Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


that may hold food, efforts are being made to reduce
the possible contact to any tin that may be in the
items. The primary target is the food can, and some
efforts are included.
Food Cans
0034 Early food cans were made of tin because of their
permanent shiny appearance and the ease with
which they could be manufactured. However, the
lack of strength to maintain their shape led to the
use of steel, which was much sturdier and less expen-
sive. However, steel corrodes easily when exposed
to air.
0035 The findings that levels of tin that were at the levels
of those originally eluted by contact with food in the
tin cans has effects on the levels of copper, iron, and
zinc, which can affect health, has made the coating of
steel for food cans a very important issue. This led to
various means to prevent corrosion, which occurred
inside the cans with contents of juices of various
foodstuffs. The early method used to prevent this
corrosion (rusting) was to plate the surface with tin.
However, any small deformations or pinholes would
permit rusting of the steel. This led to the use of
various plastics.
0036 The literature is full of procedures for protecting
steel, and most are coatings, for which patents de-
scribe in detail the ingredients and the procedure for
preparing for them. An early simple procedure was to
plate the outer coat with tin and apply an inner
thermoplastic layer with particles of tin in them.
The contents of the can would then be exposed to
only a small amount of tin, and the outer surface of
the can would be protected by the tin coating.
0037 One Japanese patent describes a corrosion-resist-
ant metal–polyester laminate formed by laminating a
hot metal sheet with a polyester film coated with a
thin aluminum layer. A number of layered coatings
without plastic have also been patented. One such
example describes a steel strip flash-electroplated
with nickel, electroplated with tin, rinsed and dried,
and then chromated.
0038 Electric-resistance seam-welded cans are painted
internally and have a tin coating with double chro-
mate layers of chromium oxide layer. They then have
a final coating of epoxy-phenolic paint and are baked
at 205
C. The patents all state that the resulting steel
is suitable for food cans.
Summary
0039 Long-term exposure of weanling rats to levels of tin
expected in foods contained in tin cans has provided
quantitative data on their effects. Copper and zinc
levels were the primary targets of exposure through
the diet. Since these elements are a vital part of many
functions, tin has an indirect effect.
0040General exposure to tin is in the inorganic form in
foods contained within cans containing tin. Origin-
ally, this exposure was through the use of tin cans,
because tin seemed to be inert to general exposure
and was an easy metal to mold into cans. However,
the relative softness resulted in many dents in hand-
ling the cans, and many cans used to be rejected
because of the dents. For this reason, the industry
turned to the use of steel, a less expensive and much
sturdier material.
0041However, the surface of steel is easily oxidized
(rust), forming an unpleasant appearance. Initially,
the steel was plated with tin to coat both the inside
and outside surfaces. The tin coat did not provide
complete protection, because small holes would
allow exposure to the air, resulting in rust spots.
What happened inside the can was not of general
concern, because the amounts of soluble tin were
not considered to be harmful. The first solution to
maintain the external appearance was to coat the
external surface with some type of plastic.
0042There have been many patents on the use of various
plastics to coat the steel, and many elements including
tin are included in the plastics. Many of the resultant
steel sheets are satisfactorily electrowelded. The
patents all state that the steel sheets are suitable for
food cans. This interest is good evidence that the
health of the consumers of canned goods is very im-
portant to showing the concern to produce safe
materials for canning.
See also: Canning: Principles; Cans and their
Manufacture; Recent Developments in Can Design;
Chromatography: Thin-layer Chromatography; High-
performance Liquid Chromatography; Copper:
Physiology; Mercury: Properties and Determination;
Zinc: Deficiency
Further Reading
Boyer IJ (1989) Review paper. Toxicity of dibutyltin and
other organotin compounds to humans and experimen-
tal animals. Toxicology 55: 253–298.
Cappon CJ (1987) GLC speciation of selected trace elem-
ents. LG-GC 5: 400–418.
Cappon CJ (1990) Speciation of selected trace elements in
edible seafood. In: Nriagu JE and Simons MS (eds) Food
Contamination from Environmental Sources. New
York: Wiley.
Chang LW (1985) Neuropathological effects of toxic metal
ions. In: Gabay S, Harris J and Ho BT (eds) Metal Ions in
Neurology and Psychiatry, vol. 15, pp. 207–230. New
York: Alan R. Liss.
5788 TIN

Donaldson JD and Grimes SM (1989) The inorganic chem-
istry of tin. In: Harrison PG (ed.) Chemistry of Tin,
pp. 118–144. New York: Chapman & Hall.
Evans CJ (1989) Industrial uses of tin chemicals. In: Harri-
son PG (ed.) Chemistry of Tin, pp. 421–453. New York:
Chapman & Hall.
Furman NH (ed) (1962) Scott’s Standard Methods of
Chemical Analysis, vol. I, pp. 1073–1093. Princeton,
NJ: Van Nostrand.
Lickiss PD (1989) Organometallic compounds of bivalent
tin. In: Harrison PG (ed.) Chemistry of Tin, pp. 221–
244. New York: Chapman & Hall.
Molloy KC (1989) Organometallic compounds of tetrava-
lent tin. In: Harrison PG (ed.) Chemistry of Tin, pp. 187–
220. New York: Chapman & Hall.
Nielson FH (1976) Newer trace elements and possible ap-
plication in man. In: Prasad A (ed.) Trace Elements in
Human Health and Disease, II. Essential and Toxic
Elements, p. 395. New York: Academic Press.
Rader JI (1991) Anti-nutritive effects of dietary tin.
Advances in Experimental Medicine & Biology 289:
509–524
Selwyn MJ (1989) Biological chemistry of tin. In: Harrison
PG (ed.) Chemistry of Tin, pp. 359–396. New York:
Chapman & Hall.
Toribara TY (1985) Preparation of CH
3
203
HgCl of high
specific activity. International Journal of Applied
Radiation and Isotopes 36: 903–904.
Toribara TY, Jackson DA, French WR, Thompson AC and
Jaklevic JM (1982) Nondestructive X-ray fluorescence
spectrometry for determination of trace elements along
a single strand of hair. Analytical Chemistry 54:
1844–1849.
Underwood EJ (1977) Trace Elements in Human and
Animal Nutrition, 4th edn. pp. 449–458. New York:
Academic Press.
Walker JQ, Jackson MT Jr. and Maynard JB (1977) Chro-
matographic Systems. Maintenance and Troubleshoot-
ing, 2nd edn. New York: Academic Press.
Willard HH and Toribara TY (1942) A volumetric method
for determining tin based on the formation of a dioxa-
latothiometastannate. Industrial and Engineering
Chemistry Analytical Edition 14: 716–721.
Willard HH and Toribara TY (1942) I. The preparation and
properties of potassium oxalatostannate. II. A study of
complex dioxalatothiometastannates. Journal of the
American Chemical Society 64: 1759–1765.
