Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

0021 Distillation Steam distillation finds application in
the analyses of volatiles from beverages and high-
water-content foods, although it is less applicable to
fats and oils. It has the disadvantage that the large
quantities of aqueous distillate require further extrac-
tion with a solvent, to separate the volatiles from the
water. Concentration of the extract is then necessary.
The formation of artefacts may also be a problem.
Vacuum distillation finds wide application in the
extraction of volatiles from fats and oils, and at low
extraction temperatures artefact formation can be
minimized. In molecular distillation, a high vacuum
(< 10
3
mmHg), is used, and the volatiles have a rela-
tively short path to travel from the surface of a liquid
oil sample to a cold surface where they condense.
Efficient collection of high-boiling-point components
is achieved because at high vacuum, the distance
between the sample surface and the cold condenser
(10–20 mm) is less than the mean free path of the
molecules. All of these distillation processes incorpor-
ate cold traps for the collection of the distillate.
0022 Extraction Direct extraction of an aqueous food
fraction with an organic solvent is of limited use,
because the extract will contain a large amount of
nonvolatile matter. However, supercritical carbon di-
oxide has been utilized as a solvent for the extraction
of aromas on both a laboratory and a commercial
scale, particularly the preparation of high-quality
essential oils. Its solvating qualities can be altered
by changing the pressure or temperature at which
the extraction takes place, and under ideal condi-
tions, supercritical carbon dioxide exhibits a strong
affinity for most aroma compounds, whereas most
nonvolatile constituents are insoluble. The ease of
removal of the solvent, after extraction, to give a
concentrated aroma extract is another attractive fea-
ture of supercritical carbon dioxide extraction.
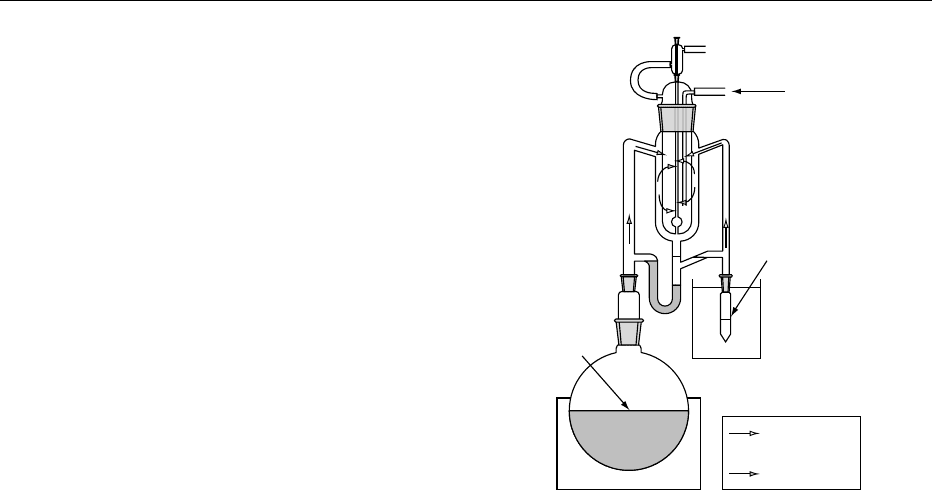
0023 One of the most widely used techniques in aroma
analysis combines steam distillation with solvent ex-
traction in a Likens–Nickerson apparatus (Figure 3),
which was first reported in 1964 for the extraction of
hop oil. The essential feature is the simultaneous
condensation of the steam distillate with an immis-
cible extraction solvent. A simple U-tube, with appro-
priate side arms, allows the return of water to the
steam distillation flask and the solvent, containing
the extracted volatiles, to a reservoir flask where it
redistils for further extraction. The technique pro-
vides a simple rapid method involving only small
volumes of solvent. By carrying out the extraction
under a reduced pressure, thermal degradation of
labile components can be diminished.
0024 The organic solvents used for extraction are chosen
on the basis of their selectivity for the volatile
compounds of interest and on boiling point. Com-
monly used solvents are diethyl ether, pentane,
isopentane, dichloromethane, and some chloro-
fluorocarbons. After each type of solvent extrac-
tion, most of the solvent must be removed to
provide an aroma concentrate suitable for GC.
Removal of traces of water, using a drying agent
(sodium or calcium sulfate) or by freezing out at
20
C, is essential before careful removal of solvent
by distillation. Final concentration to a volume of
0.1–1 ml is often achieved by purging with a gentle
stream of nitrogen.
Separation of Aroma Components
0025To determine the sensorially important compounds in
an aroma isolate, the complex mixture needs to be
separated into its components. The amount of isolate
is usually small, containing many compounds of di-
verse chemical structures, varying greatly in concen-
tration, and important components are often present
in extremely low amounts. The success of any aroma
analysis depends mainly upon the efficiency of
separation and the sensitivity of detection. GC using
bonded-phase fused-silica capillary columns is almost
universally used as the separation method in aroma
analysis. Such columns can separate complex mix-
tures, and the most commonly used stationary phases
are Carbowax 20M, a polar phase, and the two non-
polar phases, 100% poly(dimethylsiloxane) and
poly(5% diphenylsiloxane/95% dimethylsiloxane).
The stereochemistry of compounds can influence
water bath
heating mantle
flow of solvent
vapour
flow of steam
cooling
water at 5 C
solvent
sample in
water
fig0003Figure 3 Likens–Nickerson apparatus for continuous steam
distillation–solvent extraction.
SENSORY EVALUATION/Aroma 5177

aroma, e.g., (þ)-carvone possesses a spearmint aroma,
whereas ()-carvone possesses a caraway-like aroma;
capillary columns coated with chiral phases can be
used to separate enantiomers. The retention times of
an aroma compound on two columns with different
stationary phases can be helpful in its identification,
and databases containing retention data for volatile
compounds are available.
