Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


0011 A risk assessment analyzes the state of affairs at one
point in time, utilizing the best available information
at that time. As situations change, and significant new
information becomes available, a reassessment or
update should be carried out. A risk assessment can
be thought of as a live and continuing process.
Risk Assessment of Chemical Hazards
0012 In hazard identification, information as to what con-
stitutes a hazard is frequently limited. The ‘weight-of-
evidence’ approach is usually adopted, relying on a
combination of relevant sources of information, in-
cluding epidemiological studies, animal toxicological
studies, in-vitro assays, and quantitative structure–
activity relationships. In the majority of cases,
clinical, and epidemiological data are unavailable or
inadequate, and much reliance is placed on animal
studies. These would include long-term chronic stud-
ies as well as short-term acute toxicity studies. They
are designed to identify the range of toxicological
effects, such as cancer, reproductive/developmental
effects, neurotoxic effects, and immunotoxic effects.
They can also provide information on the relevance of
these effects for human risks through characterizing
the mechanism of action. In-vitro studies may pro-
vide information on genotoxicity, pharmacokinetics,
and pharmacodynamics.
0013 One particular issue with animal toxicological
studies is the need to use a relatively high dosage in
order to identify the toxicological effects. However,
realistic contamination levels in food are typically
orders of magnitudes lower, and the significance of
the identified health effects is uncertain. This can pose
major issues for hazard characterization.
0014 Animal experiments provide information on
dosage at which no ill effects occur in the test animals;
this is referred to as the ‘no observed effect level.’ A
dose–response assessment would then need to ex-
trapolate this animal data. There is much uncertainty
in this, because the nature of the hazard and mode of
human metabolism may change with dose, and the
response in humans may be different to that of the
experimental animals used. These uncertainties are
compensated for with the use of safety factors. Fre-
quently, a multiplication factor of 10 is used to ac-
commodate the differences between how humans and
animals might react to the hazard. Another factor of
10 is typically used to take account of the fact that
some humans are more sensitive than others to the
hazard in question. This yields an ‘acceptable daily
intake’ (ADI) figure. If dietary exposure is below
the ADI, it is assumed that there will be no ill effect.
This is known as the ‘threshold approach.’ For geno-
toxic carcinogens, this approach is not considered
appropriate, as it is assumed that there is a finite
risk, even with the lowest possible dose. In such
cases, a level of negligible/acceptable risk is set, fre-
quently at one in a million.
0015Exposure assessment is carried out using diet
studies. Direct monitoring of residues of the chemical
hazard (e.g., organochlorine pesticides) in human
tissues and body fluids is also gaining importance as
a means of exposure assessment.
0016Risk characterization then draws on the outputs of
hazard identification, hazard characterization, dose–
response assessment, and exposure assessment to
derive an estimate of the likelihood of adverse health
effects in human populations as a consequence of the
exposure. For threshold-acting hazards, risk is char-
acterized by comparing exposure to ADI. Risk is
notionally zero if exposure is lower than ADI. For
nonthreshold acting hazards, risk is calculated as a
function of exposure and potency.
0017As an example of how such a risk assessment can
be used, a study may be carried out on the existing or
proposed use of an agricultural or veterinary chem-
ical in association with food-production animals.
If the estimated risks are deemed unacceptable,
consideration can be given to various possible risk-
mitigation measures, such as means of reducing ex-
posure. If the risks can be reduced to an acceptable
level through such means, the chemical may be con-
sidered for registration. The setting of ‘safe/tolerance
levels’ for various chemical residues in food is another
application of risk assessment of chemical hazards.
Risk Assessment of Biological Hazards
0018Biological hazards include pathogenic strains of bac-
teria, viruses, helminthes, protozoa, and algae. Some-
times, toxins that some of these organisms may
produce, e.g., enterotoxins produced by Staphylococ-
cus aureus when growing to high numbers in food, are
also grouped with biological hazards. Of these, food-
borne pathogenic bacteria and viruses present, are by
far, the greatest concerns in regard to food safety.
0019Microbiological risk assessment follows basically
the same framework as chemical risk assessment.
