Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

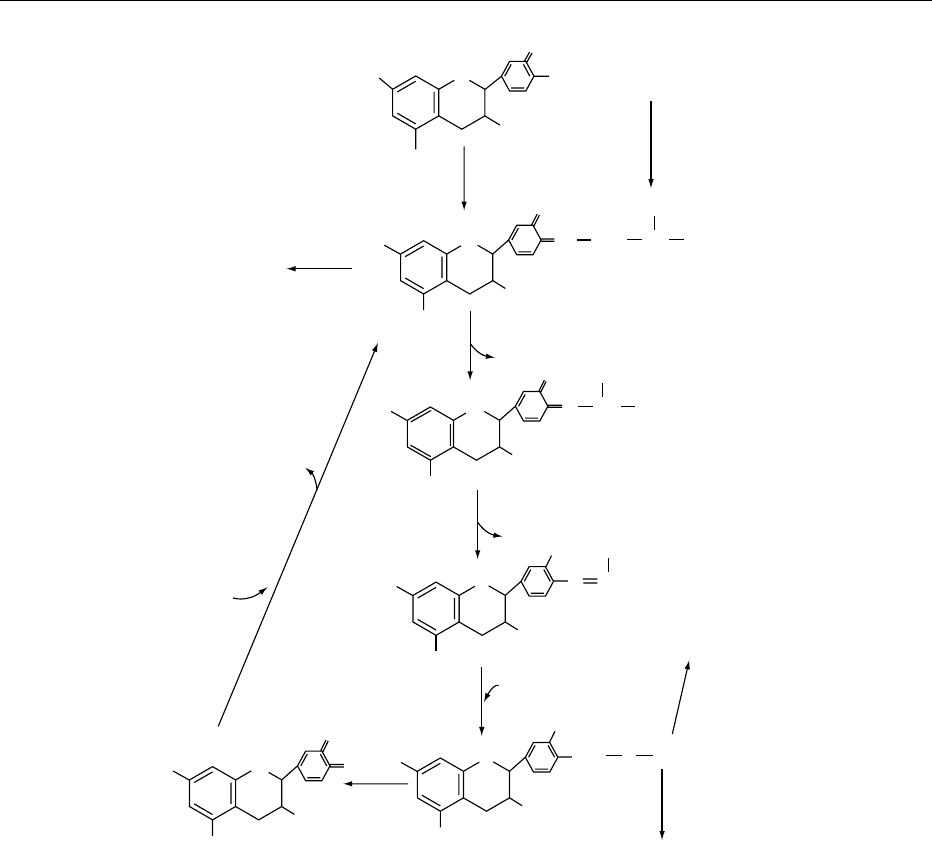
are catalyzed by polyphenol oxidase and/or peroxid-
ase in the presence of oxygen and catechins. Some of
the formed aldehydes are further oxidized to carbox-
ylic acids during firing (as the concentration of acids
is higher in fired teas) or storage, although some of
the aldehydes are certainly reduced to their respective
primary alcohols.
Lipids
0019 Lipids make up between 4 and 9% dry weight of the
fresh tea leaf and are composed mainly of free fatty
acids and fatty acid esters. Linolenic acid is the major
fatty acid in tea, but variations in this observation
have been noted. The fatty acid profile changes with
the geographical area of tea production, agronomic
practices, and variety. The levels of fatty acids change
throughout tea manufacture. During withering, the
fatty acid esters are hydrolyzed to free fatty acids. The
unsaturated free fatty acids degrade to form aroma
compounds, but the fate of saturated fatty acids
during tea processing is unknown.
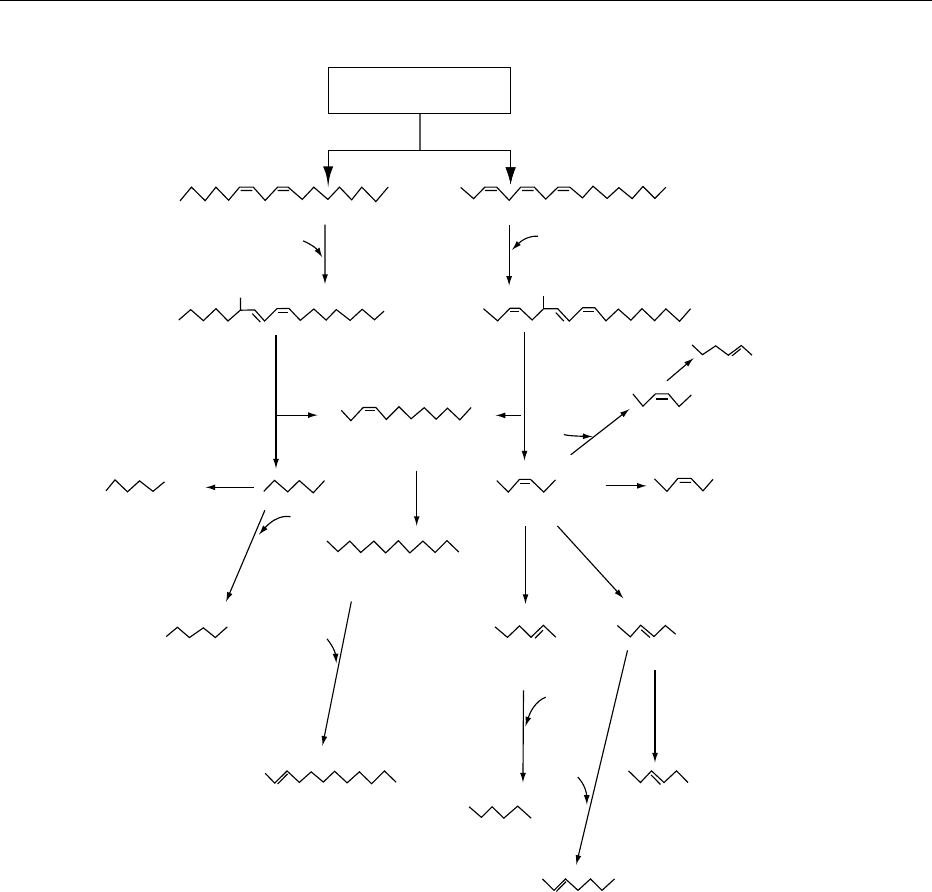
0020The mechanism for the degradation of unsaturated
fatty acids to aroma compounds is outlined in Figure 5.