TOCOPHEROLS
Contents
Properties and Determination
Physiology
Properties and Determination
D C Woollard, AgriQuality NZ, Auckland 1,
New Zealand
H E Indyk, Anchor Products, NZWP, Waitoa,
New Zealand
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Physical Properties
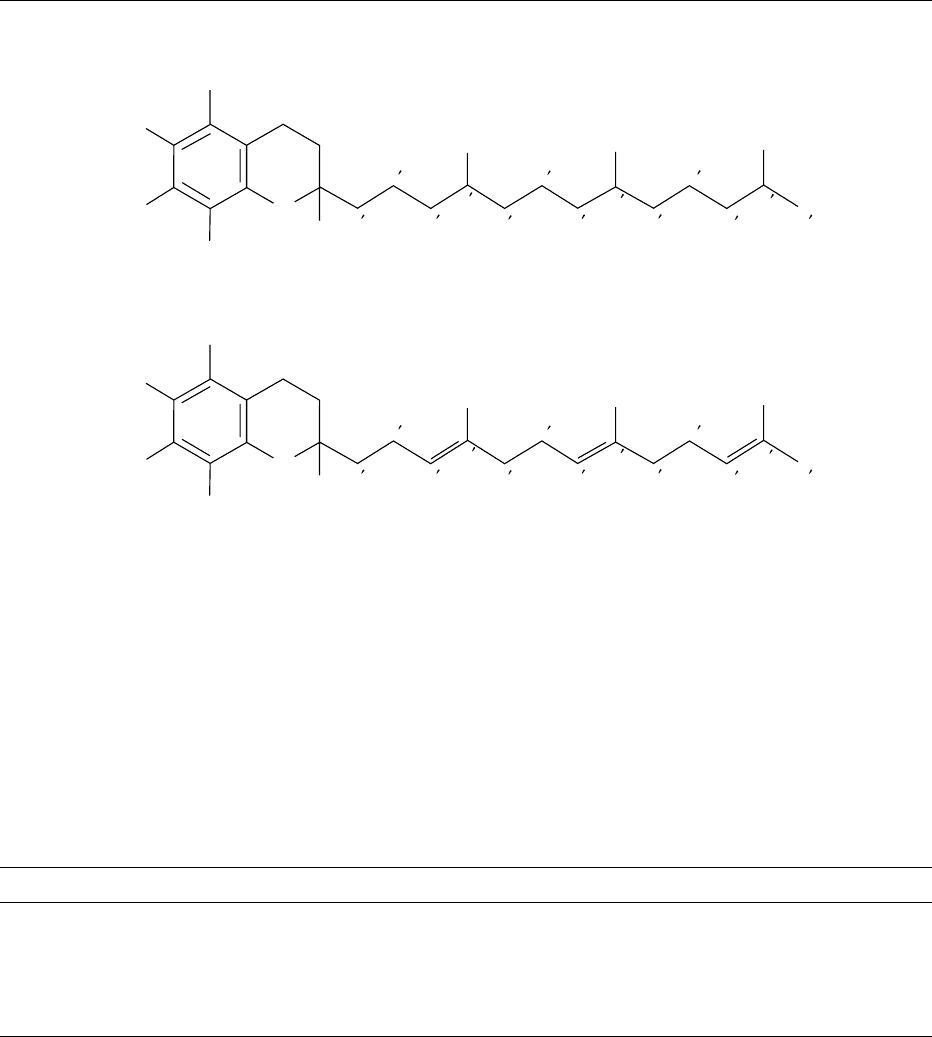
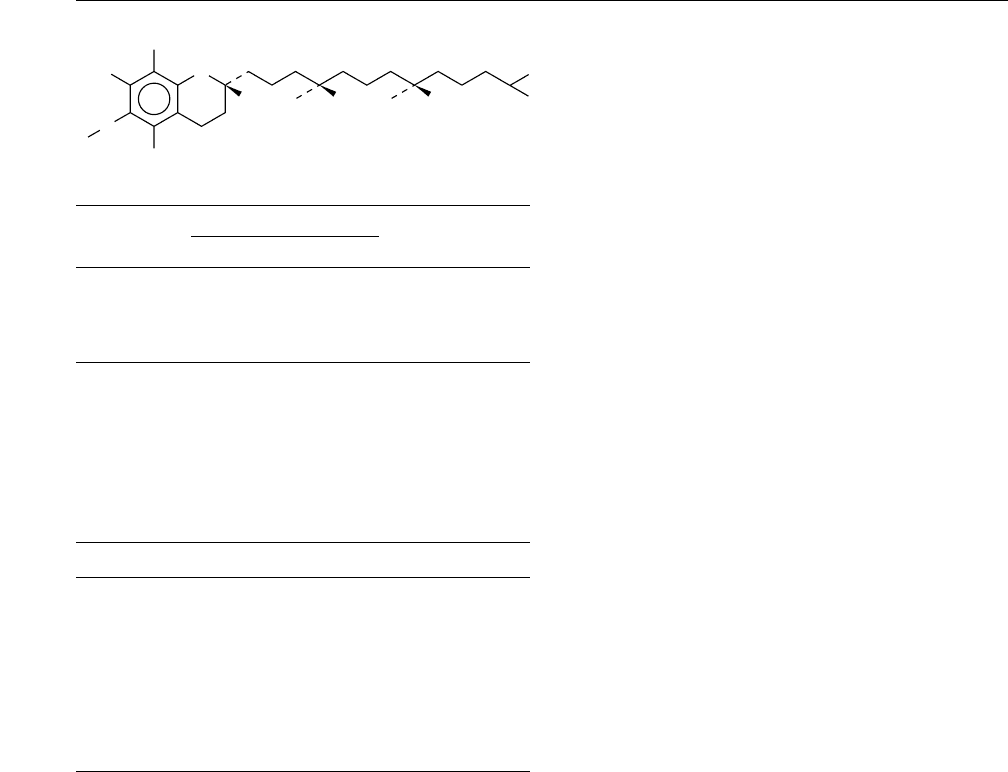
0001 The generic term ’vitamin E’ is used to describe a
group of eight structurally related tocopherol and
tocotrienol compounds (Figure 1). The parent form
is commonly referred to as a-tocopherol and system-
atically named 2,5,7,8-tetramethyl-2-(4
0
,8
0
,12
0
-tri-
methyltridecyl)chroman-6-ol, as a derivative of the
benzene-pyran ring structure.
0002 The tocopherols are insoluble in water, freely
soluble in fats and oils, and soluble in most organic
solvents. They exist as pale yellow viscous oils in
purified form but can be crystallized at subzero tem-
peratures, the melting point of a-tocopherol being
2.5–3.5
C. Each tocopherol contains three asymmet-
ric carbon atoms, making possible a total of eight
optical isomers, although the epimeric configuration
at carbon-2 dominates biological activity. Table 1
lists the major physical properties of the naturally
occurring RRR-tocopherols, as well as the synthetic
all-rac-a-tocopheryl acetate. Spectral properties and
specific optical rotations are highly dependent on the
solvent used during measurement.
