithiamins from
the gut lumen are preferentially absorbed by passive
diffusion and rapidly converted to the active vitamin
by reducing SH-compounds. These allithiamins are
because of their improved bioavailability suitable
transfer substances with which to establish high
thiamin levels in target tissues comparable with drug
targeting. The elevated blood level results in an
improved vitamin status, as measured by enzymatic
criteria.
Thiamin Antagonists
Synthetic Antivitamins
0015Like most of the B vitamins, thiamin has a rather high
structural specificity, i.e., minor modifications of
the molecule lead to a decrease or a total loss of
Thiaminium ion
Ethylthiamin
N-propythiamin
Oxythiamin
Pyrithiamin
Amprolium
Allithiamin
Ethyl
N-propyl
N-propyl
Methyl
Methyl
Methyl
Methyl
R
1
R
1
R
2
R
2
R
3
R
3
X
Amino
Amino
Amino
Hydroxy
Amino
Amino
Amino
Ethanol
Ethanol
Ethanol
Ethanol
Ethanol
Hydrogen
Ethanol
Sulfur
Sulfur
Sulfur
Sulfur
Ethene
Ethene
S-alkyl
(thiazol oxidatively
splitted at C
2
)
N
N
CH
2
CH
3
2
⬘
6
⬘
5
⬘
4
⬘
N
+
4
2
5
X
fig0003 Figure 3 Thiamin derivatives and antagonists.
tbl0001 Table 1 Thiamin content of plant and animal foods (mg per
100 g fresh weight)
a
Content Foods
1.0–2.0 Whole wheat and rye Plant foods
0.9 Para nuts
0.7–0.8 Soy flour
0.6–0.7 White bran
0.3–0.5 Whole cereal products, lentils, oats,
unpolished rice, lima beans, whole
corn meal, cashew nuts, pea nuts,
hazel and walnuts, mung beans
0.1–0.2 Parboiled rice, sorghum, beans and
peas cooked, cauliflower, potatoes,
fennel, broccoli cooked, asparagus,
soy sprouts, mushroom
< 0.1 Fruits, milk products, white flour
products
0.9 Loin pork Animal foods
0.3–0.5 Liver, kidney (calves, beef, pork), duck
0.1–0.2 Lean meat (calves, lamb, beef), eel,
tuna, salmon, mackerel, plaice and
flounder, oysters
a
Modified from Souci SW, Fachmann W and Kraut H (2000) Food
Composition and Nutrition Tables, 6th revised edn. Boca Raton, FL: CRC
Press/Medpharm Scientific.
THIAMIN/Physiology 5775

physiological activity or even to antagonistic effects.
Of the numerous analogs synthesized so far, only
the 2-ethyl- and the 2-n-propyl homologs in the
pyrimidine moiety of the molecule show any
biological activity comparable with that of thiamin.
For example, when the 4-amino group is replaced
by a hydroxyl function, oxythiamin, one of the
most potent thiamin antagonists, is obtained. An-
other prominent antivitamin is pyrithiamin, which
contains an ethylene group instead of sulfur in the
thiazolium ring. Some thiamin antagonists have
been found to exhibit antiprotozoal activity, as in
the case of amprolium, which is effective as a
prophylactic agent for coccidiosis of fowl. Some
of the modified thiamin derivatives are shown in
Figure 3.
0016 The use of antagonists results in rapid and severe
deficiency symptoms in humans and animals. Ampro-
lium is already known to affect the absorption process
already, and this has been suggested as the mechanism
for its anticoccidial action in fowls. Other antivita-
mins appear to function at the conzyme level, i.e., they
bind to the apoenzyme, but inhibit the following en-
zymatic reaction. Nevertheless, there are several dif-
ferences in the action on neurological symptoms. Only
pyrithiamin (and not oxythiamin) can block the
action potential of a single myelinated nerve fiber. It
is also well known that only pyrithiamin produces
polyneuritis. In addition, differences in neurophysio-
logical action are caused by the different abilities of
both antagonists to cross the blood–brain barrier,
which has only been verified in the case of pyrithia-
min. However, it can be shown that the amino group
of the thiamin molecule is essential for the active
transport of the vitamin from the rat small intestine
and the formation of thiamin pyrophosphate.
