Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

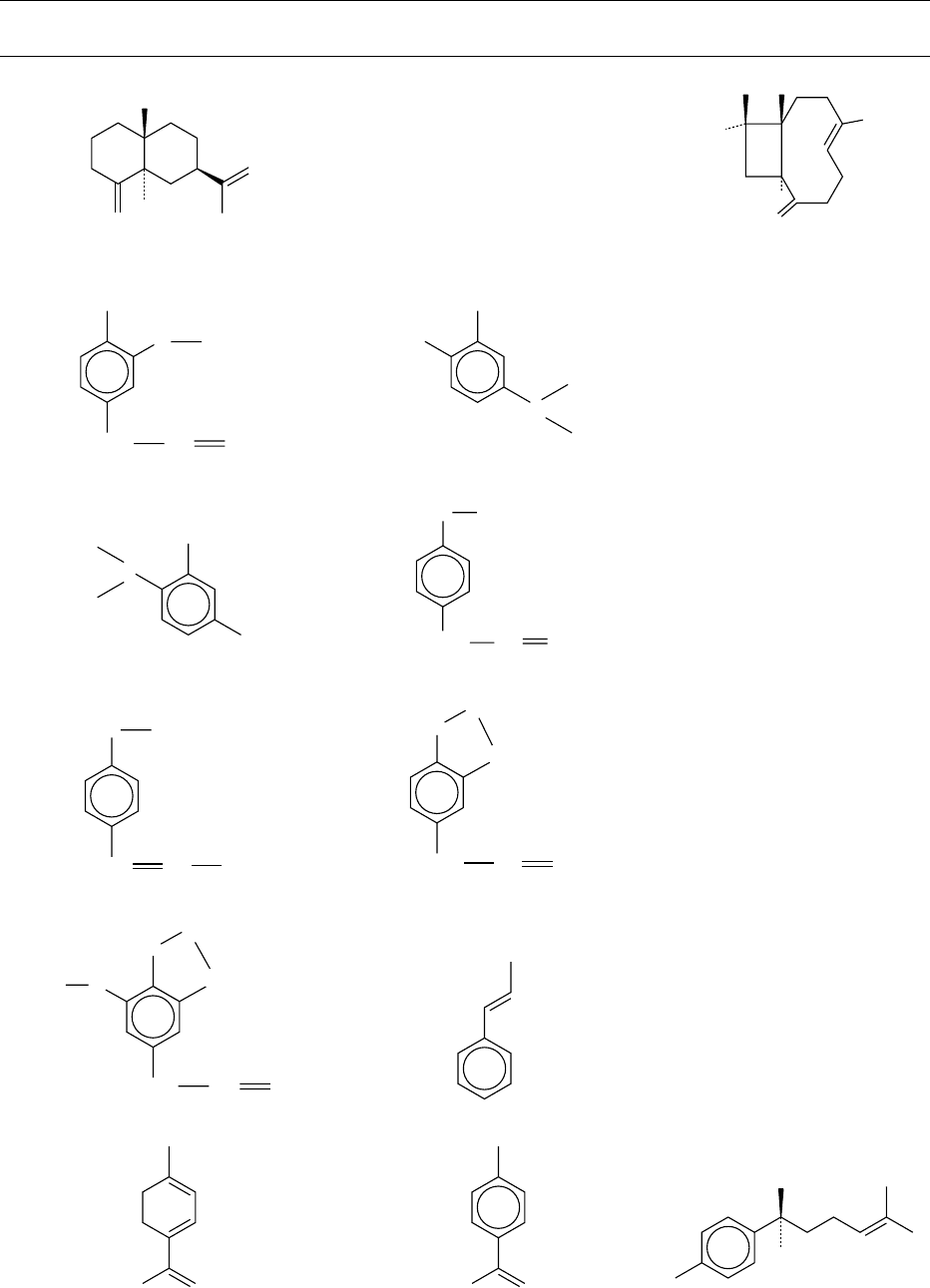
H
[48] b-Selinene
H
H
[49] b-Caryophyllene
Other
OH
O
CH
3
CH
2
CH
2
CH
[50] Eugenol
OH
CH
3
CH
3
CH
H
3
C
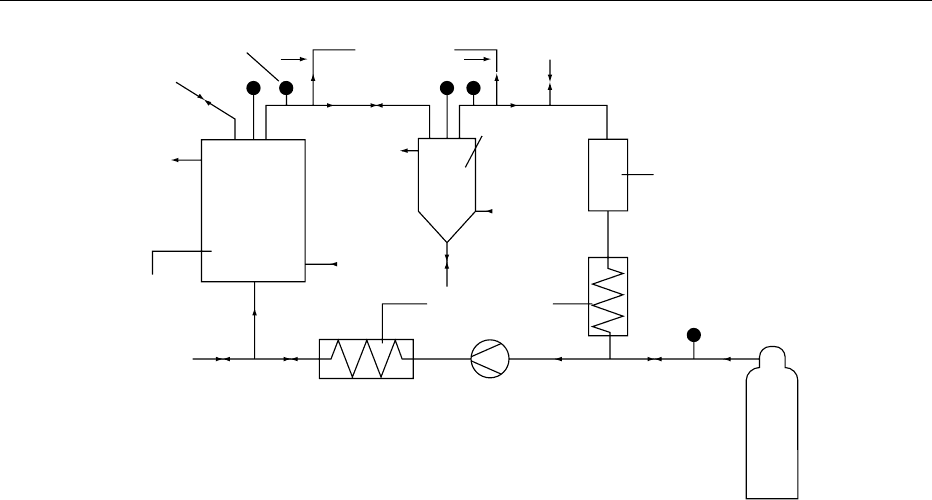
[51] Carvacrol
OH
CH
3
HC
H
3
C
H
3
C
[52] Thymol
O
CH
3
CH
2
CH
2
CH
[53] Estragole
O
CH
3
CH
3
CH
CH
[54] Anethole
O
O
CH
2
CH
2
CH
2
CH
[55] Safrole
O
O
OH
3
C
CH
2
CH
2
CH
2
CH
[56] Myristicin
CHO
[57] Cinnamaldehyde
[58] 1,3,8,p-Menthatriene [59] 1-Methyl-4-isopropenylbenzene
H
[60] (þ)-ar-Curcumene
Continued
Table 3 Continued
SPICES AND FLAVORING (FLAVOURING) CROPS/Properties and Analysis 5497
Extraction Techniques
0018 Extraction with organic solvents This technique is
frequently used, like steam distillation. It involves put-
ting aromatic plants in a static extraction vessel and
covering them with an organic solvent, such as hexane,
benzene, toluene, ethanol, or light petroleum or a
binary mixture. Once equilibrium has been reached
(or at least nearly so), the solution is separated, and
the solvent is evaporated in a vacuum to yield the
extract. Two particular methods are extraction with
water and extraction with aqueous alcohol.