0021Linoleic and linolenic acids produce hexanal and
E-2-hexenal, respectively, when the acids are added
to tea leaf extracts. Z-3-Hexenal formed from lino-
lenic acid easily isomerizes to E-2-hexenal, and also is
the precursor of Z-3-hexenol in macerated tea leaves.
Alcohol dehydrogenase reduces the aldehydes to
alcohols.
HO
HO
HO
HO
HO
HO
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
NH
O
O
O
NH
2
R
CHO
Peroxidase
or polyphenol
oxidase
Aldehyde
Alcohol
dehydrogenase
RCH
2
OH
alcohol
H
2
O
Schiff base II
Schiff base I
RCO
2
H
Acid
Heat and/or
autooxidation
O
O
O
O
N CH
R
CO
2
OH
H
2
O
−NH
3
O
O
N
R
CH
CO
2
H
Polymerized
polyphenol
−H
2
O
Quinone
H
2
N
CH
R
CO
2
H
Amino acid
Peptidase
Proteins
Polyphenol oxidase
or peroxidase
O
(−)-Epicatechin
fig0004 Figure 4 Formation of aldehydes, alcohols, and carboxylic acids from amino acids.
5748 TEA/Chemistry
0022 The linoleic acid forms 13-hydroperoxy acid,
which is an intermediate in the production of C
6
aldehydes and alcohols in tea leaves. The hydro-
peroxidation of the acid occurs in the presence of
lipoxygenase enzyme in a highly stereospecific
manner forming only l-hydroperoxy acid. Hydroper-
oxide lyase breaks down the 13-hydroperoxide acid
to C
6
aldehyde and 12-oxo-acid. The action of
this enzyme is enentioselective, breaking down only
the l-hydroperoxide acids.
0023 The formation of 9-oxo-nonanoic acid from lino-
lenic acid in tea chloroplasts, by cleavage at C-10,
suggests that Z-3,Z-6-nonadienal, Z-3,Z-6-nonadie-
nol, E-2,Z-6-nonadienal, and E-2,Z-6-nonadienol
may also be derived from linolenic acid via a similar
intermediate. Similarly, cleavage at the C-10 carbon
of linoleic acid might be expected to produce Z-3-
nonenal, E-2-nonenal and E-2-nonenol. However,
only minor amounts of E-2,Z-6-nonadienal and E-2
nonenal have been detected in tea, implying that the
hydroperoxidation of linoleic and linolenic acids
occurs predominantly at the C-13 carbon to produce
the C6 aldehydes and alcohols.
0024Low levels of palmitoleic and oleic acids have been
detected in fresh tea leaves. These fatty acids break
down to form heptanal and heptanol, nonanal, and
nonanol, respectively, during tea processing. The re-
lationship between precursor fatty acid in fresh leaf
Free falty acids
and lipids
Lipolytic acylhydrolase
CO
2
H
CO
2
H
CO
2
H
CO
2
H
CO
2
H
CO
2
H
CO
2
H
Linolenic acid
Linolenic acid
Lipoxygenase
Lipoxygenase
O
2
O
2
CO
2
H + other free
fatty acids
OOH
OOH
13-Hydroperoxylinolenic acid
13-hydroperoxylinolenic acid
Hydroperoxide lyase
Hydroperoxide
lyase
OHC
E-2-Hexenoic acid
Z-3-hexenoic acid
IF
Pyrolytic
or
autooxidation
Pyrolytic
or
or
autooxidation
Pyrolytic
autooxidation
Pyrolytic
or
autooxidation
Pyrolytic
or
autooxidation
O
2
O
2
O
2
CHO
CHO
CHO
ADH
12-OXO-(9Z )
12-OXO-(10E)
-dodecenoic acid
-dodecenoic acid
CHO
IF
IF
IF
CH
2
OH
ADH
Hexanal
Hexenal
O
2
O
2
OHC
CH
2
OH
CH
2
OHCO
2
H
CO
2
H
Z-3-hexenal
Z-3-hexenal
Diacid
HO
2
C
Hexanoic acid
CO
2
H
ADH
E-2-hexenal
E-2-hexenoic acid
E-3-hexenoic acid
E-3-hexenal
E-3-hexenal
CO
2
H
fig0005 Figure 5 Production of volatile flavor compounds from linoleic and linolenic acids.
TEA/Chemistry 5749

and derived aroma compound in the processed prod-
uct is rarely linear due to the various interactions that
take place during processing. Linolenic acid and 13-
hydroperoxylinolenic acid, for example, inhibit the
formation of n-hexanal from linoleic acid during
tea manufacture. In addition, the aroma compounds
formed have different boiling points, and more of the
lower boiling compounds are lost by volatilization
during processing.
Terpene Glycosides
0025 There has been considerable speculation on the mech-
anism of formation of monoterpene alcohols during
tea manufacture. It was originally thought that lina-
lool was a product of carotene degradation. Later, it
was suggested that terpene alcohols were produced
from oxygenated isoprenoid hydrocarbons. However,
it has now been demonstrated that linalool and
geraniol are hydrolytic breakdown products of b-
d-terpene glycosides during tea manufacture. Indeed,
many alcohols in the tea aroma are products of glyco-
side hydrolysis. In recent studies, several alcohol
glycosides have been isolated from tea leaves. These
include identified glycosides of 2-phenylethanol, all
the four isomers of linalool, geraniol, benzyl alcohol,
nerolidol, etc. These glycosides are hydrolyzed during
tea manufacture to form their respective alcohols.