0026 Preparative liquid chromatography is sometimes
performed on solvent extracts, as a first step, to
simplify subsequent separations. The most widely
used example of this is flash chromatography, where
the extract is pipetted on to the surface of a stationary
phase, such as silica gel, which is contained in a glass
column. Fractions of the extract are obtained, as
solvents of increasing polarity are passed through
the column under positive air pressure. The fractions
are analyzed subsequently by GC. (See Chromatog-
raphy: Gas Chromatography.)
Detection of Components of Sensory Significance
0027 An essential step in the analysis of aroma is to deter-
mine which volatile compounds contribute to the
aroma of the food or beverage. Sensory profiling of
the whole product can give some indication of the
important aroma characters present in the product,
and such information should be used when assessing
the contribution of the individual components of an
aroma isolate to the overall flavor quality. A widely
used technique for determining components that
contribute to aroma is GC olfactometry (GCO). The
column effluent is split between a conventional GC
detector and a vent to the outside of the oven, where
the odors emerging can be smelled and described.
Chromatogram peaks can thus be identified that cor-
respond to specific aromas, and ‘aromagrams’ can be
compiled to complement the chromatogram.
0028 To estimate the relative contributions of volatile
components towards the total aroma quality of a
food, a set of serial dilutions of an aroma extract
can be made up and analyzed by GCO. At a certain
dilution, the aroma of a particular volatile compound
will no longer be perceived. The flavor dilution factor
for that compound is defined as the highest dilution at
which that compound can be perceived by GCO. For
example, if the concentration of the extract was
halved at each dilution and the seventh dilution was
the last at which the compound could be detected, its
flavor dilution factor would be 2
7
(128). Hence, if the
aroma extract is representative of the food from
which it is derived, the most important contributors
to the aroma of the food are those with the highest
flavor dilution factors. It should be noted that com-
ponents with high flavor dilution factors might not
give GC peaks of any significant size.
0029Another way of estimating the contribution of a
volatile compound to the aroma of a food is by calcu-
lating its odor unit number. The odor unit number of
an aroma compound in a food is defined as the ratio
between its concentration in the food and its odor
threshold, as measured by sensory evaluation. Hence,
the higher the odor unit number of the compound, the
more likely it is to contribute to the overall aroma of
the food. Several literature compilations of odor
thresholds exist.
Identification of Components
0030Structure elucidation of the chromatographically sep-
arated components is the next step in the analysis of
an aroma isolate. Coupled gas chromatography–mass
spectrometry (GC–MS) allows direct analysis of the
separated components and provides the most efficient
means of volatile identification.
0031Gas chromatography–mass spectrometry The avail-
ability of coupled gas chromatograph–mass spec-
trometer systems in the late 1960s was one of the
most important developments for flavor research,
and this was followed a decade later by the introduc-
tion of efficient computerized data-handling systems.
Today, sensitivity is usually such that a full spectrum
can be readily obtained on 1 ng of a single component
in a complex mixture injected on to the GC column,
and in many cases, an identifiable spectrum can be
obtained from as little as 10 pg. Quadrupole mass
spectrometers, obtaining at least one spectrum per
second, are ideal for low-resolution GC–MS of
aroma extracts. However, double-focusing magnetic
sector mass spectrometers can acquire accurate mass
data, allowing the calculation of empirical formulae
for ions in a spectrum, which can be a great asset in
the identification of unknown compounds.
0032The characterization of unknown compounds is
greatly facilitated by comparing their mass spectra
with those of known compounds in compiled libraries
incorporated into the GC–MS data system. Current
databases contain the mass spectra of over 200 000
compounds, but special compilations containing only
volatile compounds have also been prepared, and at
least one is available in computer-readable format.
Confirmation of the identity of compounds should
always be carried out, preferably by comparing their
mass spectra and retention times with those of
authentic samples. (See Mass Spectrometry: Prin-
ciples and Instrumentation.)
0033Spectroscopic methods Nuclear magnetic resonance
and infrared (IR) spectroscopy are both extremely
valuable techniques in the identification of organic
compounds. However, they require larger sample
5178 SENSORY EVALUATION/Aroma

sizes than mass spectrometry and are not as widely
used in aroma analysis. They are primarily used to aid
structure elucidation of unknown compounds.
0034 Fourier-transform IR instruments directly coupled
to a gas chromatograph have been developed, giving
adequate vapor-phase spectra on 10–100 ng of com-
pound. IR offers functional group information and
in general is complementary and supportive of MS.
Because IR is nondestructive, it has been possible to
develop tandem GC–IR–MS systems, providing a
powerful tool for aroma analysis.
Quantification of Aroma Components
0035 Often, quantitative information on aroma com-
pounds in a food is needed, such as for the calculation
of odor unit numbers. Quantification is rarely simple,
because most extraction techniques only remove a
proportion of the aroma from the food. Sometimes,
the extraction technique may generate aroma com-
pounds, and difficulties in quantification may arise
when compounds are not resolved by GC. The most
effective means of quantification is isotope dilution
assay using GC–MS, where a known amount of a
13
C- or
2
H-labeled internal standard is added to a
slurry of the food to be extracted, in order to quantify
its nonlabeled equivalent. As the labeled and un-
labeled aroma compounds possess similar physical
properties, the proportion of each extracted will be
the same. The relationship between the labeled stand-
ard and the compound of interest can be used to
calculate accurately the amount of the compound of
interest in the food.