0019 The choice of the solvent depends on many tech-
nical and economic parameters, in particular: select-
ivity; boiling point; diffusivity; miscibility with water;
facility for recycling; safety – in general, the solvent
chosen must be of as low a toxicity as possible both
for operation of the extraction process and for con-
sumption of the product.
0020The process of extraction used also depends on the
nature of vegetable matter, such as its thermal lability,
the operating temperature ranging from ambient to
the boiling point.
0021According to the technique and solvent used, the
products are known by one of the following names:
.
0022Tinctures: obtained either by treating natural raw
materials with ethanol or ethanol–water mixtures
or by dissolving an extract in these solvents.
.
0023Oleoresins: concentrated extracts with a character-
istic odor and/or flavor, obtained from a natural
raw material.
.
0024Resinoids: concentrated nonaqueous extracts with
a characteristic odor obtained from a dried natural
raw material.
.
0025Concretes: concentrated nonaqueous extracts with
a characteristic odor and/or flavor obtained from a
fresh natural raw material.
0026Besides odorant compounds, the organic solvent
also extracts such undesirable substances as waxes
and lipids, which are responsible for the nature of
‘concretes.’ In fact, a transformation is needed from
concrete to absolute. Because waxes are not soluble in
alcohol at low temperatures (about 10
C), the con-
crete is diluted with alcohol at 30–40
C and strongly
stirred. When this alcohol solution is refrigerated at
5to10
C, waxes precipitate and are filtered off.
The filtrate is concentrated under vacuum, and after
elimination of alcohol, the absolute remains. Gener-
ally absolutes are in liquid form, though they may be
viscous or ‘pasty.’
0027CO
2
extraction This process (see Figure 2) uses high
pressure and, depending on the characteristics desired
in the product, employs either liquid carbon dioxide
(300 bar, 30
C) or supercritical carbon dioxide (350
[61] p-Cymene
O
(1)
(3)
[62] Turmerone
O
[63] ar-Turmerone
a
Compounds [4a] and [4b], which occur as cis and trans isomers in wines, are also known as linalooloxides.
b
Corresponding aldehydes genanial, neral, and citronellal also occur in foods. Citral is a mixture of neral and geranial.
c
The corresponding acid is an important aroma constituent of wine cultivars ‘Traminer’ and ‘Scheurebe.’
d
()-3,7-Dimethyl-1,5,7-octatrien-3-ol (hotrienol) is found in grape, wine, and tea aromas.
From Belitz H-D and Grosch W (1987) Food Chemistry. Berlin: Springer.
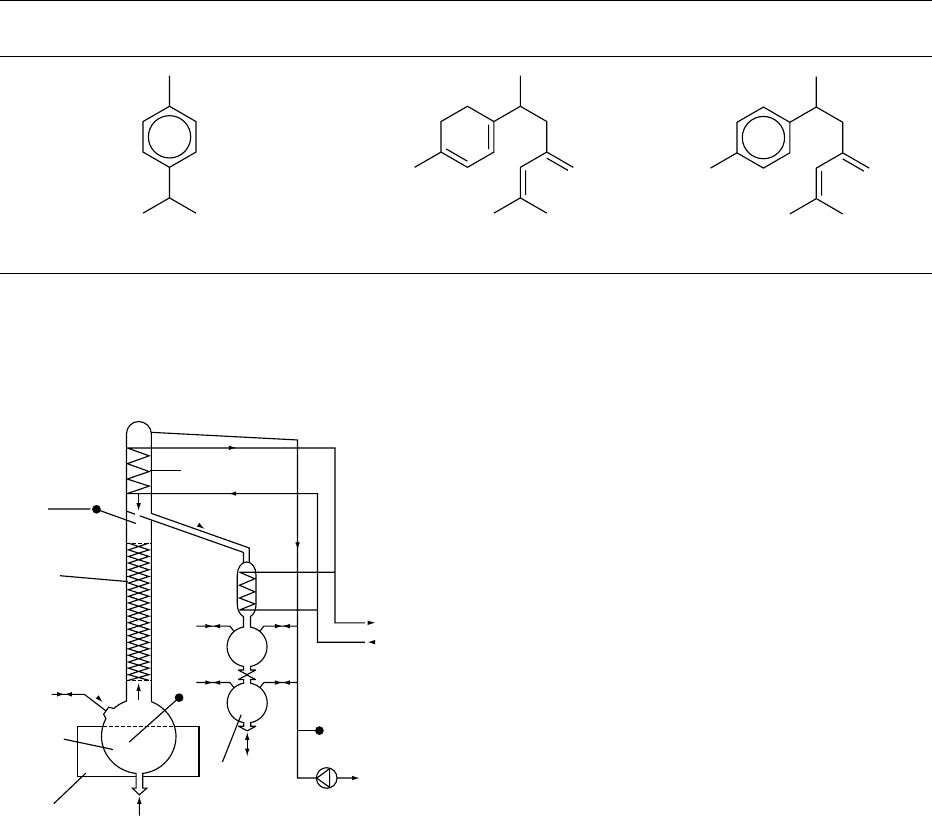
Probe
Column
Input
Raw
material
Heating
jacket
Distillate
Probe
Vacuum pump
Pressure
Water
Condensers
θ
θ'
Circulation
fig0001 Figure 1 Vacuum distillation column. Reproduced from Spices
and Flavouring Crops: Properties and Analysis, Encyclopaedia of
Food Science, Food Technology and Nutrition, Macrae R, Robinson
RK and Sadler MJ (eds), 1993, Academic Press.