However, it has its unique set of difficulties and chal-
lenges. The list of foodborne pathogens is extensive,
and pathogens can be found in abundance in the
natural environment, including that for the produc-
tion, harvesting, processing, storage, transportation,
display, retail, and preparation of food. Some of the
microbiological hazards, e.g., bacteria, can multiply
rapidly in the food as it goes through the production
and supply chain, whereas others can also produce
toxins or form heat-resistant spores under certain
conditions. The survival and proliferation of these
5016 RISK ASSESSMENT

organisms are affected by the complexity of the food
matrix, parameters of the manufacturing processes,
as well as the temperature profile to which the food is
exposed along the production supply chain. So, it is
not unusual to find that a specific pathogen in a
certain food matrix has caused many cases of food-
borne illness, whereas the same pathogen in another
food may rarely have been implicated. Moreover, the
virulence and infectivity of microorganisms can
change, depending on their interaction with the host
and the environment. Genetic material can be trans-
ferred between microorganisms, leading to the trans-
fer of characteristics such as antibiotic resistance and
virulence factors. There is also evidence of very large
differences in virulence between different strains of a
pathogen, e.g., Listeria monocytogenes, that are
almost impossible to distinguish by traditional
taxonomic methods, and there are usually no easily
identifiable virulence markers.
0020 Human susceptibility to specific pathogens can
also vary widely. For example, young, old, pregnant,
and immunocompromised consumers are highly sus-
ceptible to the foodborne pathogen Listeria mono-
cytogenes, but the healthy adult population is rarely
affected. All these factors can make hazard identifica-
tion even less clear-cut in comparison with identi-
fication of chemical hazards. Like the latter,
epidemiological studies remain the most desired
information base with which to advise biological
hazard identification. Structure–activity relationship
studies are used in the absence of, or in addition to,
reliable epidemiological data. They compare the dis-
ease agent in question with a similar agent whose
health effect is better understood.
0021 Exposure assessment aims to define the pathway by
which people will become exposed to the pathogen
in the food, how much of the pathogen they are likely
to ingest, and how frequently. This requires infor-
mation on the occurrence rate and level of the patho-
gen in the food in question. These data are usually
obtained by conducting microbiological surveys of
the food. If surveys are not carried out at the point
of consumption, predictive microbiology can be used
to ‘extrapolate’ from the survey point (e.g., the end of
production) to the point of consumption, based on
typical temperature–time conditions to which the
food is exposed, in order to estimate the dose
exposure.
0022 A dose–response assessment for microbiological
hazards presents an even greater difficulty compared
to chemical hazards. Human studies are very limited,
and animal studies are generally not used. The rele-
vance of the former is also restricted because of the
variability in susceptibility between persons. There
have been attempts to derive a dose–response
relationship from available epidemiological data,
but this approach has yet to be fully developed.
0023Risk characterization in microbiological risk as-
sessment would include one or more of the following:
1.
0024Risk estimate: depending on the purpose of the
risk assessment, this may be expressed as a risk
ranking of the hazards considered. This may be
done in terms of high-, medium-, and low-risk
categories, or in accordance with some arbitrary
scale that would also show more quantitatively the
relative risk levels. The risk estimate may also be
expressed as the probability of human illness for
defined exposure scenarios, e.g., in terms of x in a
million chance of illness or x cases of illness per
year in a specific population.
2.
0025Characterization of uncertainty, variability, and
confidence in the risk estimate: the uncertainties
associated with each step or component of the risk
assessment are identified and, if possible, quanti-
fied. Similarly, variability that reflects the hetero-
geneity of the population under consideration and
the diversity in exposure is also identified and
quantified. Confidence in the risk estimate is then
discussed with reference to the adequacy of data
and the weight of evidence.
3.
0026Sensitivity analysis. This is used to show the influ-
ence of different variables on the outcome of the
risk assessment and the range of response that may
be encountered. It can also be used to highlight the
impact on the risk estimate if various assumptions
are changed.
4.
0027Identification and evaluation of risk mitigation
options: various options for mitigating or control-
ling the major risks are identified and evaluated in
terms of their effectiveness.
The risk estimate may be expressed qualitatively,
semiquantitatively, or quantitatively, depending
on the purpose of the risk assessment, and the
availability of relevant data and resources to allow
quantification of the risk estimate. Accordingly,
the risk assessment is referred to as a qualitative,
semiquantitative, or quantitative risk assessment. A
qualitative study is much less resource-intensive and
may be adequate for some applications, such as pri-
oritization of hazards for further investigation. A
quantitative risk assessment may be necessary for
purposes such as the setting of food standards and
critical limits.
Risk Assessment of Physical Hazards
0028The possible health effects of physical hazards, such
as glass, metal, and bone fragments, are generally
obvious. There has been little demand to quantify
RISK ASSESSMENT 5017
physical hazards in a systematic way in the context of
food safety. However, attempts have been made to
quantify risks according to the size and nature of
extraneous objects.