0036 Other quantification methods include the addition
of an internal standard, not present in the food, of
a similar chemical composition to the compound
of interest, e.g., 2-methylpentanal could be used to
quantify hexanal. Conversely, standards could be
added to the extract, in order to calculate the amount
of the compound of interest in the extract rather than
the food itself. Standards can be injected into traps
containing adsorbent and, in the case of SPME,
injected on to the GC column immediately before
desorption of the fiber.
The Electronic Nose
0037 The electronic nose is an array of chemical sensors,
connected to a pattern-recognition system that
responds to odors passing over it. Different odors
cause different responses in the sensors, and these
responses provide a signal pattern characteristic of a
particular aroma. The computer evaluates the signal
pattern and can compare the aromas of different
samples, using pattern recognition. For example, if a
manufacturer uses coffees from around the world, the
electronic nose can analyze them, and their aromas
can be plotted on a multidimensional response map.
The analysis of several samples of each coffee, under
ideal conditions, can result in a group of points for
each coffee, well resolved from any other group. As
the number of samples increases, the resolution be-
tween the groups should also increase. Plotting the
sensor responses of an unknown sample on the same
map should permit its recognition, by its proximity to
one of the known samples.
0038Sensors are usually made of metal oxides or
organic polymers, although more recently, surface
acoustic waves and piezoelectric crystals have been
used. Problems may exist when samples with a high
water content are analyzed, as many of the sensors
respond strongly towards water, preventing any
sample differences being observed.
Measurement of Aroma Release during
Eating
0039Attempts have been made by several researchers to
measure the release of aroma during the eating of
food. At present, mass spectrometry using an atmos-
pheric pressure chemical ionization (APCI) interface
appears to be the most effective way of doing this. As
a subject eats a food or beverage, a sampling tube
draws the volatiles from the mouth or nose into the
mass spectrometer interface. Released aroma com-
pounds are ionized in air containing water vapor at
atmospheric pressure in the APCI source; the ions are
subsequently sampled into the high-vacuum area of
the mass spectrometer. APCI does not normally
induce fragmentation, and a strong protonated mo-
lecular ion is observed. By monitoring the molecular
ions associated with the major aroma compounds in
the sample, volatile release profiles can be measured.
Pulsed release curves are usually obtained, due to
respiration of the subject, and these can be smoothed
to show how each of the volatiles of interest behaves
during the eating process. This equipment has been
used, for example, to examine how reducing the fat
content of a food will affect its flavor or to study how
the chewing patterns of different people affect flavor
release.
See also: Chromatography: Gas Chromatography;
Combined Chromatography and Mass Spectrometry;
Essential Oils: Properties and Uses; Flavor (Flavour)
Compounds: Structures and Characteristics; Food
Acceptability: Affective Methods; Mass Spectrometry:
Principles and Instrumentation; Applications;
Spectroscopy: Infrared and Raman; Nuclear Magnetic
Resonance; Taints: Types and Causes; Analysis and
Identification
SENSORY EVALUATION/Aroma 5179

Further Reading
Acree TE and Teranishi R (eds) (1993) Flavor Science:
Sensible Principles and Techniques. Washington, DC:
American Chemical Society.
Goodenough PW (1998) The molecular biology of olfac-
tory perception. International Journal of Food Science
and Technology 33: 63–77.
Maarse H (ed.) (1991) Volatile Compounds in Foods and
Beverages. New York: Marcel Dekker.
Marsili R (ed.) (1997) Techniques for Analysis of Food
Aroma. New York: Marcel Dekker.
Meilgaard M, Civille GV and Carr BT (eds) (1991) Sensory
Evaluation Techniques, 2nd edn. Boca Raton, FL: CRC
Press.
Morello M and Mussinan C (eds) (1998) Challenges in the
Extraction and Isolation of Food Flavors. Washington,
DC: American Chemical Society.
Nijssen LM, Visscher CA, Maarse H, Willemsens LC and
Boelens MH (1996) Volatile Compounds in Food –
Qualitative and Quantitative Data, 7th edn. Zeist, The
Netherlands: TNO Nutrition and Food Research
Institute.
Pawliszyn J (ed.) (1999) Applications of Solid Phase Micro-
extraction. Cambridge: Royal Society of Chemistry.
Taylor AJ and Mottram DS (1996) Flavour Science: Recent
Developments. Cambridge: Royal Society of Chemistry.
Taste
H Valentova
´
and Z Panovska
´
, Institute of Chemical
Technology, Prague, Czech Republic
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Taste
0001 Taste, according to the International Standard Sens-
ory Analysis Vocabulary ISO 5492 (1992), is defined
as sensations perceived by the taste organ when
stimulated by certain soluble substances. Taste is
closely related to smell. The perception of odor and
taste, combined with trigeminal sensations, results in
the overall flavor. Flavor influences food acceptance
and selection of food intake, and helps us to
distinguish potentially harmful compounds. The
taste sensation is a very complex process starting at
the sensory receptor level and finishing in the central
nervous system, where it is combined with informa-
tion coming from other senses. The earliest work
concentrated on anatomical studies, and so we have
a good deal of information about the structural taste
system on the tongue.
Anatomy of the Tongue
Mouth
0002An important role in the perception of taste is played
by our mouth cavity and especially by the tongue.
Food is placed inside the mouth, where our teeth
chew it, and the saliva makes it into a solution, and
therefore, the food comes into contact with the sur-
face of the tongue. The main role for perception of
taste is played by taste papillae, taste buds, and taste
cells.
Papillae
0003Taste papillae are located on the tongue, soft and hard
palate, pharynx, and epiglottis. The papillae give the
tongue its bumpy appearance and can be classified,
according to their shape, into four types: fungiform,
foliate, circumvallate, and filiform papillae. The first
papillae appear when the human fetus is 6 weeks old,
and at around 10 weeks, the first primitive taste pores
are found. Table 1 shows a description of the different
types of papillae.