Table 3 Continued
5498 SPICES AND FLAVORING (FLAVOURING) CROPS/Properties and Analysis
bar, 50
C). The separation of extract takes place in
the gas phase by a simple separation of gas and liquid.
0028 The principle of the method is based on good solu-
bility in carbon dioxide of most odorants of vegetable
matter and semifinished products, such as concrete
or even essences. The technique has the following
advantages:
.
0029 No residual toxic solvent, particularly important
for materials used in flavors.
.
0030 Low process temperature, important when pro-
cessing unstable and heat-sensitive products.
.
0031 Selectivity (e.g., in caffeine extraction).
.
0032 Lack of fire hazards.
.
0033 Energy efficiency (no loss of organic solvents such
as hexane or benzene, which have to be redistilled
for use again).
Carbon dioxide as a solvent is relatively nonpolar, so
its solubilizing powers are comparable to, but slightly
more polar than, those of hexane. The latter is used
frequently in the production of aromatic vegetable
matter.
0034 Extraction using ultrasound This process consists of
vibrating the material by means of supersonic waves,
which, owing to resonance, disintegrates the product
matrix. More particularly, when the process is carried
out in a liquid medium, the vibrational energy applied
produces pressure variations that greatly facilitate
the dispersion of the desired products in the solvent
after they have been separated from their natural
matrices.
Expression
0035Expression of oil at low temperature is a response to
two particular features of the essential oils of citrus
plants: (1) these oils contain terpene peroxides and
polymerizable terpenes, so that they are heat-sensitive
to hydrodistillation temperatures; (2) the oils are
located in the porous pericarp, which is easily torn.
It is therefore preferable to recover the essential oils
by simple mechanical action.
0036It is the only process for treatment of aromatic
plants without using a fluid extraction phase and
involves either expressing the pericarp by water pres-
sure or crushing whole fruits in a metallic cylinder.
The essences so produced have low densities and can
be separated from the aqueous phase by centrifuga-
tion. They are also designated ‘essential oil.’
Specialized Extraction Techniques
0037Molecular distillation Molecular or short-path dis-
tillation is used to obtain colorless products; more
stable products, because of the elimination of con-
stituents with higher molecular weight (acids, pig-
ments); and more delicate notes, because of the
increase in the proportion of odorant in the oil.
0038Because of the high vacuum (0.05–0.0005 torr),
the boiling point and extraction time are greatly
Pressure
HE
HE
HE
θ'
θ
P'
HE
Heat exchanger
(HE)
Compressor
Separator
(extract)
Charcoal trap
P"
CO
2
θ'< θ
P'< P
P > 1071 psi
θ >318C
Extractor
raw material
or semifinished
product + CO
2
Safety valve
P
fig0002 Figure 2 CO
2
extraction system. Reproduced from Spices and Flavouring Crops: Properties and Analysis, Encyclopaedia of Food
Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
SPICES AND FLAVORING (FLAVOURING) CROPS/Properties and Analysis 5499
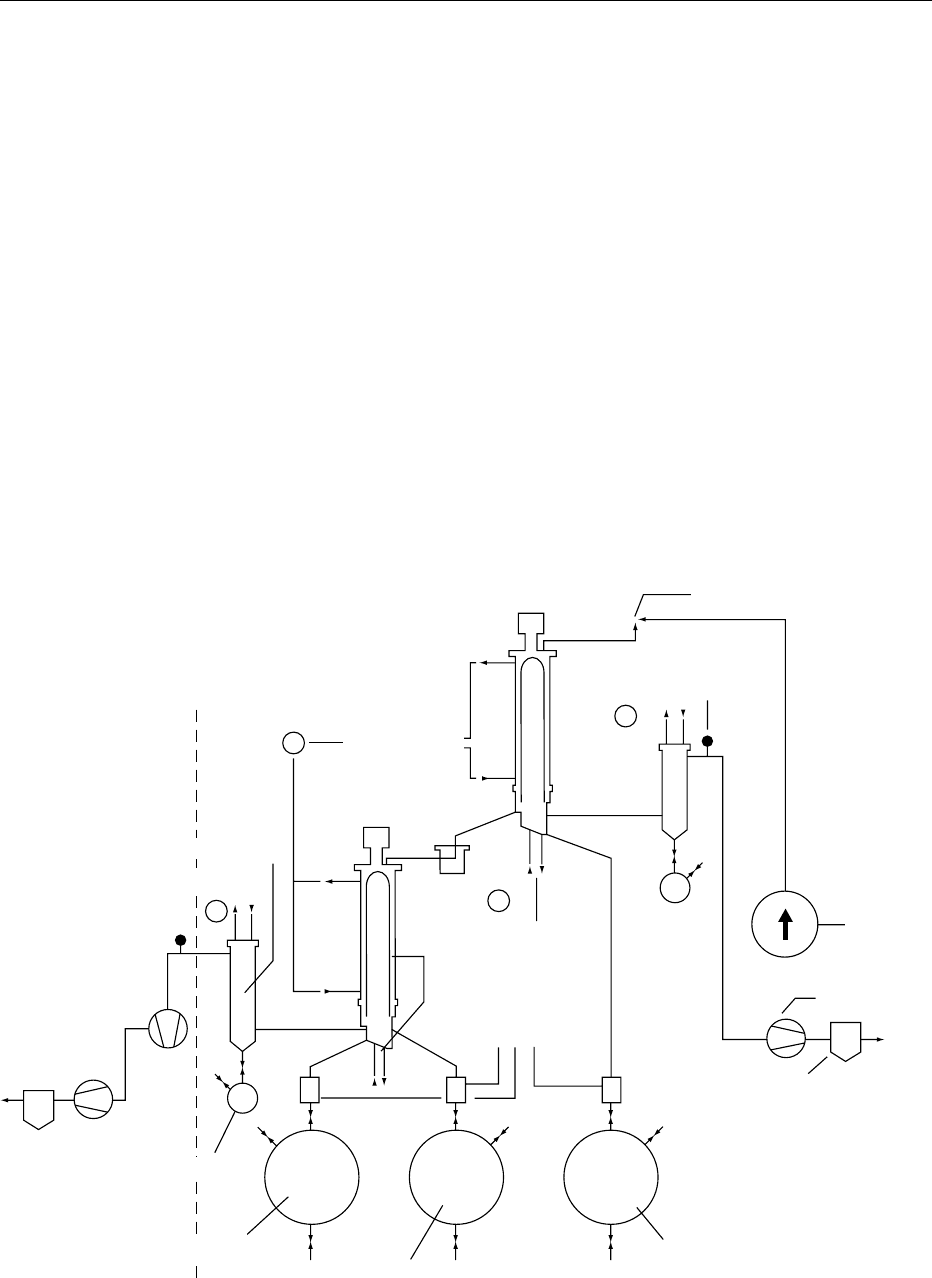
reduced, thus preventing the loss of some heat-
sensitive notes in the oil. The product to be proces-
sed is combined with a heavy, and a light solvent and is
then passed through the first short-path evaporator;
the most volatile components condense together
with the light solvent on a so-called ‘finger’ in the
middle of the evaporator and are recovered as the
first distillate (see Figure 3). The other components
condense on the walls of the evaporator and are
pumped into a second short-path evaporator. Here
again, the next most volatile components of the oil
condense on the inner ‘finger’ and are recovered
as the second distillate. The residues and the heavy
solvent condense on the walls and are recovered in the
residue tank.