Risk Assessment and Risk Analysis
0029 Risk assessment is not an end in itself and is usually
conducted for a specific purpose or objective. For
example, it may be conducted to identify the higher-
level risks of a food or manufacturing process, with
the view of controlling those risks at acceptable levels
though better policy setting, strategy formulation,
and/or implementation/improvement of process con-
trols. To achieve such an objective, risk assessment
has to feed into a process of risk management,and
both processes have to be carried out in conjunction
with risk communication. The three elements, risk
assessment, risk management, and risk communica-
tion, together are referred to as risk analysis.
0030 Risk management is the process of weighing policy
alternatives in the light of the results of risk assess-
ment and, if required, selecting and implementing
appropriate control options, including regulatory
measures. It is responding to the findings of risk
assessment and determining the most practical and
effective way of achieving the purpose or objectives
identified. Risk communication is the interactive ex-
change of information and opinions concerning risk
and risk management among risk assessors, risk man-
agers, consumers, and other interested parties. This
ensures that the purpose, objectives, considerations,
processes, findings, uncertainties, and implications of
the risk assessment and risk management are clearly
understood by all stakeholders throughout the con-
duct of the risk analysis. This tends to increase the
quality of the process owing to a broadening of the
input base for relevant information and expertise.
It increases the transparency of the risk analysis
and fosters ownership of the process and output.
The interrelationship between the elements of risk
analysis is illustrated in Figure 2.
Application of Risk Analysis
Government Policy Setting
0031 Developed countries have come to recognize food
safety as a major social, economical, and political
issue. The monetary and social costs of foodborne
illnesses to society are very significant. There is also
an increasing public awareness of food-safety issues,
particularly after a spate of high-profile food-safety
incidents, such as the BSE crisis in the UK, the dioxin
contamination episode in Belgium, and the Snow
Brand milk-poisoning case in Japan. As a result,
there is an increasing expectation that the govern-
ment fulfills a role of protecting the public from
undue exposure to food-safety hazards, and in signifi-
cantly reducing foodborne disease.
0032In 1997, President Clinton of the USA announced
his Food Safety Initiative that recognized the import-
ance of risk assessment as a scientific tool in achieving
the goal of improving food safety. Major agencies
such as the US FDA, FAO/WHO, and the European
Commission have indicated that risk analysis under-
pins the formulation of their food-safety policy.
0033Whether at company, industry sector, or national
level, food-safety hazards are numerous, whereas the
resources for improving food safety are finite. In
practice, resources must become more focused on
reducing those risks that have the greatest conse-
quences for human health. Risk assessment assists in
such decision-making by providing a scientific esti-
mation of the risks of various food hazards, and
providing risk-management options that are scientif-
ically sound. This allows the risk managers to priori-
tize and weigh up the options available. This may lead
to government agencies formulating legislative re-
quirements on food production, setting food stand-
ards, encouraging industry-based codes of practice,
and/or undertaking educational programs.
Standard Setting
0034Food standards, such as those that specify the max-
imum allowable level of contaminants in food, or the
amount of food additives permitted, are an important
instrument for the assurance of food safety. This is
particularly the case in international trade, where the
food commodity being traded is manufactured in
another country, and the history and conditions of
Risk analysis
Risk assessment
Risk management
Risk
communication
Risk
communication
fig0002Figure 2 Components of risk analysis.
5018 RISK ASSESSMENT

manufacture may not be fully known. International
trade on food is based on agreements achieved at the
World Trade Organization (WTO), including the
Agreement on Sanitary and Phytosanitary Measures
and the Agreement on Technical Barriers to Trade.
Under these agreements, the Codex Alimentarius
Commission (CAC) has the role of implementing the
Joint FAO/WHO Food Standards Program, establish-
ing international food quality and safety standards.
These standards are adopted or used as models by
many countries in their domestic food legislation and
regulations.
0035 The CAC takes a risk-based approach to setting of
standards and has enunciated the principle of using
sound scientific analysis and evidence as the basis of
all its food standards. In practice, this means the use
of risk assessment and risk analysis for the justifica-
tion and derivation of food standards. This has set the
scene for many countries also to adopt risk analysis as
a tool for developing domestic standards.
Determination of Equivalence
0036 It is the sovereign right of a county to determine a
desired level of public health protection to be
achieved in food production. The exporting country
need not apply the same regulations for processing
and production of food as the importing country,
provided that the controls used will achieve the
same level of safety. Risk assessment is a scientific
tool that can be used to assess whether the imported
food is of an equivalent level of safety as food pro-
duced locally or deemed acceptable from other
sources. This provides a means of preventing artificial
barriers to trade. In recognizing the importance of
this science-based approach to fair trade, the WTO
has required each member country’s food safety
measure to be based on risk assessment.