Pigments
0026 Fresh tea leaves contain appreciable amounts of pig-
ments, mainly chlorophyls and carotenes. Fresh tea
leaves contain about 1.4 mg g
1
dry weight chloro-
phyls a and b. During tea processing, the chlorophyls
degrade to pheophytins and pheophorbides. These
compounds play an important role in giving black
tea its shade of color. A number of breakdown
products from the phytol side-chain contribute to
the aroma complex of tea.
0027 More than 15 carotenoid pigments, dominated
by neoxanthin, violaxanthin, lutein and b-carotene,
have been identified in fresh tea leaf. These caroten-
oid compounds account for about 0.5% dry weight of
tea leaves. The carotenes decrease during tea process-
ing with the resultant production of various aroma
compounds. b-Carotene degrades to b-ionone,
whilst b-ionone, a-ionone, 3-hydroxy-b-ionone, 3-
hydroxy-5,6-epoxyionone, 3,5-dihydroxy-4,5-dihy-
dro-6,7-didehydro-a-ionone, and other terpenoid
aldehydes and ketones are degradation products
of other carotenes present in tea leaves. Dihy-
droactinidiolide, 2,2,6-trimethylcyclohexanone, 5,6-
epoxyionone, 2,2,6-trimethyl-6-hydroxycylohexa-
none, and theaspirone and possibly formed
form the primary oxidation products of carotenes,
i.e., b-ionone. b-Damascenone, a-damascone, b-
damascone, 3-oxo-b-ionone, 1,2-epoxy-1
0
,2
0
dihydro-
b-ionone, loliolide, dehydrovomifoliol, and 3,
7-dimethyl-1,5-octadien-3,7-diol are speculated to be
derived from carotenes via oxidative enzymatic reac-
tions that take place during withering and fermenta-
tion, and pyrolytic reactions during firing. The
mechanisms of these reactions have, however, not
been fully worked out. The formation of these com-
pounds is affected by the amounts of catechins present,
oxidase activity, degree of mixing of the cell contents,
and concentrations of the reactants. These factors
change with degree of wither. Loss of carotenes has
been demonstrated to increase with physical withering
and fermentation process. Further pyrolytic and
photo- and/or autooxidative reactions of carotenes
occur during firing to produce more aroma com-
pounds. The compounds produced from carotenes
have a major effect on the aroma of tea. Flavory teas
are normally produced from green leaf with high car-
otene contents.
0028As research on tea aroma continues, it is inevitable
that more mechanisms and pathways for the forma-
tion of tea aroma compounds will be identified. These
will likely involve nonvolatile precursors, which cur-
rently are largely ignored with respect to tea aroma
and quality. For example, it is known that chlorophyl
degrades to phytol and other products, but the con-
tribution of chlorophyl degradation products to tea
aroma is not known.
0029Considerable research has been directed into deter-
mining how the aroma complex changes with vari-
ations in agronomic, cultural, and manufacturing
practices. Many studies have indicated the changes
that occur in aroma composition by varying one par-
ameter or the other without any attempts to quantify
and classify the contribution of the aroma com-
pounds to quality. Generally, the aroma compounds
can be classified into two groups, i.e., those although
important for the characteristic black tea smell, are
deleterious to black tea quality when present at higher
concentrations (group I compounds), and those that
impart a sweet flowery aroma to tea, the presence of
which is considered to be highly desirable (group II
compounds). The classification of aroma compounds
in group I and group II compounds has been based on
either the odor characteristics or the retention time of
the aroma compounds during gas chromatographic
analysis. The ratio of group II to group I aroma
compounds has been used to classify teas in order of
flavor quality. In other studies, the ratio of terpenoid
to nonterpenoid compounds has been used.
0030Although these ratios provide the basis of a semi-
quantitative method for classifying teas in order of
their aroma quality, the ratios must be used with
5750 TEA/Chemistry

caution as the olfactory perception limits of the
aroma compounds differ widely. Some compounds
may be present at very low levels yet have a large
impact on aroma and vice versa. For example, methyl
epijasmonate has an aroma that is 400 times stronger
than that of methyl jasmonate at the same concen-
tration. In addition, some of the compounds con-
sidered deleterious to black tea quality are important
for green tea quality.
Chemistry of Tea Manufacture
0031 Several tea beverages exist. These beverages include
green teas, several semifermented teas, and black
teas. The chemistry occurring during their processing
varies depending on the desired final product. In
green tea processing, oxidative reactions, discussed
above, are completely discouraged. In black tea
processing, there is more extensive oxidation of the
catechins, other polyphenols, amino acids, and unsat-
urated fatty acids. Fewer oxidative reactions occur in
the processing of the semifermented teas.
Black Tea Processing
Withering
0032 Processing of tea beverages starts as soon as the
leaf is detached (plucked or harvested) from the
plant. The polyphenol oxidase activity decreases,
while the catechins levels vary. ()-Epicatechin,
()-epigallocatechin gallate, and ()-epicatechin
gallate levels decline. This decline is associated
with oxidative transformations. Caffeine levels
rise, while protein levels decline. This decline is
caused by an increase in the activity of proteolytic
enzyme activity, which hydrolyzes the proteins to
amino acids, with a concomitant increase in the
level of free amino acids. Carotenoid compounds
degrade due to photoisomerization to volatile flavor
compounds. Fatty acid esters are hydrolyzed to free
fatty acids that oxidize during fermentation through
a lipoxygenase-initiated reaction to volatile flavor
compounds associated with the green notes in tea.