Taste Buds
0004In the epithelia of the papillae, there are taste buds,
40–80 mm in size. Children have about 10 000 taste
buds, but the amount decreases with age. The average
number for adults is 2000–3000 buds. The taste-bud
density and placement of the taste buds among people
vary. For example, the density on the tongue tip
ranges from 3–512 taste buds per square centimeter,
with an average of around 115 buds per square centi-
meter. People with a higher density of buds perceive
taste stimuli more intensively. The specificity of indi-
vidual taste bud cells is being studied. It is still not
clear at the taste bud level whether there is modality
specificity. Some studies have confirmed that some
fungiform papillae contain only one taste bud, but
this could not be proved. It has also been demon-
strated that taste buds respond differently to different
tbl0001Table 1 Descriptionofpapillae
a
Types of papillae Number of
papillae
a
Taste buds
perpapilla
a
Location
Fungiform 100–400 0–22 Base and edges of
the tongue
Foliate 8–20 10 Each side of lingual
posterior
Circumvallate 3–13 100–250 V-shaped pattern
across the root of
the tongue
Filiform 1000 No taste buds All the tongue
a
Average values are given because numbers differ among authors.
5180 SENSORY EVALUATION/Taste
substances. It seems that all areas with taste buds
respond to some degree to all tastes, but there is
usually one taste to which the response is the best.
Taste Cells
0005 The tip of the taste bud has a small opening about
2–5 mm in diameter, called a taste pore, where taste
stimuli interact with taste receptors. Taste buds are
aggregates of some 40–60 elongated epithelial cells.
There are different types of cells in a taste bud: dark
cells, light cells, cells with dark-cored and synaptic
vesicles, basal cells, and perigemmal cells. It is not
clear which of the cells serve as receptor cells or if the
different cell types are specialized to detect different
ranges of the gustatory spectrum. The taste cells con-
tinually regenerate; they live for only 7–10 days and,
within this period, grow to maturity and die. The
young taste receptor cells move from the periphery
of the taste bud to its center, and the older taste cells
in the central portion of the taste buds die away and
are then pressed out from the taste bud into the
mouth through taste pores. By replacing the cells in
this way, the gustatory system is protected from
losing its sensitivity. All taste cells from one taste
bud are connected by basal synapses to about 50
afferent nerve fibers. The nerve fibers from taste
buds converge into different cranial nerves.
Cranial Nerves
0006 The sense of taste is mediated by three cranial nerves.
The anterior (front) two-thirds of the tongue and
palate is supplied by branches of facial nerves, namely
chorda tympani branch (seventh cranial nerves), the
back of the tongue by glossopharyngeal nerve (ninth
cranial nerves), the base and central region, and the
throat and larynx by certain branches of vagus nerve
(tenth cranial). All nerves subserve not only taste but
also touch, temperature, and pain sensitivity. The
lingual nerve is the branch of the fifth cranial nerve
and is responsible for the perception of pungency,
heat, cooling, and others.
0007 The taste fibers from all the sensory nerves from
the mouth come together in the brainstem (medulla
oblongata). From the brain’s medulla, the gustatory
fibers ascend by a pathway to a small cluster of cells
in the thalamus and thence to a taste-receiving area in
the anterior cerebral cortex.
0008 Two main theories try to describe how taste infor-
mation is coded in the brain. In the pattern theory, the
main role is played by taste receptor cells that respond
with different sensitivities to the different tastants.
In the labeled line theory, the four tastes are sensed
in four discrete type of receptor cells, and the infor-
mation from receptors goes to the brain through
independent channels. It seems that both types of
mechanism are involved in taste perception.
Transmission of Taste Through Nerve
Impulses
0009The transmission of electrochemical impulse through
the nerves is called transduction. The understanding
of this process has been the main focus of taste
researchers.
0010Generally, the arrival of a chemical stimulant on
the surface of a receptor changes the ionic conduct-
ance in the taste membrane and the electrochemical
impulse is produced. The transduction pathway in-
volves a number of different receptors and different
cellular mechanisms.
0011Taste stimuli may interact:
.
0012directly with the ion channel – this produces recep-
tor cell depolarization either by direct permeation
or by blockage of the ion channel;
.
0013with receptors and alter the flux of ion through the
ion channel (K
þ
,Na
þ
,Ca
þ
);
.
0014may diffuse through lipid phase and affect G pro-
tein or the endoplasmatic reticulum.
Researchers believe that each basic taste has a par-
ticular transduction pathway (Table 2) that varies
with the location of taste buds on the tongue. For
tbl0002Table 2 Suggested pathways for individual tastes
Taste Possible pathways for transduction mechanism for different
stimuli
Bitter Multiple mechanisms – depending on the structure of
the compounds:
(a) Inhibition of apical K
þ
channel (mechanism for
chinine, divalent salts)
(b) Blockage of Na
þ
amino transport system
(c) G protein-mediated activity involving
phospholipases
Sweet Specific membrane receptors are required for
transduction of sugars:
(a) Multiple sweet receptors coupled to G protein
(gastducin) and second messenger system
(b) Stimulus-gated ion channel
(c) Direct activation of G protein by sweet stimuli
Sour Does not require specific taste receptor – several
apically located ion channels may participate in sour
transduction:
(a) sodium ion channel – proton enters taste cells
through amiloride-sensitive sodium channels;
(b) channels are stimulated by protons that permit
entry of cations into the cell
Salty Does not require specific taste receptor – direct
permeation of Na
þ
ions through amiloride-sensitive
sodium channel
Umami Stimulus-gated ion channel: taste stimulus binds to a
receptor site on a receptor–ion channel complex
SENSORY EVALUATION/Taste 5181

sweet and bitter taste, there probably exist multiple
pathways that can be common for substances with a
similar structure. The transduction of the different
taste stimuli has been studied on nonhuman species,
and it is not still clear whether the same mechanism is
involved in humans.