0039 Simultaneous distillation/extraction This original
process was designated by Likens and Nickerson. It
uses two fluids; the volatile compounds are removed
by steam distillation, and a nonpolar solvent continu-
ously extracts them from the aqueous distillate.
0040The geometry of the apparatus enables the steam
carrying the volatiles and the solvent vapor to meet
in the condenser. The condensate separates into two
phases, which are directed to their respective vapor
generators. Eventually, the evaporation of the solv-
ent allows recovery of a concentrated solution of
odorant.
0041The relative position of two vapor generators
differs according to the density of the solvent used.
The method is particularly applicable in the study of
rare vegetable materials.
0042Capture of headspace Three methods have been
used as follows:
.
0043Adsorption in liquid, which allows capture of the
volatile components by passing the head space
vapors through a solvent, and then concentrating
the solution by evaporation.
.
0044The cryogenic method, which condenses the vola-
tile components in a series of refrigerated traps.
Low temperature trap
Nitrogen cooling system
Oil diffusion pump
Condensate vessel flask
2nd Distillate flask
Residue flask
1st Distillate flask
Graduated vessel
Water cooling system
Vacuum pump filters
Vacuum pump
Product
to be
processed
Pressure retention valve
Manometer
B
A
C
B
Agitator
Heat exchanger
Short-path evaporator
fig0003 Figure 3 Molecular distillation unit. Reproduced from Spices and Flavouring Crops: Properties and Analysis, Encyclopaedia of Food
Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
5500 SPICES AND FLAVORING (FLAVOURING) CROPS/Properties and Analysis

This method has been used particularly for the
capture of the volatile components of fruit.
.
0045 Adsorption of solids, which involves passing the
headspace over an adsorbent, such as XAD-4
(polystyrene) or TENAX [poly(2,6-diphenyl-p-
phenylene oxide)]. The volatiles are recovered by
thermal desorption or by elution with an organic
solvent.
Volatiles
0046 Odorants must be volatile to some extent, and this
property is common in those molecules that contain
fewer than 10 carbon atoms, for example, monoter-
penes and their derivatives. It must be noted that alde-
hydes, ethers, and esters generally have a stronger odor
than their parent hydrocarbons. This is why they are
used for head notes in the formation of perfumes,
cosmetics, and deodorants.
0047 Volatiles of higher molecular mass evaporate rela-
tively slowly at room temperature; because of this
property, such compounds provide the more persist-
ent odors.
Nonvolatiles
0048 Nonvolatiles such as sugar, salt, piperine, and caf-
feine, may affect taste, although they cannot be
detected by smell at room temperature.
Mechanism of Degradation and Staling
0049 During steam distillation of an essential oil, terpenoid
compounds are susceptible to degradation. Terpene
alcohols and esters, especially, undergo a variety of
well-known chemical reactions (hydrolysis, hydra-
tion, and cyclization), in particular under acidic
conditions. For example, low pH values induce con-
siderable hydrolysis of linalyl acetate and extensive
rearrangement reactions. It is well known that ter-
tiary alcohols and their esters are protonated in aque-
ous media to yield oxonium ions, which split to form
a tertiary carbocation; thus, degradation reactions
and rearrangements can be observed under the condi-
tions of steam distillation.
0050 In the mechanism of linalyl acetate thermal degrad-
ation, the generation of a primary allylic carbocation
– the linalyl carbocation, which creates a p-menthane
skeleton by cyclization – explains the presence of
nerol and geraniol among the degradation products.
0051 Some degradation can take place during the storage
of essential oils, especially for oxygenated compon-
ents. For example, citronellal can be resinified in a
basic medium or oxidized in contact with air in the
presence of light.
0052Since flavoring compounds are volatile, they can be
lost easily by evaporation, thus leading to staling if
the conditions of storage are not appropriate.
Microbiological Contamination
0053A number of essential oils, as well as some spices and
aroma extracts, e.g., thyme and cloves, which are rich
in phenolic compounds, possess antifungal and anti-
bacterial properties. In general, essential oils have a
good bacteriological quality, provided humidity is
not too high. In contrast, the chemical stability of
some essential oils is limited on account of oxidation,
for example, those that are rich in limonene. Such
essential oils must be protected against oxidation
during storage. (See Spoilage: Chemical and Enzym-
atic Spoilage; Bacterial Spoilage.)
0054Bacteriological contamination is a hazard for
spices and aroma extracts, particularly if they are
aqueous extracts, for example, alliaceous extracts. It
is necessary to ensure their bacteriological quality.
The chemical stability for certain extracts, such as
pepper and rosemary, is protected by the presence
of natural antioxidants. (See Antioxidants: Natural
Antioxidants.)
See also: Antioxidants: Natural Antioxidants; Browning:
Nonenzymatic; Essential Oils: Properties and Uses;
Isolation and Production; Flavor (Flavour) Compounds:
Structures and Characteristics; Phenolic Compounds;
Sensory Evaluation: Aroma; Taste; Spoilage: Chemical
and Enzymatic Spoilage; Bacterial Spoilage
Further Reading
Association Franc¸aise de Normalisation (1988) Contro
ˆ
le
de la qualite
´
des produits alimentaires – Epices et
Aromates, 2nd edn. Paris: AFN.