Terpene and other alcohol glycosides hydrolyze to
simple alcohols that contribute to tea aroma. These
transformations, which continue up to the point at
which the leaf is macerated, are collectively called
’chemical wither.’ Usually, the leaf is subjected to
moisture loss to make it more flaccid, so that ma-
ceration is easy. Moisture loss, the most visible
change in the leaf before maceration, is referred to
as ’physical wither.’ Chemical wither benefits
mostly flavory black teas, as it improves the black
tea aroma and to some extent benefits plain
black tea as it reduces the level of green taste.
Physical wither benefits both flavory and plain
black tea quality. Hard physically withered leaves
are easier to macerate and make more aromatic
black teas. Withering therefore plays an important
role in black tea processing.
Maceration
0033Maceration ruptures the leaf cell structure, exposing
the chemical constituents of the cells, mainly poly-
phenols, oxidative and degradative enzymes, lipids,
amino acids, etc., to oxygen. Most importantly, cate-
chins come into contact with the polyphenol oxidase
enzyme, initiating ’tea oxidation,’ which is errone-
ously referred to as ’fermentation.’ Several methods
are used, but the most common are the orthodox
rolling, crush, tear, and curl (CTC), and Laurie tea
processor (LTP) methods. The method used has a
significant effect on the resultant black tea. There is
less cell matrix destruction with the use of orthodox
rollers than the other two. The orthodox maceration
therefore leads to fewer oxidative reactions, and fer-
mentation is slow. Cell matrix destruction is greater
in the CTC and LTP maceration methods, leading to
more extensive oxidation, but these teas are less aro-
matic, with higher plain tea quality parameters. LTP
manufacture requires a softer physical wither than
CTC processing.
Fermentation
0034Most chemical transformations occur during the fer-
mentation phase of black tea processing. These trans-
formations are responsible for the characteristic taste
and aroma products of black tea. As illustrated in
Figures 2 and 3, the catechins and polyphenol oxidase
form the theaflavins and thearubigins. The amino
acids and fatty acids also oxidize to various volatile
flavor components. For LTP and CTC manufacture,
the process is complete after 60–120 min.
Firing (Drying)
0035Firing is necessary to terminate fermentation and to
dry tea for storage and transport. As a result of the
temperature rise, some reactions are accelerated until
the rise is adequate to denature the enzymes or mois-
ture has been adequately removed to prevent reac-
tions occurring, but a lot of changes occur to give
black tea its character.
0036As a result of firing, the color changes as a result
of the transformation of chlorophyls to pheophytins
and pheophorbides. Some caffeine is lost while the
amount of volatile flavor compounds is reduced. The
volatile flavor compounds that result from various
pyrolytic reactions are formed during firing.
TEA/Chemistry 5751
Sorting
0037 The fired black tea is sorted first by removal of fiber
then by separation into different particle sizes. The
various particle sizes define the various grades. Gen-
erally, although the grades have different chemical
compositions, quality is not solely dictated by grade.
Other Tea
Green and Semifermented Teas
0038 There are fewer chemical transformations in the pro-
cessing of the other teas compared with black teas. In
green tea processing, attempts are made to insure that
there is no oxidation, especially that of the catechins.
The process therefore starts with steaming or roasting
to deactivate polyphenol oxidase activity. The green
teas are made from tea plant varieties with a lower
catechin content than those for black tea, but this
level is sufficient to create astringency. The volatile
components of green tea are basically those of the
primary products.
0039 Partially fermented teas undergo incomplete fer-
mentation. Several types exist. Some are processed
by roasting, whereas others are subjected to high-
temperature rolling.
See also: Amino Acids: Properties and Occurrence;
Caffeine; Carotenoids: Occurrence, Properties, and
Determination; Chlorophyl; Sensory Evaluation:
Aroma; Tannins and Polyphenols
Further Reading
Owuor PO (1992) A comparison of gas chromatographic
volatile profiling methods for assessing the flavour qual-
ity of Kenyan black teas. Journal of the Science of Food
and Agriculture 59: 189–197.
Owuor PO (1995) Results from factory processing and
black tea quality research in Kenya (1982–1994). Tea
16: 62–69.
Robertson A (1992) The chemistry and biochemistry of
black tea production – the non-volatiles. In: Willson
KC and Clifford MN (eds) Tea: Cultivation to Con-
sumption, 1st edn, pp. 555–601. London: Chapman &
Hall.
Robinson JM and Owuor PO (1992) Tea aroma. In: Will-
son KC and Clifford MN (eds) Tea: Cultivation to Con-
sumption, 1st edn, pp. 603–647. London: Chapman &
Hall.
Sakata K, Gou W and Moon JH (1999) Tea chemistry with
special reference to tea aroma precursors. In: Jain NK
(ed.) Global Advances in Tea Science, pp. 693–706. New
Delhi: Aravali Books International.
Takino Y, Imagawa H, Horikawa H and Tanaka A (1964)
Studies on the mechanism of oxidation of tea leaf
catechins – formation of a reddish/orange pigment and
its spectral relationship to some benzotropolone deriva-
tives. Agriculture and Biological Chemistry 28: 64–71.
Yamanishi T (1999) Tea flavour. In: Jain NK (ed.) Global
Advances in Tea Science, pp. 707–722. New Delhi:
Aravali Books.
Processing
P O Owuor, Tea Research Foundation of Kenya,
Kericho, Kenya
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Tea
0001Beverages produced from tea leaves include black tea,
green tea, and various partially fermented teas such as
oolong and pouchong. All of these products are culti-
vated and harvested using similar procedures, but
variations in manufacturing methods determine the
final product. This article deals with tea processing
from cultivation through to packaging.
Cultivation
0002Tea was introduced into many countries of the world
from South-east Asia and grows in climates ranging
from the Mediterranean to the hot humid tropics.
Commercially viable plantations have been estab-
lished between as far north as Turkey and Georgia
(24
N), and as far south as Argentina (27
S), at
altitudes ranging from sea level to 2700 m.