0037 Equivalent systems, once established between
trading partners, can result in acceptable export cer-
tification of shipments with minimum additional con-
trol. This simplifies trade procedures and reduces or
eliminates the need for stringent and prescriptive
trading conditions.
Underpinning HACCP
0038 Hazard Analysis Critical Control Point (HACCP) has
been recognized worldwide as the desired approach
to achieve safe food production. There is a close
relationship between hazard analysis within the con-
text of HACCP and risk assessment/risk analysis.
Much of the information required for hazard analysis
is also required for the hazard identification and
hazard characterization steps of risk assessment,
such as identifying the nature, source, prevalence,
and anticipated level of hazards. However, HACCP
and risk assessment are different in terms of their
scope, application, and focus.
0039HACCP is generally carried out at company level
for a specific food product manufactured by a specific
process and may be specific to a single factory, or even
one particular production line. The objective is to
manage and control the identified significant hazards
at acceptable levels, thus ensuring production of safe
food from the production line in question. Hazard
analysis in HACCP generally does not systematically
quantify risks, although a prioritization of hazards
is involved, which can be considered an intuitive
estimation of relative risk levels.
0040Risk assessment and risk analysis are normally
carried out by government agencies, industry peak
bodies, and the like. They have a broader focus than
one specific manufacturing process and find applica-
tion at a policy or strategic level. Usually, the study is
carried out for a specific hazard in the context of a
food commodity group, e.g., Salmonella enteritidis in
egg and egg products. Sometimes, it is also carried out
for a specific industry sector or food commodity
grouping, e.g., significant hazards in seafood pro-
duced in a specific region. Systematic quantification
(to the extent possible) of the risks of significant
hazards is a fundamental element of such a study.
The risk estimates provided by a risk assessment
will give guidance in prioritization and strategic plan-
ning for the management of the risks. The proposed
risk mitigation options then feed into the risk-
management process to formulate practical work
plans and risk control measures.
0041Owing to the commonality between HACCP and
risk assessment/risk analysis, there is also an import-
ant interrelationship between them. Hazard identifi-
cation conducted as an element of risk assessment is
generally done more systematically and thoroughly,
by virtue of the greater resources and expertise usu-
ally made available for such studies. In risk assess-
ment, the hazards are also scientifically evaluated in
terms of risks. This distinguishes those hazards that
pose a significant threat from others that are no more
than perceptions or are of a low impact. Therefore,
risk assessment, where available, should provide the
foundation for HACCP. The latter should address
all the hazards of higher risks identified in the
risk assessment and adopt one or more of the risk-
management options provided, customized appropri-
ately for the specific circumstances wherein the
HACCP plan operates.
Benefits and Limitations
0042Risk assessment, with its clearly defined framework,
provides the necessary discipline to ensure rigorous
RISK ASSESSMENT 5019

problem definition, through examination of relev-
ant data, assimilation of existing knowledge, and
identification of variability and uncertainties. This
provides for a structured, scientific, and objective
inquiry into issues of food safety. Apart from pro-
viding a quantification of food-safety risk, a risk-
assessment study also produces a report that serves
as a database of relevant information, a documenta-
tion of considerations justifying the risk estimate and
risk-management options, as well as a listing of crit-
ical information gaps. The transparency, objectivity,
and scientific basis of the study and its findings en-
gender critical thinking and understanding, and pro-
vide a firm foundation for sound decision-making,
strategic planning, and effective action.
0043 The major problem in conducting a risk assess-
ment, in particular quantitative risk assessment, is
the frequent lack of relevant data, whether epidemi-
ological, prevalence, and/or dose–response data. It is
also frequently necessary to make broad assumptions
on which the robustness of the study rests heavily. As
a consequence, this means that the conclusions of a
risk assessment are sometimes accompanied by large
uncertainties. In most cases, the generation of neces-
sary additional data is not feasible or is very labor-
and time-intensive.
0044 The lack of relevant data may also mean that the
study can only be qualitative, or at best semiquanti-
tative. As such, the full benefits of a quantitative risk
assessment cannot be realized, and the findings may
be little more than what is already known based on an
intuitive estimation of risk or impressions. In such
cases, the main value of the risk assessment is re-
stricted to having documented all relevant consider-
ations in a systematic and transparent manner and
identifying the knowledge gaps that need to be filled
for future analyses.