Bicchi C and Sandra P (1987) Capillary Gas Chromatog-
raphy in Essential Oils Analysis. Heidelberg: Springer.
Cu Jian Qin (1990) Extraction de Compositions Odorantes
Ve
´
ge
´
tales. PhD thesis, Institut Polytechnique de Tou-
louse No. 393.
International Standards Organization (1984) Essential Oils
– Terminology. Murcia: ISO.
Meyer-Warnod B (1984) Natural essential oils – extraction
processes and application to some major oils. Perfumer
& Flavorist 9(2): 93–104.
Morin Ph and Richard H (1984) Thermal degradation
of linalyl acetate during steam distillation. In: Pro-
ceedings of The 4th Weurman Flavor Research Sympo-
sium, Dourdan, France, pp. 563–576. Amsterdam:
Elsevier.
SPICES AND FLAVORING (FLAVOURING) CROPS/Properties and Analysis 5501

SPOILAGE
Contents
Chemical and Enzymatic Spoilage
Bacterial Spoilage
Fungi in Food – An Overview
Molds in Spoilage
Yeasts in Spoilage
Chemical and Enzymatic
Spoilage
N F Haard, University of California, Davis, CA, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The storage life of foodstuff is limited by the occur-
rence of chemical reactions which alter edible quality,
including deterioration in optimal color, appearance,
texture, aroma, flavor, nutrition, safety, and functional
properties. When food undergoes deterioration in
quality, it is not always clear whether the reaction
causing the problem is of nonenzymatic, enzymatic,
or microbial origin. For example, development of bad
odor in stored coleslaw prepared with mayonnaise and
sour cream was first attributed to microbial spoilage.
However, investigation of the problem revealed that
endogenous enzymes in the cabbage were activated
by the anaerobic conditions in the package and were
responsible for the formation of offensive- smelling
volatiles. Likewise, enzymatic reactions rather than
microbial spoilage was shown to be the primary cause
of early spoilage in chilled, properly handled fish, by
studying the initial quality loss of the flesh in sterile and
nonsterile fish. In most cases, chemical and microbial
spoilage acts in concert. For example, in cold-smoked
salmon microbial activity causes production of charac-
teristic spoilage off-odors and off-flavors while auto-
lytic enzymes from the fish tissue areresponsible for the
characteristic texture deterioration. Moreover, there
are also many instances where chemical, enzymatic,
and microbial spoilage reactions are interactive
processes. For example, products of nonenzymatic
browning have been shown to inhibit other enzymatic,
nonenzymatic, or microbial spoilage reactions.
Chemical Reactions that Contribute to
Spoilage
0002 Chemical spoilage includes enzyme-catalyzed reac-
tions as well as nonenzymatic reactions. In general,
enzyme-catalyzed reactions are of primary concern in
untreated plant and animal tissues and nonenzymatic
reactions predominate in properly processed food-
stuffs. There are, however, some exceptions to this
general rule. In addition, enzymatic and nonenzy-
matic spoilage reactions may act in concert. For
example, discoloration of the surface of red meats is
caused by a nonenzymatic reaction, oxidation of myo-
globin (Fe
2þ
) to form metmyoglobin (Fe
3þ
). However,
enzymatic reactions involved with respiration at the
tissue surface can lower oxygen concentration and
indirectly promote nonenzymatic oxidation of myo-
globin. Some muscle tissues also contain the enzyme
metmyoglobin reductase, which catalyzes the reduc-
tion of metmyoglobin back to myoglobin. Likewise,
the nonenzymatic denaturation and aggregation of
myosin primarily cause texture deterioration during
frozen storage of certain species of fish. However,
these reactions may be accelerated by the action of
trimethylamine oxide demethylase, which forms for-
maldehyde, or by phospholipase, which forms free
fatty acids. It is normally possible to distinguish be-
tween enzymatic and nonenzymatic catalyzed spoil-
age reactions by conducting simple experiments
(Table 1).
Physiological Processes
0003Some spoilage reactions are of biological origin, i.e.,
are ‘of or pertaining to living organisms,’ and these
include sets of enzyme-catalyzed reactions. For
example, a set of biological reactions described
under the general heading ‘postharvest physiology
of fruits and vegetables’ are important because they
may contribute to deteriorative processes such as
plant senescence, wound response, chilling injury,
and other types of stress response. Physiological
processes in fruits and vegetables also lead to
desirable changes such as fruit ripening, wound
healing after harvest, and the reduction of reducing
sugars during the ‘conditioning’ of potato tubers.
Likewise, ‘postmortem physiology of food myosys-
tems’ describes a set of reactions that normally
cause a desired conversion of muscle to meat.
5502 SPOILAGE/Chemical and Enzymatic Spoilage

However, in some cases postmortem reactions may
contribute to poor-quality meat, e.g., ‘burnt tuna,’
‘pale, soft, exudative pork,’‘cold shortening,’ and
‘thaw rigor.’
0004 Spoilage reactions in the case of harvested and
stored plant and animal tissues are, for the most
part, catalyzed by enzymes. Myriad enzymes occur
naturally in biological tissues used by humans for
food. There is remarkable similarity in the basic
metabolism of biological cells. However, the
amounts and different kinds of specific enzymes pre-
sent in a given cell may be quite distinctive because
of heritable characteristics, intrinsic factors such as
age of the source organism, and extrinsic factors
such as preharvest stress, growing conditions, and
diet. Only a small set of enzyme-catalyzed reactions
has thus far been clearly identified with food spoil-
age. Many important deteriorative processes in food
are caused by families of enzymes operating in se-
quence and catalyzing a net change in the tissue, such
as synthesis of lignin in asparagus. Some other
examples of multistep reactions, which contribute
to losses in food quality, are summarized in Table 2.