0003All vitamin E congeners show weak UV absorp-
tion (290–300 nm) and unusually narrow band-
widths. However, they exhibit strong native
fluorescence, emitting radiation at 324–330 nm, the
practical use of which has increased with advances
in detector design. The esters of vitamin E have
TOCOPHEROLS/Properties and Determination 5789
bathochromically modified spectral properties as a
consequence of electronic interactions with the chro-
manol chromophore, and reduced extinction coeffi-
cients and quantum yields relative to the parent
alcohols. The tocopherols and tocotrienols show a
strong infrared band at 8.6 mm and O–HandC–H
stretching frequencies at 2.8–3.0 and 3.4–3.5 mm,
respectively.
Chemical Properties
0004The tocopherols and tocotrienols share a common
chemical structure based upon tocol. Tocol consists
of a chromanol nucleus with substitution at the 2- and
6-positions. A methyl group and a phytyl side-chain
are bonded to C-2, while the hydroxy functionality
on C-6 is available for esterification. Tocol itself is not
tbl0001 Table 1 Physical properties of the tocopherols
a-T b-T g-T d-T a-T acetate
Formula C
29
H
50
O
2
C
28
H
48
O
2
C
28
H
48
O
2
C
27
H
46
O
2
C
31
H
52
O
3
Molecular weight 430.69 416.66 416.66 402.62 472.73
Boiling point (
C, 10 kPa (0.1 atm)) 210–220 200–210 200–210 184
Optical rotation (a
546
in ethanol, RRR-isomers) þ0.32
þ2.9
þ3.2
þ3.4
þ3.2
l
max
(nm) in ethanol 294 297 298 298 285.5
Extinction coefficient, E
1cm
1%
, in ethanol 71.0 86.4 92.8 91.2 43.6
See text for the abbreviated names used.
HO
6
7
R
1
R
2
R
3
8
5
9
10
4
3
2
O
1
2
3
4
5
6
7
8
9
10
11
12
13
HO
6
7
R
1
R
2
R
3
8
5
9
10
4
3
2
O
1
2
3
4
5
6
7
8
9
10
11
12
13
Tocopherols
Tocotrienols
α-Tocopherol and α-Tocotrienol
β-Tocopherol and β-Tocotrienol
γ-Tocopherol and γ-Tocotrienol
δ-Tocopherol and δ-Tocotrienol
R
1
R
2
R
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
H
HH
H
fig0001 Figure 1 Structure of the tocopherols and tocotrienols.
5790 TOCOPHEROLS/Properties and Determination

widely regarded as an E-vitamin but shares some of
the properties of the group, particularly regarding its
antioxidant role, and occurs naturally in low concen-
tration.
0005 The tocopherols are derived from tocol by the
addition of methyl groups on the three available
carbons (5,7,8) of the benzene ring. Trimethylation
produces a-tocopherol, the most active E-vitamin as
defined by rat fertility and other biological activity
studies. In general, any structural modifications from
a-tocopherol markedly reduces biological activity,
including side-chain unsaturation, side-chain length,
oxidation of the chromanol ring, and loss of any
methyl group. While C-8 methylation is essential, b-
and g-tocopherol isomers (5,8- and 7,8-dimethyltocol,
respectively) have reduced bioactivities of *30% and
*12%, and d-tocopherol (8-methyltocol) exhibits
only 1% biopotency relative to a-tocopherol. Other
mono- and dimethyltocols are also known to occur, as
either minor natural components or synthetic bypro-
ducts, but are not classified as E-vitamins owing to
their lack of any measurable physiological response.
0006 Each of the four tocopherols has a related toco-
trienol (abbreviated T3) through phytyl side-chain
unsaturation at C-3
0
,7
0
and 11
0
, resulting in reduced
biopotency: a-T3 (25%), b-T3 (5%), g-T3 (< 1%)
and d-T3 (< 1%) relative to a-tocopherol.
0007 Optical asymmetry at C-2 yields stereoisomers, of
which the d-form is biologically more active by a
factor of 2.38 compared with the l-isomer. Thus,
naturally occurring a-tocopherol, which exists exclu-
sively as the d-isomer has a higher efficacy than the
synthetic racemic equivalent.
0008 The tocopherols have two additional chiral carbon
atoms (4
0
and 8
0
), and naturally occurring d-a-toco-
pherol is more correctly described as 2R,4
0
R,8
0
R-
a-tocopherol (abbreviated RRR-a-tocopherol). The
stereochemistry of the phytyl ’tail’ is known to govern
biological properties, believed to be a consequence
of in vivo interaction with chiral biological
membranes. Thus, the natural RRR-a-T isomer is
approximately three times more effective than its
stereochemically inverted relative SRR-a-T (referred
to as 2-epi-a-tocopherol). While tocotrienols possess
no side-chain asymmetric centers, geometrical isom-
erism becomes a possibility, although only the trans-
isomers have been reported. The influence of cis
geometry on biopotency is therefore unknown.
0009 The tocopherols are vulnerable to aggressive forms
of oxygen, leading to the formation of biologically
inactive monomeric and dimeric quinones. These
reactions are accelerated by UV light, heat, high
pH, and certain transition elements, whereas in the
absence of oxygen, the E-vitamins are relatively stable.
Lability is decreased by esterification at the 6-hydroxy
substituent, presumably through stabilization of the
phenolic ring structure. Consequently, most nutri-
tional uses of vitamin E involve a-tocopherol esters.
0010To account for the variation in biological activities
of the isomers, the concentration of vitamin E is often
defined in international units rather than by weight.
The international unit (IU) is expressed relative to
synthetic all-rac-a-tocopheryl acetate, where 1 IU is
equivalent to 1 mg, based upon fetal resorption after
oral administration to rats. The information agrees
fairly well with rat hemolytic anemia studies. On this
basis, 1 unit is provided by 0.91 mg of all-rac-a-
tocopherol and 0.67 mg of RRR-a-tocopherol. The
bioactivities of other tocopherols and tocotrienols
are then calculated against these data by applying
the appropriate conversion factors.
0011As a consequence of their phenolic structure, the
tocopherols and tocotrienols are potent antioxidants,
conferring protection to vulnerable lipids in bio-
logical tissues and foods. Such antioxidant behavior
is presumed to depend on pyran ring cleavage to
form the hydroquinone intermediate, followed by
oxidation to tocopherol quinone. These and other
oxidation products (tocopheroxides, epoxides, and
polymers) are receiving increased attention.