Basic Tastes
0015 Over the centuries, the numbers of primary tastes
have varied from two – sweet and bitter – to more
then 10. Studies carried out to date have distinguished
four taste categories: sweet, salty, bitter, and sour
taste. Now, some of the scientists also include be-
tween basic tastes a less-established taste, ‘umami.’
International Standards ISO 5492 in paragraph 3.22,
under basic taste, states: ‘basic taste: any one of the
distinctive tastes: acid, bitter, salty, sweet, alkaline,
umami, metallic.’
Sweet Taste
0016 Sweet taste is one of the favorite tastes and therefore
the most studied. There is evidence that prenatal
infants detect and like sweet in utero and that
human infants are born with a preference for sweet
taste.
0017 Scientists tried to solve the problem of sweet taste
through the chemical structure of sweet compounds,
but substances that have a sweet taste are found in all
chemical classes. They are usually associated with
multiple hydroxyl groups, and a- but not b-org-
amino acids. Salts such as beryllium or lead salts,
simple organic compounds such as chloroform, and
alcohols can have a sweet taste. The main groups
are carbohydrates, with sweetness decreasing in a
homologous series sugars > oligosaccharides > poly-
saccharides. Other sweet organic compounds include
amino acids, peptides, proteins, compounds with
NH
2
groups such as amides, ureas, and hydrazides.
Benzene compounds with one single NO
2
group are
often sweet, and those with more NO
2
groups
are usually bitter. Small changes in the stereochemie
of atoms within a molecule may affect its taste. The
chemical structure of the sweet compound also plays
an important role in the determination of the area of
the tongue, where the sweet taste is perceived. For
example, the taste of inorganic salts and nitrogen
compounds is perceived on the front of the tongue,
some dihydrochalcons on the back, and some sweet
proteins such as thaumatine and nonelline on the
sides.
0018 From the time when, in 1914, the need for discrete
functional groups of a sweet compound was recog-
nized, several theories tried to explain the sweet taste.
In Shallenberger’s hypothesis sugar molecules must
possess more hydrogen-bonded functional groups
per carbon. The molecules interact with the sweet-
taste receptor sites of a protein through hydrogen
bonds. The question is if there is a receptor for
sweet taste or if a different structural class has its
own special receptor and how they interact with the
sweet compound. It seems that this question will be
answered soon. Recently, scientists discovered a
sweet receptor gene, T1R3, thought to be the likely
basis for the tongue’s sweetness sensor. One of the
newest theories is multipoint attachment theory. This
theory assumes the presence, probably a seven-pass
transmembrane receptor, of at least eight fundamen-
tal recognition sites. The multiple theory is supported
also by other findings, such as synergy effects of
sweeteners, the adaptation of the response to some
sweeteners, but not to others, the sensitivity of differ-
ent parts of the tongue to the different sweeteners,
and human variability in sensitivity to different
sweeteners.
0019Studies with a new approach to studying the mech-
anism of sweet taste also appeared during the last
decade. Schallenberger in 1993 described the role of
symmetry. Sweetness is elicited through a bilaterally
symmetrical and concentrated dipolar interaction
between glycophore and the receptor. Also, the role
of partial molar and specific volumes has been ex-
plored in sweet taste chemoreception. Some scientists
focused on the role of water that could be the vehicle
by which stimulus molecules are transported to the
receptor environment and oriented towards it. Recent
studies suggest that there is a different transduction
mechanism for natural sugars and synthetic sweeten-
ers that could be activated by different G proteins
from those activated by sucrose. Although the sweet
taste may be present in hundreds of compounds,
fewer than 30 are permitted for use. The sensory
reference standard for sweet taste is sucrose. No
other sweetener is perceived to be identical; for
example, glucose produces moderate burning and a
bitter taste. Humans are more sensitive to synthetic
sweeteners. A comparison of the sweet taste of
synthetic sweeteners with that of sucrose is given in
Table 3. Some synthetic sweeteners have a bitter and
metallic taste, so they are used in mixtures.
Bitter Taste
0020Bitter taste is closely linked to sweet taste. Some
compounds can have both a sweet and bitter taste,
and slight structural modifications can alter their
intensities. Bitter compounds require polar (electro-
philic or nucleophilic) and hydrophobic groups and
must be at least slightly soluble in water. Among
the best-known bitter substances are alkaloids
(often toxic) such as quinine, caffeine, and strychnine.
5182 SENSORY EVALUATION/Taste
Alkaloids are often found in many plants, and so
bitter taste is thought to be a mechanism for poison
avoidance by animals. Also, salts that contain the
cesium or rubidium cation or the iodide anion are
bitter. Other bitter-tasting compounds include pep-
tides, amino acids, ureas, thioureas, terpenoids, and
polyphenols. The ability to perceive bitter substances
has been related to their lipid solubility, which can be
altered by pH. Bitterness is perceived towards the
back of the tongue, but some compounds are per-
ceived also on the tip. Transduction of bitter tastants
can involve multiple mechanisms, depending on the
structure of the bitter compound. One group of bitter
compounds may be detected by several different
mechanisms. The existence of more bitter receptors
is confirmed by a single Mendelian gene, which
affects the ability to taste one particular class of bitter
compounds. Recently, remarkable progress has been
made in establishing the nature of bitter taste. Two
groups of scientists have confirmed the existence of
bitter taste receptors. They identified a large family of
G-protein-linked receptors (40–80 members). These
receptors are organized in clusters.