Thiaminases and other Antithiamin Factors
0017 Two thiamin-cleaving enzymes have been identified,
called thiaminase I and thiaminase II.
0018 Thiaminase I is found in shellfish, clams (but not
oysters), some freshwater fish viscera, crustacea, and
certain ferns, but very few higher plants. Also, certain
species of Bacillus and Clostridium, which are com-
ponents of the human and animal intestinal flora,
have been found to produce this enzyme. The enzyme
catalyzes an exchange reaction, in which the thiazol
moiety of the molecule is displaced by another N-
containing base or a SH-compound. The effect of
this compound was first observed when silver foxes
were fed raw fish waste, resulting in a deleterious
thiamin deficiency disease called Chastek paralysis.
Thiaminase I may also be produced by the rumen
microflora of ruminants or by plants, and, in the
presence of suitable cosubstrates, e.g., niacin, or pyr-
idoxine, and certain antihelmintics, seems to be
responsible for the ruminant CNS disorder, polio-
encephalomalacia.
0019Thiaminase II is of bacterial origin (predominantly
Bacillus, Candida, and Oospora) and breaks down
the free vitamin, but not the thiamin pyrophosphate,
into pyrimidin and thiazol components. More preva-
lent are thermostable thiamin-inactivating factors of
plant origin, e.g., polyphenolic substances such as
flavonoids and catechol derivatives in fermented tea,
ferns, sweet potatoes, and betel nuts, and, in small
quantities, in other leaves, fruits, and roots. These can
decompose the thiazol component of the vitamin,
accelerate the oxidation to the disulfide form, or
form unabsorbable adducts with thiamin. Rats fed
on food high in polyphenolics have been shown to
develop deficiency symptoms as a result of a marked
decrease in levels of cerebral thiamin and thiamin-
dependent enzymes. Likewise in man, high tea con-
sumption leads to a deficient thiamin status, but
ascorbic acid, if present, completely inhibits the thia-
min-inactivating processes.
Status Assessment
0020In terms of biochemical status, urinary vitamin excre-
tion and blood enzymes are suitable criteria. Urinary
thiamin excretion is, as with most B vitamins, largely
dependent on intake and therefore provides an indi-
cation of the recent dietary intake but does not ad-
equately reflect the body stores. At intake levels
below 0.5 mg of thiamin per 1000 kcal per day
(0.1 mg MJ
1
) thiamin excretion varies only slightly,
and the proportion of metabolites in the urine exceeds
the vitamin content itself. Because urinary excretion
follows a diurnal rhythm, 24 h urine or the creatinine
excreted is taken as the basis for status assessment.
Occasionally, the status can be interpreted more ac-
curately by a thiamin load test. After administration
of a 5-mg dose, vitamin excretion is measured for the
following 4 h. A deficient status can be assumed if less
than 20 mg is excreted during this period.
0021Erythrocytic transketolase activity (ETKA), the
rate-limiting enzyme in the pentose phosphate path-
way, and its activation coefficient, a
ETK
are often used
as an indication of functional status as they give a
better idea of body stores than urinary excretion.
According to the saturation deficit of the apoenzyme,
the transketolase activity can be stimulated in vitro by
the addition of TDP. The ratio of stimulated to basal
activity, which increases with the degree of deficiency,
indicated by the activation coefficient (AC) or a
ETK
,is
commonly taken as a prognostic status index. The AC
is better as a comparison of status in groups, because
5776 THIAMIN/Physiology

of the large interindividual variation in basal enzyme
activity. Moreover, it can be assumed that apoenzyme
levels are not affected by the vitamin deficiency.
Nevertheless, in alcoholics, either the apoenzyme syn-
thesis or the linkage to the coenzyme TDP seems to be
impaired, and the transketolase assay may indicate
misleading results about the true status. Furthermore,
confounding factors in the enzymatic assay, irrespect-
ive of the thiamin status, may include certain cancers,
uremic neuropathy, diabetes, and treatment with
certain anticancer drugs (fluorouracil, acytoxin) or
diuretics, such as furosemide.