The detailed mechanisms and biochemical controls
for many of these multistep reactions are not com-
pletely understood. For example, until the advent of
rDNA technology it was generally believed that
the increased solubility of pectin and the associated
texture softening of fruit are caused by hydrolysis of
a-1,4 galacturonate linkages in pectin, a reaction
catalyzed by polygalacturonases (EC 3.2.1.15; EC
3.2.1.67). However, blocking the synthesis of poly-
galacturonases in ripening tomato fruit was not
completely effective in preventing the softening of
transgenic fruit. Since pectin contains small amounts
of other sugar residues in addition to a-1,4 galactur-
onate linkages, it now appears that other, not yet
identified, mechanisms play a role in tomato fruit
softening.
Other Endogenous Enzymes
0005One-step enzyme-catalyzed processes may also
contribute to quality deterioration, particularly in
processed foods. For example, for orange juice, in
which a stable colloidal suspension is desired, the
action of endogenous pectin methylesterase (PME;
EC 3.1.1.11) is undesirable since demethylation of
pectin, catalyzed by this enzyme, leads to separation
of serum from particulates in juice. Heat processing
of orange juice to inactivate PME is undesirable since
a nonenzymatic chemical reaction adversely affects
the delicate flavor of the product. Hydrolases, like
PME, as well as oxidoreductases are the most studied
enzymes in connection with food spoilage. Examples
of enzymes and their contribution to quality loss in
specific commodities are summarized in Table 3.
Nonenzymatic Reactions
0006In the case of heat-treated or other processed food-
stuffs where enzymes have been destroyed or their
activity has otherwise been arrested, nonenzymatic
chemical reactions play a more important role in
food spoilage than enzyme-catalyzed reactions. As
with enzyme-catalyzed reactions some, but not all,
nonenzymatic changes that occur during food pro-
cessing and storage will result in loss of quality.
Examples of nonenzymatic reactions that can lead
to deterioration in food quality are listed in Table 4.
The two nonenzymatic reactions in foods which
appear to be of most widespread importance are
Maillard browning and lipid oxidation. These reac-
tions are discussed in detail in other sections.
Factors Influencing Chemical Reactions in Food
0007The rate of chemical reactions in foodstuffs is a func-
tion of one or more variables, including pH, tempera-
ture, ionic strength, concentration of reactants,
presence of catalysts, mobility of reactants, oxidation
tbl0001 Table 1 Some general characteristics that can distinguish enzymatic and nonenzymatic catalyzed reactions
Treatment Characteristics
Thermal inactivation As proteins, most enzymes are inactivated by a brief heat treatment, while catalysts of nonenzymatic
reactions are normally heat-stable
Dialysis As macromolecules, enzymes are nondialyzable, while catalysts of nonenzymatic reactions are normally
low-molecular-weight and removed by this treatment
Specificity The number of reactants and products in enzymatic reactions are normally less than in the
corresponding nonenzymatic reaction. Enzyme catalysis and inhibition are characterized by
stereospecificity
Proteolysis Enzyme catalysts, as proteins, are often inactivated by treatment with proteolytic enzymes
pH While nonenzymatic reactions are often acid- or base-catalyzed, enzymatic reactions are characterized
by a relatively narrow pH optimum
Temperature coefficient The Q
10
of nonenzymatic reactions is normally much lower than that of enzymatic reactions
SPOILAGE/Chemical and Enzymatic Spoilage 5503
reduction potential, competing reactions, decompart-
mentation of reactants, and the physical state of the
reaction milieu. One of the most important variables
influencing chemical changes in food is temperature.
The Arrhenius relation gives the influence of tempera-
ture on reaction rate:
k ¼ k
E
a
=RT
o
where k is the reaction rate constant, k
o
is the pre-
exponential constant, E
a
is the activation energy in
kilojoules per mole, R is the gas constant, and T is the
absolute temperature in Kelvin. E
a
is normally much
lower for enzymatic reactions than for nonenzymatic
reactions. Thus, in general, the rate of a nonenzy-
matic reaction is more sensitive to temperature
change than is an enzymatic reaction. Enzymes, like
other proteins, are subject to thermal denaturation.
Accordingly, the range of temperature in which cata-
lytic rate increases with a rise in temperature is limited
by the enzyme’s thermal inactivation temperature.
Because of these two considerations, the ‘master’
chemical reaction, i.e., that which limits the storage
tbl0003 Table 3 Some endogenous enzyme-catalyzed reactions that contribute to food spoilage
Enzyme ECnumber Food Importance
Lipoprotein lipase 3.1.1.34 Milk Releases short-chain fatty acids from milk fat leading to hydrolytic
rancidity
Alkaline protease 3.4..24.40 Milk Since this enzyme is stable to heat it may contribute to gelation in
products processed at ultra high temperatures
Thiaminase 2.5.1.2, 3.5.99.2 Shellfish Loss of thiamin in fermented products
Phospholipase 3.1.1.4, etc. Fish Releases fatty acids in frozen product causing denaturation of muscle
proteins and texture deterioration
Trimethylamine oxide
demethylase
4.1.2.32 Fish Releases formaldehyde in frozen gadoid fish that contributes to protein
aggregation and texture deterioration
Lipoxygenase 1.13.11.12, etc. Legume seeds Formation of specific hydroperoxides can lead to bleaching of
pigments, offensive flavor formation, as well as texture change and
loss of nutrients
Peroxidase 1.11.1.1, etc. Vegetables Decomposition of hydroperoxides with generation of free radicals
appears to cause bleaching of pigments, off-flavors, etc.
Ascorbic acid oxidase 1.11.1.11 Citrus Results in loss of vitamin C activity in orange juice
Chlorophyllase 3.1.1.14 Green vegetables Removal of the phytol side chain from chlorophyll appears to be part
of the degreening process during plant senescence
Polyphenol oxidase 1.10.3.1 Fruits, shellfish Enzymatic browning
tbl0002 Table 2 Some multistep enzyme-catalyzed reactions that contribute to food spoilage
Net reaction Contribution to food spoilage
Glucosestarch Loss in sweet taste of some vegetables, e.g., sweetcorn
GlucoseCO
2
þ H
2
O Respiration rate is directly related to spoilage rate of fruits, vegetables, and seed crops
Starchglucose þ fructose Cold sweetening of some vegetables leads to excessive Maillard browning, e.g., potato
tuber
Glycogenlactic acid þ H
2
O Glycogenolysis and glycolysis are of central importance in postmortem myosystems.