0021 One of the most interesting bitter compounds that
has been studied in the past decade is 6-n-propyl-
2-thiouracil (PROP). Individuals detect this com-
pound with very different sensitivities that could be
inherited.
Salty Taste
0022 Salty taste is the last of the basic tastes that a newborn
child perceives (at approximately 4 months). It is
mainly associated with the taste of NaCl. At first, it
was thought that the saltiness of NaCl is caused by
Cl
anions, but later, scientists preferred the theory
that saltiness is produced by Na
þ
cations and that
anions modulate the intensity of the saltiness (larger
anions reducing saltiness more than smaller anions).
The molecular weight of the cation does not influence
the saltiness, but anions with a low molecular weight
are predominantly salty, whereas those of a higher
molecular weight tend to be bitter. The salts of
heavy metals such as mercury have a metallic taste.
The degree of saltiness decreases in the following
order: ammonium (most salty), potassium, calcium,
sodium, lithium, and magnesium salts (least salty).
This order can vary for different animals. Some salts
in low concentration can taste sweet, NaCl tastes
sweet in concentrations below 0.04 M, KCl below
0.02 M, and in some salts, especially lead acetate
and beryllium chloride, the sweet taste dominates. It
was found that people are more sensitive to NaCl at
the tip than on the more posterior region of the
tongue and that NaCl taste thresholds are system-
atically related to the size and region of tongue
stimulated.
0023Although sodium chloride is essential for life and is
the second most-used additive, it is believed to
contribute to hypertension and some cardiovascular
diseases. Therefore, scientists are looking for possible
substitutes. Other chlorides have a salty taste but
cannot be used as a good substitute because of their
bitter and sour taste or toxic effects. Recently, pep-
tides with a salty taste (l-ornithyltaurine, glycine
methyl, or ethylester hydrochloride) and also differ-
ent dipeptides were examined. The food industry tries
to avoid using salt by replacing it with herbs, spices,
and organic acids.
Sour Taste
0024Sour taste is acceptable when mild but becomes un-
pleasant when strong. It helps us to avoid unripe fruit
and damage our tissue with acids. In humans, acids
induce salivation, which increases bicarbonate secre-
tions, the major buffering agent in saliva.
0025From a chemical point of view, acids can be distin-
guished by their pH index (describes the activity of
protons in a solution) as a weak pH 3–7 and strong
pH 1–3. We cannot use this distinction when we look
at sour taste. Weak organic acids (e.g., the acetic acid
in vinegar) taste more sour than mineral acid at the
same pH.
0026The perception of sour taste is influenced not only
by the activity of the proton, but also by the quality
and character of the anion. The role of anion has not
been studied in detail at receptor level. Sour taste in
food is mainly connected with citric, malic, oxalic,
and tartaric acids in fruits and lactic acid in yogurt
and other dairy and meat products. Propionic acid is
found in Emmental cheese, and acetic acid is used in
the canning industry.
Umami
0027In 1909, the Japanese scientist Ikeda described a
special delicious or savory taste and used the Japanese
word ‘umami.’ This taste is familiar in the East, where
seaweed and mushroom are used as ingredients,
tbl0003 Table 3 Relative sweetnesses for several artificial sweeteners
compared with 3% sucrose solution
Sweetener Date of discovery Relative sweetness (sucrose ¼1)
Saccharin 1879 300–500
Dulcin 1883 70–350
Cyclamate 1937 30–60
Aspartame 1965 100–200
Acesulfame-K 1967 130–200
Sucralose 1974 600
Alitame 1979 2900
SENSORY EVALUATION/Taste 5183

because they have a high content of glutamate. This
taste is sometimes called a savory or meat-like taste,
because meat also has a high content of glutamate.
Extensive scientific tests carried out in the twentieth
century indicated that the umami taste is different
from the four classical tastes and that it is connected
with the l form of monosodium glutamate (MSG).
Later, nucleotides such as guanosine-5
0
-monopho-
sphate (GMP) and inosine-5
0
-monophosphate (IMP)
were also considered to have umami flavor.
0028 The occurrence of peptides (around 30), usually
dipeptides and tripeptides, with a possible umami
taste has also been reported, but this has not been
confirmed by other scientists. Some dipeptides have a
salty or bitter taste, or their taste is described as a
salty/umami or sour/umami taste.
0029 Faurion in 1991 found that the MSG taste is recog-
nized by more than one type of glutamate receptor.
Recently, the genes for proteins that serve as the
molecular receptors for umami taste were identified.
Other Tastes
0030 Other tastes such as metallic, soapy, alkaline, and
astringent are described for the use of sensory evalu-
ation but have not been studied at the receptors level.
0031 Astringency taste Astringency is a tactile taste felt as
a dry, rough feeling in the mouth and contraction of
the tongue tissue. It usually involves the formation of
aggregated precipitates between tannins or poly-
phenols and proteins in the saliva. Tannins positively
influence the taste of tea, coffee, cocoa, wine, and
beer but have a negative effect in immature fruit
(bananas). Lawless and co-workers developed a vo-
cabulary for astringency taste to describe its multiple
perception characteristics. They defined astringency
as a combination of three tastes: drying (the lack
of lubrication or moistness), roughing (the rough
texture in the mouth) and puckery (the drawing or
tightening sensation). There are also specific terms
such as sappy, harsh, woody, powdery, and gritty
that are used for describing astringency taste in wine
and beer.