Occurrence and Forms in Food
0012The dietary intake of vitamin E in the general popu-
lation appears to be sufficient to meet the US Recom-
mended Daily Allowances of 3 mg (infants) to 12 mg
(adult males). Avitaminosis E is comparatively rare
in well-nourished communities because of large
physiological reserves in tissues and organs, although
neuromuscular and other disorders have been associ-
ated with dietary deficiencies, particularly in chil-
dren. (See Cells; Dietary Requirements of Adults.)
0013The tocopherols are well distributed in nature, the
richest and most varied isomeric sources being vege-
table oils and products derived from them, such as
margarine. It has been common practice with these
products to speciate the various vitamers during
analysis (Table 2). Other valuable sources of vitamin
E include nuts, cereals, green vegetables, fruits, and
foods of animal origin such as eggs, dairy products,
and meats (Table 3). In practice, the contribution of
the non-a vitamers in most foods is sufficiently small
after biopotency factors are applied that they are
regularly excluded from estimations of total vitamin
E status. Animal tissues contain vitamin E almost
exclusively as a-tocopherol, with the exception of
egg yolk, where g-tocopherol is found in significant
concentrations. There is a fairly wide disparity in
literature data, probably as a consequence of genetic,
geographical, seasonal, and processing factors as well
TOCOPHEROLS/Properties and Determination 5791
as variations in analytical techniques. Refer to
individual foods.
0014 Tocopherols and tocotrienols are labile materials,
and their stability during food processing and storage
has been subject to intensive investigations. Storage
of foods under anaerobic conditions and the incorp-
oration of other synthetic or natural antioxidants are
common strategies to optimize the preservation of
their vitamin E status.
Role as Antioxidants
0015 Oxidation of polyunsaturated lipids operates through
a free-radical pathway that not only facilitates food
spoilage but creates substances of potential toxi-
cological significance that can have damaging
consequences upon ingestion. The tocopherols and
tocotrienols combat this process both in vitro and
in vivo by trapping the hydroperoxide intermediates
and preventing their propagation. The fate of the
stable vitamin E phenoxy radicals so produced is
variable, with formation of monomeric quinone,
which can react further with a secondary radical
forming a dimer. Under certain conditions, ascorbate
is considered capable of synergistically repairing the
phenoxy radical and regenerating the tocopherols.
(See Antioxidants: Natural Antioxidants; Oxidation
of Food Components.)
0016It has been confirmed that antioxidant activity of
the tocopherols follows the same order as their
biological potencies (a>b>g>d) and that this may
be rationalized on a stereoelectronic basis. Thus, a
higher resonance stabilization of the phenoxy radical
is achieved in a-tocopherol where ring planarity is
optimal relative to the other congeners.
0017Vegetable oils and margarines are particularly
prone to oxidation, owing to their high content of
polyunsaturated fatty acids but fortuitously also
possess substantial native vitamin E, which functions
protectively. However, additional stabilization is
often considered necessary, particularly for refined
oils where the tocopherols become depleted during
the harsh manufacturing conditions. Although animal
fats and foods rich in them are predominantly satur-
ated and less sensitive to oxidative processes, the
addition of tocopherols is usually of benefit since
natural vitamin E contents are low. (See Margarine:
Methods of Manufacture; Vegetable Oils: Types and
Properties.)
0018The ratio of tocopherol to polyunsaturated fat is an
important criterion in determining optimum oxida-
tive protection offered by vitamin E and also the
toxicological safety of the fat. Excessive concentra-
tions of vitamin E are reported to accelerate lipid
oxidation, although there is debate about the detail
tbl0003 Table 3 Vitamin E content of selected foods
a
Food
Tocopherol (mg per 100 g)
a-T
b
b-T g-T d-T
Peanut 7.0–8.3 8.0
Spinach 1.8–2.5 0.1 1.0
Lettuce 0.2–0.4 0.04 0.1
Parsley 0.8–1.2 0.1
Celery 0.3–0.5 0.1 0.4
Peas 0.2–0.5 0.05 0.2
Carrot 0.4–0.5 0.02 0.01
Tomato 0.4–0.9 0.03 0.1
Potato 0.05–0.1
Apple 0.3–0.6 0.02 0.07
Blackberry 3.0–3.5 4.7 4.5
Banana 0.2–0.3
Wheat flour 0.7–0.9 0.6
Beef, raw 0.4–0.8 0.02
Chicken, cooked 0.2–0.4 Trace
Halibut 0.4–1.3 Trace Trace
Milk 0.05–0.13 0.01
Butter 1.0–2.0 0.05
Margarine 10.0–15.0 25.0 3.0
Egg white, raw 0.07–1.95 0.04 0.2 0.01
a
a-Tocopherol values are expressed as typical ranges, whereas
representative values are reported for the less biologically active
congeners. Blanks indicate a lack of data or the absence of traceable
levels. Tocotrienol contributions are not reported as they are
predominantly absent from these foods.
b
To convert to IU per 100 g, multiply by 1.49 (as RRR-a-tocopherol).
tbl0002 Table 2 Vitamin E content of selected vegetable oils
Oil
Tocopherol (mg per100 g) Tocotrienol (mgper100 g)
a-T b-T g-T d-T a-T3 b-T3 g-T3 d-T3 Total
Coconut 0–2 0–1 0–1 0–1 0–1 0–4 1–5
Soya bean 1–35 0–4 40–240 15–95 60–330
Corn 2–55 0–30 25–250 2–8 0–24 0–5 0–40 0–2 30–340
Wheat germ 120–150 40–70 0–25 0–25 0–2 6–18 150–200
Sunflower 40–80 1–4 0–5 0–1 45–90
Palm 0–20 1–4 0–30 0–3 4–70 0–10 10–130
Olive 10–25 Trace 10–27
Safflower 20–45 3–10 25–50
Blanks indicate a lack of reported data, implying concentration below limits of detection.
5792 TOCOPHEROLS/Properties and Determination

and extent of this process. As expected, antioxidant
and prooxidant properties differ between the various
tocopherols and tocotrienols. Vitamin E acetate
cannot be used as an antioxidant because this prop-
erty is lost upon esterification.
Use in Food Fortification
0019 As indicated previously, the tocopherols are among
the most important fat-soluble antioxidants used in
the prevention of food spoilage. However, vitamin E
has other recognized biological functions and conse-
quently is added to a variety of foods for the manage-
ment of community health. This has particular
relevance for infant nutrition and for sections of the
population where inadequate nutrition is suspected.
To maintain vitamin efficacy, the free phenols are not
used during food fortification unless an antioxidant
function is also required. (See Food Fortification.)
0020 The most common nutritional additive is synthetic
all-rac-a-tocopheryl acetate owing largely to its
greatly enhanced oxidative stability. Technological
problems during the fortification process can be
overcome by utilizing the oily vitamin in a water-
dispersible ’beadlet’ form, contained within a gelatin
coating. The vitamin can then be blended as an emul-
sion into liquid foods, usually prior to spray drying.