The rate and extent of associated pH decline directly (e.g., water-holding capacity, protein
denaturation) and indirectly (e.g., rate of other enzymatic and nonenzymatic reactions)
to influence quality
ATPhypoxanthine The rate and extent of adenosine triphosphate catabolism in myosystems influences meat
quality in several ways, e.g., the acceptability of fish is directly related to the
accumulation of hypoxanthine
Methionineethylene The plant hormone ethylene is synthesized by the ‘methionine cycle’ during specific
developmental change or as a consequence of wound injury. In turn, increased
ethylene can initiate biosynthesis of enzyme cascades, e.g., chlorophyll destruction or
lignin biosynthesis
Insoluble pectinsoluble pectin Texture softening and increased vulnerability to saprophytic microorganisms and physical
damage in some fruits and vegetables, e.g., ripe tomato
Phospholipidaldehydes, ketones,
free radicals
Lipoxygenase cascades can decrease the amounts of essential nutrients, and cause
off-flavors
Collagenpeptides, amino acids Postmortem degradation of collagen is catalyzed by a family of enzymes and can influence
physical integrity, appearance, and yield of fish fillets
5504 SPOILAGE/Chemical and Enzymatic Spoilage

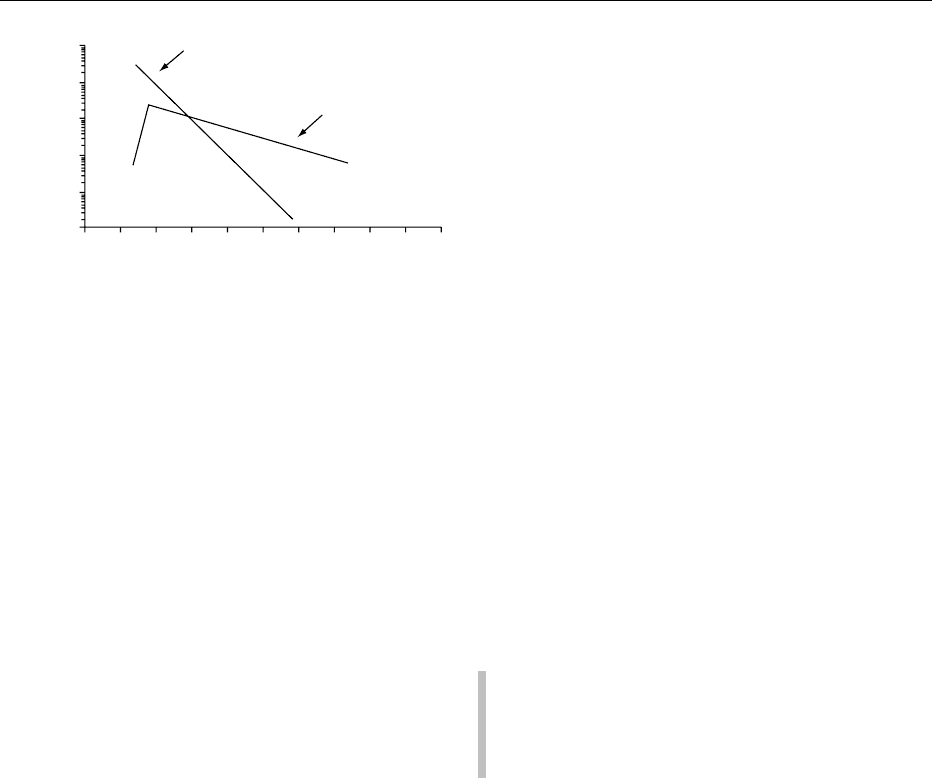
life of a given product, normally differs with the
temperature at which the product is stored (Figure 1).
It is also important to recognize that E
a
for a given
reaction in a particular temperature range is also a
function of other parameters (e.g., pH, glass transi-
tion). The extent to which other parameters influence
thermal response depends on the particular reaction
under consideration.
0008 The extent and direction to which chemical trans-
formations are influenced by parameters other than
temperature depend on the reaction under consider-
ation. For example, removal of oxygen from fish tissue
decreases the rate of enzymatic or nonenzymatic lipid
oxidation and the development of off-flavors, but
at the same time it increases the rate of enzymatic
trimethylamine oxide (TMAO) demethylation and
associated protein aggregation. Likewise, reducing
the water concentration of food by dehydration may
decrease the mobility of water-soluble reactants, but
it does not serve the same function for fat-soluble
reactants and may even promote lipid oxidation by
exposing lipid substrates to oxygen and increasing the
availability of a metal catalyst.
0009It is also important to consider that, food-processing
and storage conditions, designed to minimize one
detrimental reaction, may inadvertently alter other
tbl0004 Table 4 Some nonenzymatic reactions that can lead to loss in food quality
Reactant(s) Product orresult Importance
Chlorophyll, H
þ
Pheophytin, Mg
2þ
Loss of Mg
2þ
from chlorophyll results in olive-brown
discoloration of green vegetables. Reaction occurs rapidly at low
pH and high temperature
All-trans b-carotene Tr a n s –cis rearrangement Isomerization of carotenoids is promoted by light or heat and results
in isomers, which absorb light in shorter wavelength and with
lower extinction coefficients, and loss of provitamin A activity
Cysteine H
2
S, NH
3
, acetaldehyde Thermal degradation of sulfur-containing amino acids can directly
produce aroma and also lead to other reactions involving the
products
RCHO, RNH
2
Maillard browning The Maillard reaction is one of the most important reactions in foods,
affecting color, flavour, nutrition, and possibly safety
Amylose Crystallization The alignment of linear starch chains by hydrogen bonding to form
insoluble precipitates, a process called retrogradation, is important
in processed products containing gelatinized starch,
e.g., bread staling
Anthocyanin, SO
2
Decolorization The addition of SO
2
to the 4-position of anthocyanins to form a
bisulfite addition product results in loss of color
Organic acid, Ca
2þ
Ca-chelate Organic acids like phytic acid or citric acid can destabilize
polygalacturonate in the middle lamella by sequestering Ca
2þ
and
thereby influence texture
Ascorbic acid 2-Furaldehyde, CO
2
The nonenzymatic degradation of vitamin C occurs by an acid/metal
ion-catalyzed reaction or by an oxidative mechanism. In addition
to loss in nutritive value, carbonyl degradation products, such as
diketogulonic acid, can contribute to Maillard browning
Fatty acid, O
2
Autooxidation Autooxidation is a free radical reaction which can be catalyzed by
metal ions; the reaction rate increases with degree of unsaturation;
a primary effect on quality is formation of off-flavors; however,
all quality indices, including nutrition, color, texture and safety
may be influenced under appropriate conditions
Protein Denaturation Loss of protein native structure can lead to protein aggregation and
loss in functional properties with the possibility of influence on all
quality indices under appropriate reaction conditions
Myoglobin,
thiol compound, TMAO
Green tuna Precooking tuna containing a high content of trimethylamine oxide
(TMAO) can lead to green discoloration of tuna by an oxidation
reduction reaction
Aspartame, peptides, nitrite Mutagenic compounds Naturally occurring dipeptides and the artificial sweetener aspartame
can form mutagenic compounds after nitrosation. Aspartame is also
unstable under alkaline conditions and heat
Arginine 1-Methylguanidine High-temperature (e.g., 150–210
C) treatment of arginine results in
formation of mutagenic compounds, such as those found in cooked
grain-based foods
Thiamine, H
þ
or OH
2-Methyl-4-amino 5-aminethyl
pyrimidine, etc.