0003Successful commercial cultivation of tea requires
a minimum annual rainfall of about 1400 mm when
irrigation is not carried out. Rainfall needs to be well
distributed with at least 120 mm per month. Pro-
longed drought adversely affects tea growth, and in
such conditions, irrigation is advocated. The inter-
action of soil texture and rainfall distribution is an
important factor to be considered when assessing the
suitability of an area for tea. Tea does not tolerate
water-logged conditions.
0004Ambient temperatures of 12–30
C are considered
ideal for growing tea. Temperatures above 30
C,
accompanied by low humidity, have been shown to
inhibit active growth. The optimum soil temperature
for active growth within the feeder root depth is
20–25
C. Using long-term average yield data from
Kenyan tea estates, situated at altitudes between 1500
and 2250 m, it has been shown that annual tea pro-
duction falls by 200 kg of black tea per hectare
for every 100 m rise in altitude. This has been attrib-
uted to decreases in air and soil temperature. The
tea plant cannot withstand frost conditions. Night
5752 TEA/Processing

temperatures below 6
C require that measures be put
in place to mitigate frost, which could lead to the
wilting of tea leaves.
0005 Tea grows most successfully in acidic soils at pH
values ranging from 4.0 to 5.8. A wide range of soil
types are suitable for cultivation, including old
sedimentary soils derived from gneiss or granite,
soils formed from the wash of old sandstone, alluvial,
peat, and soils derived from volcanic rock.
0006 Propagation is carried out either from stumps gen-
erated from seeds or from cuttings. Propagation from
seeds takes at least 3 years from seed planting to the
production of viable plants capable of transplanting
to the field, whereas plants generated vegetatively
require a minimum of 6 months before they are
ready for transplanting. However, when the plants
are vegetatively produced under very cold conditions,
the period needs to be lengthened. Vegetatively pro-
duced plants have the additional asset that they are
usually of predictable yield and quality. Grafting
plants with other desirable characteristics can further
enhance these attributes.
0007 After transplanting, the young plants are left to
establish for at least 18 months in the field before
commercial plucking (harvesting) can commence.
During this time, the plant is manipulated through a
process described as ’bringing into bearing’ to form a
plucking table. This is achieved by tipping and peg-
ging. The tea is subsequently harvested regularly, with
pruning every 3–5 years, thus maintaining a height at
which the leaves and buds can be plucked easily.
During this time, the tea is treated with regular appli-
cations of fertilizers, particularly nitrogen.
Harvesting
0008 Harvesting (plucking) of tea involves manual or
mechanical removal of the terminal young tender
portions of peripheral shoots. In most countries, the
recommended plucking standard is two leaves and a
bud. However, under commercial production, it is
difficult to select leaves of one plucking standard
exclusively, and it is usual to find varying proportions
of the more mature leaves in the harvested crop. The
quality of the final product deteriorates with increase
in mature leaf content.
0009 After plucking, depending on climatic conditions,
new shoots take between 40 and 100 days from bud
break to develop into pluckable shoots. In general,
the colder or drier the production area, the slower is
the shoot growth. The intervals between plucking
rounds vary accordingly. The appropriate plucking
interval is determined by optimizing between the
extent of new shoot development and the level of
overgrowth of shoots that have been left after the
previous plucking. Hand plucking is the preferred
method of harvesting tea as it enables the selection
of the quality requirements of two leaves and a bud.
Compared with mechanical harvesting, however, hand
plucking is slow. Mechanical harvesting is carried out
in some countries where labor is either expensive or in
short supply. This can be carried out using modified
hedge trimmers, motorized machine pluckers oper-
ated by one or two people, or a self-propelled ma-
chine capable of negotiating the lines of tea bushes.
Black Tea Manufacture
Withering
0010The changes that occur in the green leaf from the time
it is detached from the plant to the time of maceration
or rolling are collectively known as ’withering.’ These
changes can be categorized into physical and
chemical processes and are thought to be important
in the manufacture of tea.
0011The main physical process that occurs during
withering is moisture loss, leading to changes in cell
membrane permeability. These changes are indispens-
able in orthodox black tea manufacture as they pre-
condition the leaf for maceration or rolling. For teas
made by alternate methods of maceration, such as
those using the Legg-cut, the Laurie Tea Processor
(LTP), or Crush Tear and Curl (CTC) (see section on
Maceration), physical withering may not be obliga-
tory. During physical withering, the stomata on the
lower surface of the leaf begin to shut gradually, but
continue to influence the rate at which water is lost.
Air temperature, atmospheric vapor pressure, and air
velocity and direction all affect the rate and degree of
physical wither. The many biochemical changes oc-
curring during withering are referred to as ’chemical
wither.’
0012Several methods of withering have been developed.
Traditionally, withering was carried out on banks of
trays in thin layers so that the largest possible leaf
surface would be exposed to air. Alternatively
withering facilities consisted of open lofts built some
distance from the factory, or on the upper, open
storeys. These methods have largely become obsolete,
as they were inefficient, time-consuming, and incap-
able of coping with increased crop production.
0013In most modern factories, withering is now carried
out in troughs that can hold between 2000 and
3000 kg of green leaf, loaded to a depth of 25–
40 cm. The troughs have dual-direction fans that
either drive air into the withering troughs or draw
air through them. Under low humidity, physical
withering can be achieved using ambient air, even if
the troughs are slightly overloaded. However, at high
TEA/Processing 5753

humidity and/or when the troughs are overloaded
because of a greater quantity of crop, stream-heated
air is necessary to assist physical withering. Excessive
use of heat during the withering process impairs black
tea quality. Chemical withering is time-dependent. It
requires a minimum of 6 h from the time the leaf is
detached from the plant during plucking. Chemical
withering beyond 20 h impairs plain black tea quality.