0032 Metallic taste Metallic taste is elicited by some
metal salts, as a standard is used iron (II) sulfate
heptahydrate in concentration 0.00475 g l
1
. The
taste threshold for copper (using copper sulfate and
copper chloride) in different types of drinking water is
2.4–3.8 mg l
1
, depending on the type of water.
0033 Alkaline taste An alkaline, soapy taste is associated
with potassium carbonate (potash), a substance that
was used for the production of soap.
0034Pungency The trigeminal nerve is stimulated by
chemical-induced sensations such as pungency. A
pungent taste is characterized by spices such as hot
pepper (in which the pungency substance is the alkal-
oid piperine), ginger, Spanish red pepper (capsaicine),
and clove (eugenol). Vegetables from the Brassicaceae
family such as horse-radish, black and white mustard,
and cabbage have a pungent taste owing to their
isothiocyanates content. Pungency is also the main
sensory attribute of benzoic acid (3–36 mM). Some-
times, other sensations are described, such as the bite
of carbon dioxide, the sting of horseradish, and other
sensations derived from spices.
Factors Influencing Perception of Taste
Color
0035Several studies have examined the effect of color on
taste perception. The results depend on conditions,
concentration of taste substances, and intensity of
color. Generally, red and orange increase the thresh-
old for bitter, sour, and sweet taste, and green and
yellow increase the threshold for sour. The effect of
color on salty taste has not been studied.
Viscosity
0036The influence of viscosity has been studied mainly for
sweet compounds, but it is clear that it depends on the
taste substances and thickening agents. Viscous solu-
tions can suppress taste because of a lower diffusion
rate and poorer access to the receptors, but they can
enhance the taste because the time during which the
compounds bind to the receptors is longer. For
example, in a chocolate drink, sweetness increases
with increasing concentrations of lecithin. Usually,
the sweetness of sucrose is lower in liquid than in
aqueous solutions. The threshold of pure substances
in aqueous solutions is different from the threshold
values in other liquids.
Temperature
0037Temperature effects must be studied separately for
every taste and in a wide range of temperatures.
Sweet and bitter tastes are more affected by tempera-
ture than salty or sour tastes. Increasing the tempera-
ture usually increases the sweetness of the solution.
For some compounds, a V-shaped function has been
described, meaning that the minimum is near the
center of the temperature range. For example, the
sensitivity for NaCl was greatest in the range of
22–37
C and lower at 0 and 55
C. The optimum
temperature for the perception of bitter taste is
10
C, and the bitter aftertaste is longer than for
5184 SENSORY EVALUATION/Taste

other tastes. The sweetness of sugars is reduced in
cool solutions and the optimum is 35–50
C.
Odors
0038 Smell and taste are closely related. Therefore, sev-
eral studies have concentrated on the relation-
ship between perception of an odor and the taste
of the solution to which the odor is added. It has
been confirmed that flavorings added to a solution
of sucrose increase or suppress the perception of
sweet taste. For example, the flavor of caramel, mar-
ajuca, and strawberry enhance sweetness, whereas
angelica oil and damascone suppress sweetness.
Also, caramel odor suppresses the sourness of citric
acid.
Interactions Between Basic Compounds
0039 Food is a complex of chemical substances, and there-
fore, it is important to study not only the individual
responses of the basic taste but also the responses and
interactions in a mixture. Although this problem has
been studied extensively and in a previous review, it is
difficult to summarize general conclusions. Usually,
only binary mixtures in aqueous solutions are stud-
ied, and their results cannot be extrapolated directly
to food.
0040 Moreover, the interaction between specific chem-
icals in a binary mixture must be studied also, because
compounds from one taste group, such as bitter
compounds, do not behave in the same way. Also, all
interactions must be studied over different concen-
tration ranges. An important role is played not only
by the type of mixture, but also by the type of experi-
mental work and differences in human sensitivities.
The interactions in mixtures can be linear – suppres-
sion (perceived as less intensive), enhancement (per-
ceived as more intensive) – and non-linear – masking
and synergism.
0041 The results can be summarized as follows:
.
0042 salts and acids enhance each other at moderate
concentrations but suppress each other at higher
concentrations;
.
0043 some salts enhance the sweet taste (potassium acet-
ate, potassium chloride, sodium chloride);
.
0044 sour substances inhibit sweetness and vice versa;
.
0045 bitter compounds and acids can either enhance or
suppress each other, depending on the concentra-
tion;
.
0046 bitter taste is generally suppressed by sodium salts,
and saltiness is unaffected;
.
0047 bitter and sour tastes are suppressed by nucleo-
tides, and salty and sweet tastes are enhanced by
nucleotides;
.
0048umami substances in water solution do not influ-
ence basic taste: there is only synergism between
umami substances.
Examples of Some Interesting Compounds
0049Caffeine and other methylxanthines can enhance
some sweeteners with a bitter component (acesul-
fam-K, thaumatin, steviosid, sodium saccharin), but
not compounds such as aspartame, sucrose, fructose,
and calcium cyclamate.
0050Certain amino acids, including d-phenylalanine, l-
and d-histidine, and l-arginine are also enhanced by
caffeine. Sweetness is inhibited, for example, by
sodium (þ)-2-(methylphenoxy)phenoxy propionate,
triterpene saponins (gymnemic acid, holodulcin, zizi-
phens), cyclohexyl acetic acid, indole acetic acid, and
a-naphthol sulfate.
0051Molecules that inhibit more than one taste are
called multisaphoric molecules. The glycoproteins
miraculin (without taste) and curculin (sweet taste)
have a special property, because they convert the taste
of acids from sour to sweet.