Milk powders, dietetic, and infant formulae are often
supplemented in this way, facilitating a uniform dis-
tribution of the additive. In other products, the ingre-
dients may be mixed in the dry state, either because
the nature of the food is not conducive to wet-
blending procedures or because the vitamin E has a
diminished stability owing to low fat content. Cereals
and skim-milk powder products are regularly supple-
mented in this manner. The major disadvantage with
this protocol is the potential for vitamin hetero-
geneity. Particle-size control and correct mixing,
packaging, and storage conditions are therefore ne-
cessary to minimize uneven distribution of the vita-
min beadlets.
0021 Vitamin E succinate can be used if severe hetero-
geneity problems are encountered since this ester is a
solid at room temperature and more easily dry-
blended into foods and pharmaceuticals. A disadvan-
tage with the use of the succinate ester is its lower
bioavailability. The third commercially available
vitamin E ester, tocopheryl nicotinate, is used in
cosmetics rather than as a food additive.
Isolation and Analysis
0022 Vitamin E poses analytical challenges because of its
multiplicity of forms and its tendency to oxidize.
Traditional bioassays (e.g., resorption gestation,
encephalomalacia, myopathy, and erythrocyte hem-
olysis tests) do not discriminate between the various
vitamers (vitamin isomers) in foods. They are tedi-
ous and relatively imprecise techniques, although
they do exhibit true, host-specific, activity data.
Lipid peroxidation tests for the accumulation of
hydroperoxides or aldehydes in animals have also
been used to indicate vitamin E status. (See Vitamins:
Determination.)
0023Physicochemical tests have become more popular
because of their ease of use, speed, and precision
compared with bioassays. The traditional chemical
test has been the Emmerie–Engel reaction, which
detects the presence of fat-soluble reducing agents
through conversion of trivalent iron to the red ferrous
bipyridyl complex. The accuracy of this and related
colorimetric procedures is, however, compromised by
spectral interferences of other antioxidant materials
such as biologically inactive tocols, retinoids, carote-
noids, nonvitamin chromanol compounds, and syn-
thetic food antioxidants. The success of the method is
therefore dependent upon the degree of purity of the
vitamin E analytes prior to colorimetry. The problem
remains that the eight E-vitamins are not differenti-
ated and will be quantified as a single analyte. These
same difficulties remain, albeit to different degrees,
whether detection is by UV absorption, fluorescence,
or electrochemical techniques. In each case, a chro-
matographic step is required in the analytical scheme
in order to remove interferences and, ideally, to sep-
arate the vitamers. Traditional column or thin-layer
chromatographic (TLC) techniques have been used
extensively for this purpose. (See Chromatography:
Principles; Thin-layer Chromatography; Spectro-
scopy: Visible Spectroscopy and Colorimetry.)
0024Instrumental chromatographic techniques have
now become the methods of choice, with separation
and detection being performed concurrently. Gas–
liquid chromatography (GLC) using various packed-
or open-tubular capillary columns, coupled with flame
ionization detection, allows a quantitative analysis of
individual tocopherols and tocotrienols. A unique at-
tribute of capillary GLC is its recent success in separat-
ing the diastereoisomers of all-rac-a-tocopherol and
the potential of stereoactive chiral columns to resolve
all eight stereoisomers. (See Chromatography: Gas
Chromatography.)
0025The free phenols can be analyzed by GLC, where
elution occurs in order of increasing methylation
(d < g < b < a), although it is preferable to derivatize
the analytes to their more volatile trimethylsilyl ethers
or alkyl esters to improve the chromatographic qual-
ity. However, coelution of the b-andg-isomers is
often a problem unless very long capillary columns
are used. In the analysis of foods, a more significant
TOCOPHEROLS/Properties and Determination 5793
problem exists from interferences by other lipid con-
stituents, predominantly sterols. It is therefore usual
to fractionate the crude extracts by TLC or column
chromatography prior to the assay in order to facili-
tate resolution and interpretation.
0026 High-performance liquid chromatography (HPLC)
offers several advantages over GLC by avoiding the
need for multistage clean-up or derivatization tech-
niques. Either normal- or reversed-phase modes can
be used to achieve a successful separation. Important
advantages of normal-phase chromatography are its
ability to separate the b- and g-isomers and its solvent
compatibility with oil-rich vitamin E extracts. Never-
theless, reversed-phase analysis is technically more
robust and remains in common use, as illustrated in
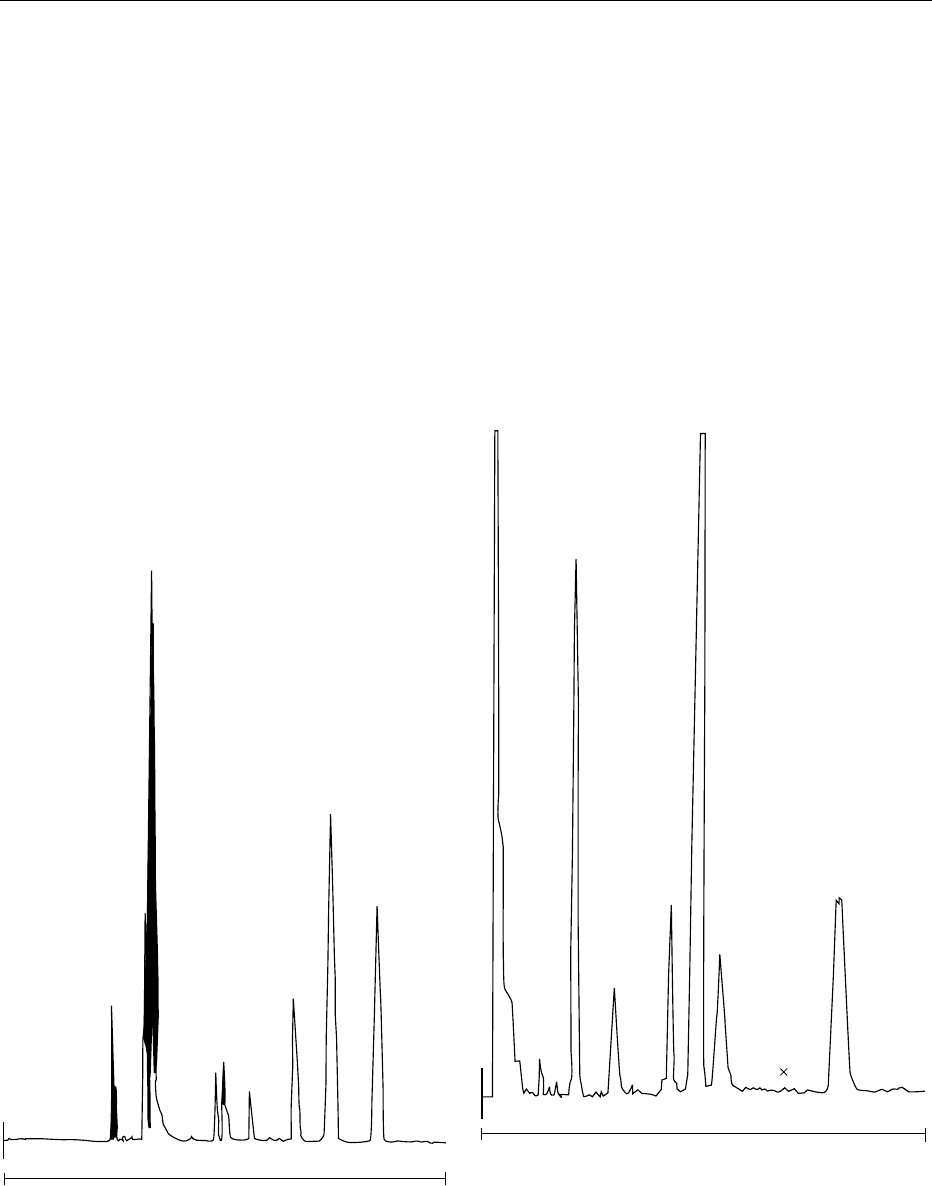
Figure 2 for an infant formula analysis. It is also
amenable to gradient techniques, allowing concur-
rent determination of tocopherols and tocopheryl
acetate as well as degradation products such as to-
copherol quinone. Recently, variants of micellar
capillary electrophoresis (CE) have been applied to
fat-soluble vitamin separations. Although represent-
ing an alternative to HPLC techniques, the applica-
tion of CE to the hydrophobic vitamins is currently in
its infancy. (See Chromatography: High-performance
Liquid Chromatography.)