0014 Despite these improvements, withering still takes
up considerable factory space, and newer methods
continue to be developed. One such method involves
storing the leaf in a holding tank with minimal mois-
ture loss for about 6 h to achieve chemical wither. The
leaf is then spread on withering troughs, or a moving
belt witherer, and the moisture is rapidly reduced
by the use of warm air. This process is known as
’two-stage withering.’ Other methods of withering
used in black tea manufacture include the use of
drum withering, tunnel withering, Tocklai continu-
ous withering machines, Russian withering machines,
and automatic continuous operating installations.
Recent studies indicate that in the unorthodox
methods of producing plain black tea, physical
withering is not essential to produce high-quality
black tea. Some factories are therefore now process-
ing plain black tea after achieving chemical withering
but without physical withering.
0015 Factors that affect withering include the leaf type,
leaf conditions, standard of plucking, thickness of
spread, length of wither, drying capacity of air, and
temperature of air used in drying.
Maceration and/or Rolling
0016 Black teas are often referred to as ’orthodox’ or
’unorthodox’. These descriptions are derived from
the method of leaf distortion employed during
manufacture.
0017 In orthodox manufacture, leaves that have
achieved physical withering are subjected to rolling.
During conventional rolling, the leaf is damaged in
such a way that it becomes twisted, and the semi-
permeable membrane of the leaf is distorted, allowing
the cell juices to be expelled to cover the leaf surface.
This allows the juices to mix with the cellular
enzymes in the presence of oxygen, and the chemical
reactions necessary for fermentation will commence.
The orthodox method of manufacture is particularly
desirable in the processing of flavory black teas, but
this method is slow and requires a large amount of
factory space to process high volumes of leaves.
0018 Although some factories still make tea using the
orthodox (rolling) system, other methods of macer-
ation have been developed and introduced into many
factories. Such methods include Legg-cut, CTC,
Rotorvane, LTP, Triturator, and other miscellaneous
methods. These methods are particularly desirable for
processing plain to medium-quality black teas. They
are generally faster than the orthodox methods and
can handle high volumes of leaves more easily.
0019The CTC method is widely employed and consists
of two engraved metal rollers close together running
in opposite directions. The machines work like a
mangle, with one roller rotating approximately
70 rpm and the other 700 rpm. The leaf is cut, torn,
and rolled in the small gap between the serrated sur-
faces of the rollers. Using this method, leaf distortion
is much greater than with the most efficient orthodox
rollers, fermentation is faster, and liquoring proper-
ties are improved.
0020Another popular method of maceration involves
use of the Rotorvane. The rotor, consisting of seg-
ments, revolves in a cylinder of 15.20 or 37.5 cm
diameter and is fitted with vanes propelling the leaf
towards the discharge against resistors projecting
from the casing. Maceration is achieved by rubbing
and shearing inside the cylinder, and fermentation
occurs simultaneously.
0021The LTP is another successful machine for macer-
ation. This machine resembles a hammer mill and
employs a centrifugal fan to induce and discharge
leaves.
0022Most modern factories use a Rotorvane plus three
CTC machines in series. However, there are factories
that still carry out orthodox manufacture, and a
few use the LTP. In general, orthodox teas have a
superior aroma to CTC or LTP teas. However, CTC
and LTP teas have higher levels of theaflavins and
thearubigins and therefore have more color and are
brighter and brisker than orthodox teas. (See Phenolic
Compounds.)
Fermentation
0023In black tea processing, fermentation is defined as the
chemical transformations occurring as a result of
breakup of the cell membrane due to maceration.
0024Factories manufacturing tea by orthodox methods
generally ferment on tables or trays. In factories that
employ CTC, LTP, and Rotorvane systems, fermenta-
tion is carried out in batches using troughs or trolleys,
or continuously on moving machines. In the case
of batch fermentation, the troughs or trolleys are
connected to an air supply by a duct, which can be
humidified if necessary to reduce the temperature
of the fermenting tea (dhool). A trolley can contain
110–130 kg of macerated leaf. The advantage of a
trolley system is that the temperature of the dhool
can be controlled more precisely than other systems.
However, it has the disadvantage that the timing
5754 TEA/Processing
of fermentation duration is manual and is subject to
error.
0025 A number of systems have now been developed, in
which fermenting is carried out on a continuous fer-
menting machine. In most of these systems, the fer-
menting tea moves on a perforated belt through which
air is passed. The speed of the belt determines the
throughput and fermentation time. In other continu-
ous methods, the dhool is fed into an open-topped,
semicircular tank with two rows of rotating paddles
that push the dhool forward in a mechanism similar
to that of a screw. The speed of the rotation governs
the rate of throughput and duration of fermentation.
The temperature of the dhool is normally controlled
by external fans. The paddles that continuously turn
expose the dhool to air. Although, in most continuous
fermenting systems, temperature control can be diffi-
cult, the timing of fermentation duration is usually
very precise.
0026 The liquor characteristics of the black tea can be
determined by controlling the temperature and lime
of fermentation. Generally, the lower the fermenta-
tion temperature, the better the black tea. Usually, the
ambient air blown through fermenting dhool supplies
adequate oxygen. Fermenting under enriched oxygen
atmosphere therefore does not improve the liquor
quality provided that the fermentation duration is
timed correctly.
Firing (Drying)
0027 Firing of tea is the process that reduces the moisture
content of fermented tea from about 60% to below
4% and renders the product in a form suitable
for storing. Firing terminates fermentation by de-
activating the enzymes by subjecting the dhool to
high temperatures. Inlet temperatures in the driers
usually range from 140 to 98
C with outlet tem-
peratures ranging from 45 to 82
C. During firing,
considerable amounts of volatile aromatic com-
pounds are lost.
0028 Firing can be carried out using conventional driers
in which the dhool is fed on to a perforated moving
belt and is discharged off after the tea is dry. In most
modern factories, fluidized bed driers are used. In
these driers, hot air is blown into the drier, and this
moves the dhool by the process of fluidization. Gen-
erally, fluidized bed driers have a higher throughput
than conventional driers.