Cause of the Differences in Feeling Taste
0052There are more reasons why people differ in their
perception of taste. Some can be explained by differ-
ences in anatomy and psychology among people,
others by some illnesses or by the process of aging.
The most-studied factor that influences taste percep-
tion is aging. Among older people at about the age of
60, there is normally a decline in the sense of taste,
especially sour and bitter.
0053Disorders associated with taste are less common
than with smell, because taste is innervated by three
cranial nerves compared with a single cranial nerve
for olfaction. Ninety per cent of patients complaining
of a reduction or loss of taste had a reduction or loss
of smell. This was confirmed by findings that people
who had lost their sense of smell (anosmic) can dis-
tinguish between four basic tastes. Changes in the
sense of taste can be either temporary or permanent.
0054Decreased perception of taste is called hypogen-
eusia. This loss was initially thought to be due to a
reduced number of taste buds, but recent data indi-
cate that a decline in the number of receptors may be
responsible. A persistent taste in the mouth in the
absence of stimulation is called dysgeusia, and com-
plete loss of the sense of taste is called ageusia (gusta-
tory anesthesia).
0055Loss of the sense of taste can be due to acute and
subacute diseases: seasonal allergies, head injury,
acute viral illness, idiopathic causes, and also the
case of hormonal disorder, tumors in the brain,
cancer, disease of the gums or teeth, nerve damage,
SENSORY EVALUATION/Taste 5185
and so on. Burning mouth syndrome is an oral pain
disorder occurring primarily in women who have
difficulties with identifying salty and sour tastes.
The intake of certain drugs (for example anesthetics
such as pantocaine and novocaine) and exposure to
chemical substances can also influence our perception
of taste. For example, chlorhexidine, an antiseptic,
can decrease the bitterness of quinine, and it is the
only known blocker of the human salty taste.
0056 Some people cannot taste the bitter substance
phenyl thiocarbamide (taste blindness), and people
with congenital idiopathic hypoparathyroidism
cannot recognize sweetness.
Methods of Sensory Analysis
0057 The basis of sensory analysis is the perception of food
attributes through the direct use of human senses. The
main tool, therefore, is an assessor, who must be
selected and trained. There are standardizing methods
for training, but it is necessary to check, refresh
and update the performance of assessors. Sensory
methods must be standardized on international, re-
gional, or national scales. Of all the sensory methods,
the most useful for studying taste are detailed below.
Methods for Investigating Sensitivity of Taste
0058 Taste is studied by different methods of sensory
analysis that require good methodological and ex-
perimental control. The International Standard ISO
3972 describes the method of investigating sensitivity
of taste. First, the assessors identify the taste of
reference substances (for example, the concentration
for reference sucrose is 5.76 g l
1
, and for caffeine
0.195 g l
1
). It has been proven by practical tests
that these concentrations are detected by 50% of
novice assessors. Another approach is to determine
the threshold. We recognize three types: detection
threshold (stimulus threshold) is the minimum value
of a sensory stimulus needed to bring about a sensa-
tion. In a recognition threshold, accessors must iden-
tify this sensation, and the difference threshold is a
value of the smallest perceptible difference in the
physical intensity of a stimulus. The threshold values
from different studies differ between authors, but the
results also vary between individuals and the results
from one individual can vary, depending on their
health condition and mood, and so on. Among all
tastes, bitter generally has the lowest detection
threshold for naturally occurring compounds (see
Table 4). The threshold is usually measured by using
pure substances in aqueous solutions. The tempera-
ture of solutions and aqua used must be specified,
because there are differences between values obtained
from distilled water, tap water, and deionized water.
Time–intensity Technique (TI)
0059The time–intensity technique is the only technique to
measure changes in perceptual characteristics with
time. The idea of measuring sensation intensity ori-
ginated in the 1960s but now has become widely used
with the development of computerized systems for
data collection.
0060The concept of TI is founded on the assumption
that intensity and time are two independent dimen-
sions of sensory space. During measurement, curves
are produced, with time on the horizontal axis and
sensation on the vertical axis, and these curves can
indicate how long it takes to reach the maximum
intensity for that taste, the overall maximum
intensity, the total duration of sensation, the rate of
extinction, and the rate of appearance.
New Analytical Methods for
Determination of the Taste
0061Sensory analysis is a time-consuming and expensive
method that requires trained assessors or experts.
Attempts have been made to replace this method by
various chemical and instrumental methods. An ‘elec-
tronic tongue’ has recently been developed to meas-
ure taste (based on pulsed voltametry). The Japanese
electric tongue is composed of several lipid/polymer
membranes that transform the information of taste
substances into an electric signal.
0062Analytical methods can be useful but still are less
objective than sensory analyses, because the former
measure only stimuli and not sensations.
tbl0004 Table 4 Values of detection thresholds for individual tastes
Sweet Threshold
(mol dm
3
)
Salt Threshold
(mol dm
3
)
Sour Threshold
(mol dm
3
)
Bitter
a
Threshold
(mol dm
3
)
Saccharose 0.011–0.017 Sodium chloride 0.03 Citric acid 0.00078 Caffeine 0.00002–0.002
Lactose 0.065–0.072 Calcium chloride 0.017 Acetic acid 0.00183 Quinine 0.0000001–0.00001
Maltose 0.038 Ammonium chloride 0.004 Tartaric acid 0.00053 (þ)-Catechin 0.002
D-glucose 0.065–0.08 Sodium fluoride 0.005 Malic acid 0.00082 L-Leucine 0.015
Saccharin 0.000000023 Magnesium chloride 0.015 Hydrochloric acid 0.0078 Magnesium sulfate 0.005
a
A range is given because the values for bitter taste differ between authors.
5186 SENSORY EVALUATION/Taste