0027Direct UV detection is commonly employed for
samples with reasonable vitamin E concentrations.
The difficulty with this mode of detection is the
potential for other materials to interfere and also the
low extinction coefficients of the tocopherols and
tocotrienols. There is consequently a regular need to
increase the sophistication of sample preparation,
which can cause problems with such labile analytes.
1
2
3
4
5
6
0
15
Time (min)
+
fig0003Figure 3 Normal-phase HPLC chromatogram of a saponified
dietary formulation. Waters Radial-PAK 5-mm silica column with a
mobile phase of 10% diisopropylether in hexane. Flow, 2.0 ml
min
1
. Detection by fluorescence at 290 nm (excitation) and
325 nm (emission). Peak identification: 1 ¼a-tocopherol; 2 ¼
a-tocotrienol; 3 ¼b-tocopherol; 4 ¼g tocopherol; 5 ¼b-toco-
trienol; 6 ¼d-tocopherol; ¼elution position of g-tocotrienol;
þ¼elution position of d-tocotrienol.
0
Time (min)
15
1
2
3
fig0002 Figure 2 Reversed-phase HPLC chromatogram of a saponified
fully oil-filled infant formulation. Waters Radial-PAK 5-mmC
18
column with a mobile phase of methanol (100%). Flow rate,
1.0 ml min
1
. Detection by fluorescence at 295 nm (excitation)
and 330 nm (emission). Peak identification: 1 ¼d-tocopherol;
2 ¼b- and g-tocopherol; 3 ¼a-tocopherol.
5794 TOCOPHEROLS/Properties and Determination
0028 Through the use of more specific detection modes,
sample preparation can be further simplified together
with substantial increases in sensitivity. Fluorescence
detection at 292 nm (excitation) and 325 nm (emis-
sion) is a simple procedure with modern detectors,
and encounters very few interference problems pro-
viding the separation is optimized. Excitation can be
performed at 210 nm, but this is less common, except
where spectral overlaps in the detector are unavoid-
able, as with filter-based instruments. Oxidative
electrochemical detection (at 0.7–0.8 V) provides a
further order of magnitude improvement in sensitiv-
ity compared with fluorescence but is less commonly
used owing to operational difficulties and the need for
a supporting electrolyte, incompatible with normal-
phase separations. Similarly, recently reported
LC-MS techniques have been restricted to research
laboratories. (See Spectroscopy: Fluorescence.)
0029For most food products, it is generally necessary to
precede any analysis, whether traditional or instru-
mental, with a clean-up stage aimed largely at remov-
ing bulk lipids, rupturing lipoprotein interactions and
releasing supplemental vitamin E. Alkaline digestion
(saponification), followed by solvent extraction of
unsaponifiable material, is the usual technique
employed where the free tocopherols are measured.
If necessary, the vitamin-rich extract can then be
concentrated by evaporation and subjected to further
purification techniques. Figure 3 illustrates a complex
food product analyzed under these conditions, using
normal-phase HPLC. Six of the eight vitamin E con-
geners are visible, the elution order being a-T < a-T3
< b-T < g-T < b-T3 < g-T3 < d-T < d-T3.