Grading and Sorting
0029 Prior to grading, stalks are removed by the use of
electrostatic separators. The process is effective
because of the higher moisture content of stalks as
compared with leaves after emergence from the drier.
0030Grading of leaves is generally carried out using
mechanically oscillated sieves fitted with meshes of
many different sizes. In some machines, the sieves are
arranged in banks of diminishing mesh size, such that
the outfall of the upper sieve falls on to the lower.
0031The products of the various siftings constitute the
different grades (Table 1). The grade specification
is entirely artificial. However, they are generally
recognizable by their appearance; for instance, bro-
ken orange pekoe contains a high proportion of buds,
orange pekoe is characterized by the abundance of
twisted tender stalk, and pekoe and souchong grades
tend to be more compact and dense. In recent years,
the pattern of grading has been altering in the direc-
tion of smaller teas.
0032The sieve standards adopted for regulating grades
differ in the various countries and even in different
districts in the same country. However, there are
efforts to standardize the sieve sizes.
0033Winnowing in one form or another is routinely
employed and, according to the size and density of
the particles, separates fannings and dust, carrying
away the fibrous residue that is of no commercial
value as a grade.
Packaging and Storage
0034Tea is hygroscopic, and if not adequately packed and
stored, it can readily absorb considerable amounts of
moisture, leading to a deterioration in quality.
0035Most tea is transported in bulk, and the common
forms of packaging employed are either tea chests or
multiwalled paper sacks. Tea chests are normally
made out of plywood, lined on the inside with
aluminum foil, which acts as a moisture barrier.
tbl0001Table 1 Black tea grades
Leaf grades
TGFOP Tippy golden flowery orange pekoe
TFOP Tippy flowery orange pekoe
FOP Golden flowery orange pekoe
OP Orange pekoe
FP Flowery pekoe
Broken grades
TGFBOP Tippy golden flowery broken orange pekoe
TGBOP Tippy golden broken orange pekoe
GFBOP Golden flowery broken orange pekoe
TBOP Tippy broken orange pekoe
GBOP Golden orange
FBOP Flowery orange pekoe
BOP Broken orange pekoe
BP Broken pekoe
BPS Broken pekoe souchong
PS Pekoe souchong
S Souchong
BM Broken mixed
BT Broken tea
TEA/Processing 5755

Multiwalled paper sacks have flat hexagonal ends to
facilitate stacking and consist of a minimum of two
plies of Kraft paper with an additional layer of alumi-
num foil on the inside of the sack to prevent moisture
migration. Materials used for the construction of the
sacks should be free from taint or odor. Both sacks
and tea chests are designed to hold 40–60 kg of manu-
factured tea depending on grade.
0036 Retail packaging of tea is wide and varied, but the
common consideration is the requirement to keep out
moisture. Tea should be stored in conditions that
minimize the absorption of moisture, preferably at a
low humidity. If teas are stored for long periods,
however, moisture absorption and subsequent loss
of quality are inevitable. (See Storage Stability: Par-
ameters Affecting Storage Stability.)
Green Tea Manufacture
0037 Green tea is manufactured from fresh leaves that have
not been fermented. There are various methods of
green tea manufacture, but they all depend on stop-
ping enzyme activity in the green leaf. Withering is
not mandatory in green tea manufacture.
0038 In China, green tea manufacture begins by roasting
the leaf in a hot iron pan for a few minutes, followed
by hand rolling on a table. The leaf is then subjected
to two or more further roastings and rollings.
0039 In Japan, the leaf is steamed for 15–20 s in a revolv-
ing cylinder provided with an agitator. The steamed
material is cooled by a fan or by air on a belt conveyor
and then subjected to primary heating and rolling. The
leaf may undergo further heating and drying before
passing through secondary (final) rollers. The green
tea is then dried to a moisture content of about 3–4%.
Partially Fermented Tea Manufacture
0040 Oolong tea is manufactured in a similar manner to
green tea, but with the following variations. The fresh
leaf is withered at room temperature for approxi-
mately 16 h, or at 40
C for 2 h followed by another
4 h at room temperature. In both cases, during the last
4 h, the leaf is rolled by hand for a period of 30 min
every hour. This is followed by roasting (parching or
pan frying) at about 160
C for about 20 min. The tea
is rolled further and then fired.
0042 The manufacturing process for pouchong tea
is slightly different than for oolong. The leaf is
withered in the sun (solar withering) for 15 min,
during which time it is turned over once. This is
followed by indoor withering for 3 h, during which
time the leaf is turned over three times. The leaf is
then pan-fried at 160
C for 20 min, rolled by hand
for about 20 min, and dried at 80–85
C for 40 min.
Low-grade pouchong tea is often scented by blending
with jasmine flowers to enhance the flavor (jasmine
tea). Other partially fermented teas such as teek-
wang-yin and pan-fried longjing are also manufac-
tured in a similar manner.
Other Tea Products
0043Earl Grey is flavored with the peel oil of bergamot, a
citrus fruit, which is added by spraying on to black tea
before final packaging. Jasmine flowers are some-
times added to manufactured black tea in the country
of origin, and these impart a characteristic floral note.
Lapsang souchong tea is a Chinese black tea flavored
with natural smoke.
0044Instant teas are prepared in the producer countries
by infusing the undried leaf and then evaporating
the liquor by either freeze-drying, spray-drying, or
vacuum-drying. All these drying procedures avoid
using excessive heat and therefore reduce the loss of
flavor components. Instant tea is also produced in the
USA and UK, but details of the processes are secret or
protected by patents.
0045In recent years, decaffeinated teas have appeared
on the market. The tea is decaffeinated with methyl-
ene chloride or other chlorinated solvents and super-
critical carbon dioxide. (See Coffee: Decaffeination.)