Vitamin B
1
is destroyed by various reactions, including acid- or
base-catalyzed reactions, redox reactions, photolysis, and reaction
with bisulfite
SPOILAGE/Chemical and Enzymatic Spoilage 5505
parameters which, in turn, may function to promote
the same detrimental reaction. For example, while
freezing of food lowers temperature and water activ-
ity, and thus is expected to lower the rate of reaction,
it may serve otherwise to accelerate the rate by unex-
pected changes in pH, ionic strength, or reactant
availability.
0010 Moreover, storage or processing strategy designed
to minimize a reaction that adversely affects storage
life may accelerate other reactions that cause a differ-
ent, but equally unacceptable, quality. For example,
pasteurization of kiwi fruit juice (pH 3.5) inactivates
enzymes and spoilage organisms that contribute to
spoilage; but at the same time, heat treatment causes
rapid pheophytinization of chlorophyll and loss in the
characteristic green color of the product. Because of
the complexity of food systems it is often difficult to
predict the consequences of processing and storage
conditions on quality. Accordingly, efforts to minim-
ize the contribution of chemical reactions to food
spoilage require an empirical approach, at least in
part.
See also: Browning: Nonenzymatic; Enzymatic –
Biochemical Aspects; Enzymatic – Technical Aspects and
Assays; Enzymes: Functions and Characteristics; Uses in
Food Processing; Spoilage: Bacterial Spoilage
Further Reading
Bradley DG and Min DB (1992) Singlet oxygen oxidation
of foods. CRC Critical Reviews in Food Science and
Nutrition 31: 211–236.
Chien-Yi W (1994) Chilling injury of tropical horticultural
commodities. HortScience 29: 986–988.
Eskin NAM (1990) Biochemistry of Foods, 2nd edn. New
York: Academic Press.
Fennema OR (ed.) (1996) Food Chemistry, 3rd edn. New
York: Marcel Dekker.
Haard NF (1994) Technological aspects of extending prime
quality of seafood: a review. Journal of Aquatic Food
Product Technology 1: 9–27.
Haard NF (1997) Food as cellular systems: impact on qual-
ity and preservation. In: Taub IA and Singh P (eds) Food
Storage Stability, pp. 39–74. Boca Raton: CRC Press.
Haard N (1997) Product composition and the quality of
frozen foods. In: Erickson MC and Hung Y-C (eds)
Quality in Frozen Foods, pp. 275–295. New York:
Chapman and Hall.
Haard NF and Simpson BK (eds) (1999) Seafood Enzymes.
New York: Marcel Dekker.
Huis INT and Veld JHJ (1996) Microbial and biochemical
spoilage of foods: an overview. International Journal of
Food Microbiology 33: 1–18.
Jayaprakasha HM, Jayaraj-Rao K and Lokesh-Kumar WA
(1997) Studies on the influence of water activity (A
w
)on
the stability of foods – a critical appraisal. Journal of
Food Science and Technology, India 34: 273–285.
Richardson T and Finley JW (eds) (1985) Chemical
Changes in Food During Processing. Westport: AVI.
Rizzi GP (1997) Chemical structure of colored Maillard
reaction products. Food Reviews International 13: 1–28.
Wong DWS (1989) Mechanism and Theory in Food
Chemistry. Westport: AVI.
Bacterial Spoilage
E A Zottola, (retired) University of Minnesota, St. Paul,
MN, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001Food for humans is obtained from plants and
animals. The nutrients in these foods serve not only
human needs but are also vital to the growth and
survival of bacteria. Thus, if food is not immediately
utilized or preserved after harvest, it spoils. Bacterial
spoilage is inherent in foods. The spoilage process
begins as soon as the food is harvested or slaughtered,
and if steps are not immediately taken to prevent
spoilage, the food will become unacceptable for
human use. Spoilage of food can be caused by many
factors: enzyme activity, physical damage, chemicals,
rodents, insects, and metabolic activities of micro-
organisms, bacteria, yeasts, and molds. Foremost
among the causes of food spoilage is the growth and
utilization of the food by bacteria. Food is spoiled by
bacteria when it becomes esthetically unacceptable to
the user. What is spoiled to one may be a delicacy
to another. It may have a foul odor or off-flavor, or be
discolored, slime may be apparent, or there may be
visible microbial growth on the food. These obvious
0.0001
0.001
0.01
0.1
1
Rate constant (k )
3 3.1 3.2
1/T (K
−1
) 10
3
3.3 3.4
Enzymatic reaction
Nonenzymatic reaction
fig0001 Figure 1 Typical temperature dependence of enzymatic and
nonenzymatic reactions. Reproduced from Spoilage: Chemical
and Enzymatic Spoilage, Encyclopaedia of Food Science, Food
Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ
(eds), 1993, Academic Press.
5506 SPOILAGE/Bacterial Spoilage