0030Some simpler matrices, particularly oils, can alter-
natively be assayed by sample dilution, clarification
and direct injection, whereas other high-fat products
(e.g., dairy products) may lend themselves to simpli-
fied fat-extraction procedures without the need for a
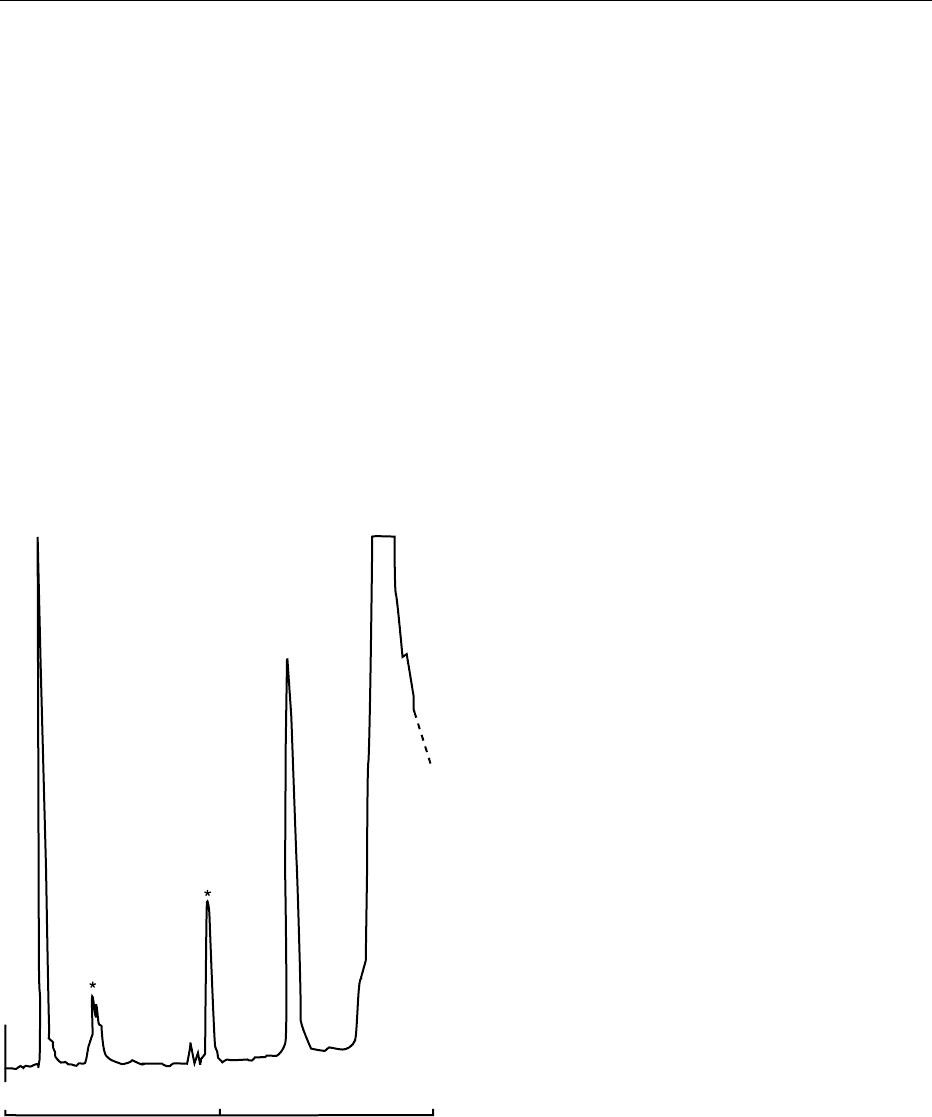
saponification step (Figure 4). This approach has the
advantage of leaving any supplemental esters intact
and eliminating most of the oxidative difficulties
associated with the parent alcohols. Unfortunately,
a-tocopheryl acetate has poorer UV absorption
than a-tocopherol and greatly reduced fluorescence
properties, whereas normal-phase chromatography is
necessary in order to tolerate the high triglyceride
loading that would quickly foul C
18
columns. The
alternative vitamin E additive, a-tocopheryl succin-
ate, has no known fluorescence and is susceptible to
peak distortion, both of which encumber chromato-
graphic analysis, unless the sample is subjected to ion-
suppression techniques or measured as a-tocopherol
after saponification.
See also: Antioxidants: Natural Antioxidants; Cells;
Chromatography: Principles; Thin-layer
Chromatography; High-performance Liquid
Chromatography; Gas Chromatography; Dietary
Requirements of Adults; Food Fortification;
Margarine: Methods of Manufacture; Oxidation of Food
Components; Spectroscopy: Fluorescence; Visible
Spectroscopy and Colorimetry; Vegetable Oils: Types
and Properties; Vitamins: Determination
Further Reading
Ball GFM (1988) Fat-soluble Vitamin Assays in Food
Analyses. London: Elsevier.
Bates CJ (2000) Vitamins: fat and water soluble: analysis.
In: Meyers RA (ed.) Encyclopedia of Analytical Chemis-
try, pp. 1–35. Chichester, UK: Wiley.
Diplock AT (1985) Vitamin E. In: The Fat-soluble Vita-
mins, pp. 154–224. London: Heinemann.
01020
Time (min)
α-Tocopheryl
acetate
Lipids
fig0004 Figure 4 Normal-phase HPLC chromatogram of a fortified UHT
milk without the use of saponification. Waters Radial-PAK 5-mm
silica column with a mobile phase of 0.08% isopropanol in
hexane. Flow rate of 2.0 ml min
1
is programed to 8 ml min
1
after 15 min to elute lipid components. Detection by UV at
280 nm at 0.01 aufs. The identification of a-tocopherol acetate is
indicated. The retinyl esters (endogenous and supplemental) are
also visible (*).
TOCOPHEROLS/Properties and Determination 5795

Eitenmiller RR and Landen WO (1999) Vitamin Analyses
for the Health and Food Sciences, pp. 109–148. Boca
Raton: CRC Press.
Frankel EN (1998) Lipid Oxidation. Dundee: The Oily
Press.
Friedrich W (1988) Vitamin E. In: Vitamins, pp. 217–283.
New York: Walter de Gruyter.
Machlin LJ (1991) Vitamin E. In: Machlin LJ (ed.) Hand-
book of Vitamins, 2nd edn, pp. 99–144. New York:
Marcel Dekker.
Nelis HJ, De Bevere VORC and De Leenheer AP (1985)
Vitamin E tocopherols and tocotrienols. In: De Leenheer
AP, Lambert WE and De Ruyter MGM (eds) Modern
Chromatographic Analysis of the Vitamins, pp. 129–
200. New York: Marcel Dekker.
Sheppard AJ, Prosser AR and Hubbard WD (1972) Gas
chromatography of the fat-soluble vitamins: A review.
Journal of the American Oil Chemists Society 49:
619–633.
Wagner AF and Folkers K (1975) Vitamins and Coenzymes,
pp. 363–388. New York: Robert E. Krieger.
Physiology
W A Pryor, Louisiana State University, Baton Rouge,
LA, USA
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Background
0001 Vitamin E is the most important fat-soluble antioxi-
dant present in human tissues. It is found in the lipid-
rich areas of cells, such as cell membranes and fat
depots. Its role is thought to be the protection of
polyunsaturated fatty acids (PUFAs) such as linoleic,
linolenic, and arachidonic acids, which are present in
lipids, against the types of oxidative damage to which
these highly unsaturated fatty acids are so readily
susceptible. (See Antioxidants: Natural Antioxidants;
Fatty Acids: Properties.)
History
0002 In 1923, Evans and Bishop reported that rats failed
to reproduce if given a diet containing rancid lard,
unless it was supplemented with a factor, called
factor X, found in lettuce or whole wheat. Grad-
ually, over the next 20 years, it became clear that
factor X was associated with more than just repro-
ductive failure in the rat. For example, a series of
observations were reported linking this factor to
encephalomalacia in the chick and nutritional mus-
cular dystrophy in guinea-pigs and rabbits. In 1937,
Olcott and Emerson reported that the tocopherols
are antioxidants.
0003The first 15 years of the study of vitamin E were
made difficult by the absence of a concentrated source
of the vitamin or a knowledge of its structure and
chemical make-up. However, in 1936, Evans and his
group reported the isolation from wheatgerm of an
’alcohol’ which had biological activity consistent
with that of a tocopherol, and they suggested the
correct chemical empirical formula for a-tocopherol
C
29
H
50
O
2
(The free, unesterified tocopherols con-
tinue to be referred to as ’alcohols,’ whereas they are
actually phenols; in fact, alcohols are not, in general,
antioxidants, whereas phenols are.) In 1938, the
structure of a-tocopherol was deduced by Fernholz.