See also: Coffee: Decaffeination; Phenolic Compounds;
Storage Stability: Parameters Affecting Storage Stability
Further Reading
Anonymous (1986) The Tea Research Foundation of
Kenya Tea Growers Handbook, 4th edn. Nairobi:
Eleza Services.
BSI (1982) British Standard Glossary of Terms Relating
to Black Tea. BS 6325. Milton Keynes, UK: British
Standards Institute.
Eden T (1976) Tea. In: Tropical Agricultural Series, 3rd
edn. London: Longman.
Orchard JE and Owuor PO (1989) Black tea quality. Effects
of manufacturing practices on tea quality. Tea 10:
117–160.
Owuor PO (1990) Some advances in the improvement of
tea quality through chemical and biochemical research
in Kenya. Tropical Science 30: 307–319.
Owuor PO and Obanda MA (1996) The impact of
withering temperature on black tea quality. Journal of
the Science of Food and Agriculture 70: 288–292.
Owuor PO and Obanda M (1997) Advances in withering
technologies and future strategies to cope with high tea
production in Kenya. Tea 18: 184–193.
Owuor PO and Obanda M (1998a) Influence of enriched
oxygen atmosphere during fermentation on plain clonal
black tea quality parameters development. Tropical
Science 38: 165–170.
5756 TEA/Processing

Owuor PO and Obanda M (1998b) The changes in black
tea quality parameters due to plucking standards and
fermentation time. Food Chemistry 61: 435–441.
Owuor PO and Orchard JE (1989) Changes in the bio-
chemical constituents of green leaf and black tea due
to withering. A review. Tea 10: 117–160.
Owuor PO, Orchard JE and McDowell IJ (1994) Changes
in the quality parameters of clonal tea due to fermenta-
tion time. Journal of the Science of Food and Agriculture
64: 319–326.
Takeo T, Tsushida T, Mahanta PK, Tashiro M and Imamura
Y (1985) Food chemical investigations on the aroma of
oolong tea and black tea. Bulletin of the Natural Re-
search Institute of Tea, Japan 20: 91–180.
Werkhoven J (1974) Tea Processing. Agricultural Services
Bulletin, No. 26. Geneva: Food and Agriculture Organ-
ization of the United Nations.
Willson KC and Clifford MN (eds) (1991) Tea: Cultivation
to Consumption. London: Chapman & Hall.
Analysis and Tasting
P O Owuor, Tea Research Foundation of Kenya,
Kericho, Kenya
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Tea Quality
0001 The term ’quality,’ as used in the tea trade is some-
times subjective and somewhat illusory. Largely, the
tea tasters set the quality standards, and most scien-
tific methodologies are based on what tea tasters
consider as important. Tea tasters have set a large
vocabulary of descriptive terms with which to assess
the different parameters considered to contribute to
tea quality. The most significant quality parameters
are associated with smell, taste, and appearance.
Considerable effort has been made to correlate vari-
ous constituents of the fresh leaf, processed tea, or
infusions with tea sensory evaluation. However, a
particular characteristic that may be highly desirable
in tea destined for a specific market could well be
considered detrimental in a tea required by another
market. Requirements also vary between the different
processed products, e.g., the green, fresh aroma that
is essential for green tea quality would be considered
a defect in a black tea. Furthermore, a tea that may be
described by a tea taster as an overall ’quality’ tea
may not satisfy the target market demands: therefore,
quality does not necessarily equate with profit.
Indeed, whereas producers tend to manufacture teas
with desirable quality characteristics, in many in-
stances, tea prices are determined by supply and
demand. This does not, however, imply a lack of
quality tea. Producers of teas with most desirable
characteristics usually realize better values, especially
when tea supply outstrips demand.
0002With the exception of a few specialty brands,
most black tea is bought in bulk by tea buyers and
then blended to suit target market demands. After
blending, the tea loses its identity, and the producer
has no control over the quality of the final product.
A tea buyer is well aware of the quality of the
teas required for blending for their own particular
market, and the availability of teas of such quality
will determine whether they buy and the prices they
are prepared to pay. Occasionally, this can result in a
high demand for lower-quality teas, whereas the pre-
mium-quality grades remain unsold or fetch relatively
low prices. A producer might thus be tempted to
concentrate on production of lower-quality teas, but
the vagaries of the market are such that the emphasis
may well have been reversed by the time their product
reaches the market, and, more seriously, such a policy
results in a general decline in quality, which damages
the image of tea as a beverage. It is essential for
producers to maximize the quality of all their grades,
particularly as tea is invariably overproduced.
Tea Tasting
0003As with many products, tea requires constant quality
evaluation during its processing, marketing, pack-
aging and storage. Tea tasting has evolved, over cen-
turies, as the most appropriate method to the needs
of the trade. This is particularly so as instrumental
methods of analysis tend to be slow and laborious,
whereas tea tasting is fast and can evaluate a large
number of samples within a short duration. The
following method is specifically for black teas, but
similar sensory and visual appraisals are also
employed for other processed tea beverages.
0004The technique involves the description and evalu-
ation of teas both before and after infusion in boiling
water. A batch of tea samples is laid out in a row, and
each sample is assigned an earthware or China bowl
and a cup fitted with a lid, both of which are of a
standard capacity. Tea equivalent to the weight of
a silver sixpenny piece, i.e., 2.83 g (0.1 oz or 44
grains), is placed into each cup. Boiling water is
added to the cups to produce a 2% brew, and the lid
applied. After infusing for 5 or 6 min, the liquor is
poured into the bowl, and the infused leaf is shaken
from the cup, pressed free of excess moisture, and
placed in the inverted lid, which is then placed on
top of the cup.
0005The dry leaf is examined and evaluated for color
grade or uniformity of particle size and form, degree
TEA/Analysis and Tasting 5757