0022 Recently, in view of improved high-performance
liquid chromatography techniques, the TDP content
in erythrocytes has been suggested to be a better
indicator of the body stores compared with ETKA
and independent of confounding factors. The criteria
of the thiamin status indices are listed in Table 2.
Thiamin Requirement
0023 Regarding the essential role of thiamin-dependent
enzymes in carbohydrate and energy metabolism,
the requirement for this vitamin is usually related to
energy intake. Irrespective of the catalytic function of
enzymes emerging basically unchanged from a bio-
chemical or metabolic reaction, controlled studies in
man have demonstrated that static as well as func-
tional status criteria (transketolase activity and urin-
ary excretion) deteriorate with increasing energy
uptake even when the thiamin supply remains un-
changed, thus indicating an increased vitamin turn-
over. The dependence on energy metabolism seems to
be particularly evident in a marginal vitamin status.
0024 Previously dietary fat was postulated to have a
’sparing effect’ on thiamin requirements. This effect
is, however, comparatively small, even with major
increases in fat in the diet. Furthermore, in a starva-
tion or semistarvation status when the vitamin intake
is zero and increasing amounts of fatty acids are
metabolized to satisfy the body’s energy needs, the
tissue stores of thiamin are rapidly depleted.
0025International committees recommend safe levels
of intake amounting to 100–130 mg of thiamin per
megajoule, equivalent to 0.4–0.54 mg of B
1
per
1000 kcal. A critical intake point appears to be ap-
proximately 0.05 mg of B
1
per megajoule (0.2 mg per
1000 kcal), below which urinary excretion is low, and
the first clinical signs of deficiency may appear. A
minimum intake of 1 mg per day is recommended to
provide an adequate margin, even for those consum-
ing less than 8 MJ (2000 kcal) daily. The additional
values for pregnancy and lactation mostly exceed the
calculated additional requirement of pregnant and
lactating women. These extra values insure optimal
pre- and postnatal development. In the past, clinical
deficiency symptoms of infants were sporadically ob-
served in spite of an inconspicuous appearance of the
mother (infantile beri beri; see Table 4).
0026Owing to the low storage capacity, regular thiamin
intake is important. Although, within the margin of
physiological requirements, the organism attempts to
prevent considerable thiamin losses by partial tubular
reabsorption and intracellular fixation as TDP, the
biological half-life time of this vitamin is assumed to
be 9–18 days. After this period, when thiamin uptake
is restricted, early nonspecific deficiency symptoms
occur.
0027The recommended daily allowances for several
European countries together with the USA’s dietary
reference intakes are listed in Table 3.
Deficiency Diseases
0028In thiamin deficiency, primarily, organs and tissues,
such as the central and peripheral nerve system and
the heart are affected showing an increased energy
turnover or close carbohydrate dependency. Clinical
signs described in most animals refer to lesions in
these tissues.
0029Since Eijkman’s observations in fowls, the degener-
ation of the peripheral nerves was the first patho-
logical symptom of thiamin deficiency noted in
tbl0002 Table 2 Reference values of thiamin status parameters
a
Deficiency
Indicator Adequate Marginal Severe
Thiamin excretion in urine
(mg per gram of creatinine)
> 66 27–66 < 27
ETKA–AC < 1.15 1.15–1.25 > 1.25
TDP content in erythrocytes
(ng per milliliter of sediment)
> 38.5 29.5–38.5 < 29.5
a
From Finglas PM (1994) Thiamin. International Journal for Vitamin and
Nutrition Research 63: 270–274 and Frank T, Bitsch R, Maiwald J and Stein
G (2000) High thiamine diphosphate concentrations in erythrocytes can be
achieved in dialysis patients by oral administration of benfotiame.
European Journal of Clinical Pharmacology 56: 251–257.
tbl0003Table 3 National RDAs for vitamins
Country B
1
(mg per day)
Poland 1.9–2.0
Belgium, Denmark, Finland, France, Hungary,
Luxemburg, Portugal, Spain
1.4
Greece, Norway 1.1–1.4
Czech Republic, Sweden 1.2
Austria, Germany, Ireland, Switzerland 1.0–1.3
The Netherlands, USA 1.0–1.2
Italy, UK 0.8–1.2
THIAMIN/Physiology 5777