The need for a pure, concentrated, and available
source of the vitamin for nutritional studies was satis-
fied in 1939 when Karrer, of the Basel (Switzerland)
laboratories of Hoffmann–La Roche, and then Smith
and his group in America, reported the synthesis of
the vitamin. (See Phenolic Compounds.)
0004The first international symposium on vitamin E
was held in London in April 1939, and 15 papers
were presented. In the current era, there have been
major international symposia on the vitamin in
various countries almost every year, each with 50 or
100 papers that appreciably add to our expanding
knowledge of this fascinating vitamin.
Natural and Synthetic Forms of
Tocopherol
0005The side-chain of the vitamin (see Figure 1) contains
three carbons that are asymmetric; therefore, there
are eight possible isomers of each of the tocopherols.
Several forms of a-tocopherol are sold. One is
a material that is derived from natural sources,
called RRR-a-tocopherol, which has the stereo-
chemical arrangement of methyl groups shown in
Figure 1; another is the synthetic vitamin called all-
rac-a-tocopherol, which is a mixture of all eight
stereoisomers. Both the naturally derived material
and all-rac-tocopherol are marketed as the free alco-
hol, the acetate ester, and the somewhat less bioavail-
able succinate ester. Some vegetable sources of
tocopherols contain as much or more g-tocopherol
as a-tocopherol. The relative biopotencies of the
various forms of tocopherol are shown in Table 1.
Amounts of the tocopherols are expressed as
either international units (IU) or equivalents of
a-tocopherol (a -TE). The relationship between these
terms is as follows: 1 mg of d-a-tocopherol (the nat-
urally derived material) equals 1 a-TE; this is the unit
used in Recommended Dietary Allowances (10
th
edn.) by the Food and Nutrition Board of the Na-
tional Research Council (National Academy Press,
1989). The IU is used in commerce for labeling
5796 TOCOPHEROLS/Physiology
vitamin preparations and food, and is defined as
follows: 1 mg of d-a-tocopherol equals 1.46 IU. (See
Dietary Requirements of Adults.)
Vitamin E Requirements
0006 A recommended dietary allowance (RDA) for various
food factors was first recognized in the USA with the
publication of the first edition of the book on RDA
requirements in 1946. A specific RDA for vitamin E
was first recommended in the sixth edition (1964), in
which the concept of the RDA was broadened and
redefined from the 1946 idea – enough ’to insure
good nutrition’–to an amount necessary ’to permit
full realization of . . . potential.’ Thus, in the 1964
edition, the concept was expressed that vitamins
might have a pharmacological use beyond that neces-
sary merely to prevent vitamin deficiency diseases.
The 1997 edition sets the need for vitamin E as 10
a-TE per day for males and 8 mg for females, which is
equal to 10 mg of the natural RRR-a-tocopherol (see
Table 1).
0007The vitamin E requirement for humans increases as
their diet includes more PUFAs because of the suscep-
tibility of PUFAs to undergo autoxidation. A value of
0.4 for the ratio of the intake of RRR-a-tocopherol
(in mg) to the intake of PUFA (in g) in the diet has
been suggested to be adequate for adult humans.
Thus, if a person eating a 2000-cal per day diet con-
sumed 35% of their calories as fat, of which 40% was
unsaturated (typical of today’s American diet), they
would require a daily intake of about 13 mg of
a-tocopherol.
0008The intake of vitamin E for Americans on typical
diets has been estimated to vary between 5 and 20
a-TE. The requirement of 10 a-TE for men and 8
a-TE for women (who are generally smaller) was
established as the RDA because it is believed that
very few Americans have overt vitamin E deficiency
symptoms, and the amount ingested in a ’normal’
diet must therefore be sufficient. This rather unsatis-
factory, backward and ad hoc reasoning is thought
to be the only approach possible for establishing an
RDA for vitamin E, for which deficiency symptoms
are only obvious in humans who have severe mal-
absorption. There is an increasing body of evidence,
however, that vitamin E may provide benefits (such
as protection from chemically induced cancers, cat-
aracts, and ischemic heart disease) that require
higher daily intakes; scientific studies of the useful-
ness of vitamin E in these contexts generally use
amounts of vitamin E up to about 800 mg per day,
which is generally agreed to be safe for humans.
(See Cancer: Diet in Cancer Prevention.)
Metabolism
0009As more tocopherol is ingested, a smaller fraction is
absorbed, such that the uptake of tocopherol by
tissues varies with the logarithm of the tocopherol
intake. Thus, ingesting four times the amount of
the vitamin will raise tissue levels by only twofold.
Triglycerides enhance tocopherol absorption, and
PUFAs inhibit it. If the acetate or succinate is ingested,
it is hydrolyzed prior to absorption through the gut
wall. Since the hydrogen atom of the alcohol group is
intimately involved in the antioxidant properties of
the vitamin, it is not unexpected that only the free
alcohol is biologically active. For high doses, a higher
blood level is achieved using the free alcohol than the
acetate. Absorption of vitamin E is maximal in the
median portion of the small intestine, and none is
absorbed in the large intestine. Vitamin E is thought
to be absorbed through the gut epithelial cells as
a lipid–bile micelle, together with fatty acids,
tbl0001 Table 1 Biopotency of various forms of tocopherols
IU mg
1
a-TE mg
1
RRR-a-Tocopherol 1.46 1.00
RRR-a-Tocopherol acetate 1.36 0.91
RRR-a-Tocopherol succinate 1.21 0.81
All-rac-a-Tocopherol 1.10 0.74
All-rac-a-tocopherol acetate 1.00 0.67
All-rac-a-tocopherol succinate 0.89 0.59
RRR-b-Tocopherol 0.30 0.20
RRR-g-Tocopherol 0.15 0.10
RRR-d-Tocopherol 0.01 0.01
Adapted from Machlin LJ (ed.) (1991) Handbook of Vitamins. New York:
Marcel Dekker.
R
H
H
H
O
O
R''
R
CH
3
CH
3
CH
3
1'
4'
8'
2
7
5
8
fig0001 Figure 1 Structures and stereochemistry of the four
tocopherols. Reproduced from Tocopherols: Physiology, Encyclo-
paedia of Food Science, Food Technology and Nutrition, Macrae R,
Robinson RK and Sadler MJ (eds), 1993, Academic Press.
tbl0003 Table 3
Tocopherol Substitutionpattern Stereochemistry
RR
0
R
00
a CH
3
CH
3
CH
3
2R,4
0
R,8
0
R
b CH
3
HCH
3
2R,4
0
R,8
0
R
g HCH
3
CH
3
2R,4
0
R,8
0
R
d HHCH
3
2R,4
0
R,8
0
R
TOCOPHEROLS/Physiology 5797