0045 Quantitative risk assessment requires the use of
simulation modeling and/or animal studies. These
models or studies cannot always be validated, and
the reliability of the assessment findings would
depend heavily on the validity of the modeling or
the relevance of the animal studies. Related to this is
the large variation in the susceptibility of people to
foodborne hazards. Relevant data are generally much
more available for the general population than for
minority groups that may be more susceptible. Con-
sequently, the risk levels determined may be an under-
estimation for certain consumer sectors.
0046 A substantial quantitative study is very costly. For
example, some quantitative microbiological risk as-
sessments have taken up to 30 person-years to com-
plete. This means that only resource-rich agencies,
organizations, or collaborative teams are able to
undertake such studies. However, in recognition of
this, many major risk-assessment reports have been
made freely available through the scientific literature
and the Internet. This has allowed others to make use
of the collated information and findings.
0047While the risk assessment process is both objective
and systematic, subjectivity cannot be avoided in
using the findings in risk management. This is because
an acceptable level of food-safety risk is neither well
defined nor universally agreed upon. Ultimately, the
risk-management decision of what is an acceptable
risk for a particular situation has to take account of
cost–benefit analysis and public perception.
0048Risk assessment, being a scientifically based pro-
cess, is designed to assess true risks to human health.
However, the public’s reaction to food safety is largely
based on perceptions. Frequently, what the public
perceives as unacceptable hazards in food and reacts
emotively to is not necessarily proven as a major
cause of foodborne disease. Nevertheless, issues sur-
rounding these perceived hazards could bring about
severe backlash on businesses, industries, and govern-
ment by consumers and export markets. From a busi-
ness viewpoint, the perceived risks are as real as the
true food-safety risks. However, perceived risks are
outside the domain of scientific food-safety risk as-
sessment but may be dealt with as a commercial risk
by other techniques.
See also: Food and Agriculture Organization of the
United Nations; Food Safety; Hazard Analysis Critical
Control Point; Legislation: International Standards;
Additives; Contaminants and Adulterants; Codex; World
Health Organization
Further Reading
Buchanan RL and Whiting RC (1998) Risk Assessment: A
means for linking HACCP plans and public health.
Journal of Food Protection 61(11): 1531–1534.
Codex Committee on Food Hygiene (1999) Principles and
Guidelines for the Conduct of Microbiological Risk
Assessment. CAC/GL-30. Codex Alimentarius Commis-
sion.
Codex Committee on Food Hygiene (2001) Draft Response
to the Codex Executive Committee Regarding Clarifica-
tion of the Terms ‘Hazard Analysis’ and ‘Risk Analysis’.
Thirty-fourth Session, Bangkok, Thailand.
FAO (2000) Report of the Joint FAO/WHO Expert
Consultation on Risk Assessment of Microbiological
Hazards in Foods. Rome: FAO.
Frewer LJ, Howard C, Hedderley D and Shepherd R (1997)
The elaboration likelihood model and communication
about food risks. Risk Analysis 17(4): 759–770.
Hathaway SC (1997) Development of food safety risk
assessment guidelines for foods of animal origin in
international trade. Journal of Food Protection 60(11):
1432–1438.
5020 RISK ASSESSMENT
Herber RFM, Duffus JH, Christensen JM, Olsen E and Park
MV (2001) Risk assessment for occupational exposure
to chemicals. A review of current methodology. Pure and
Applied Chemistry 73(6): 993–1031.
ILSI Risk Science Institute (2000) Revised Framework for
Microbial Risk Assessment. Washington DC: ILSI Press,
International Life Sciences Institute.
International Commission on Microbiological Specifica-
tions for Food (ICMSF) Working Group on Microbial
Risk Assessment (1998) Potential application of risk
assessment techniques to microbiological issues related
to international trade in food and food products. Journal
of Food Protection 61(8): 1075–1086.
Lammerding AM and Paoli GM (1997) Quantitative risk
assessment: an emerging tool for emerging foodborne
pathogens. Emerging Infectious Diseases 3(4): 1–7.
McNab WB (1997) A literature review linking microbial
risk assessment, predictive microbiology, and dose–
response modelling. Dairy, Food and Environmental
Sanitation 17(7): 405–416.
Potter ME (1996) Risk assessment terms and definitions.
Journal of Food Protection 59 (supplement): 6–9.
Van Schothorst M (1997) Practical approaches to risk
assessment. Journal of Food Protection 60(11): 1439–
1443.
WHO (1995) Report of a Joint FAO/WHO Expert Consult-
ation Application of Risk Analysis to Food Standards
Issues. Geneva: WHO.
WHO (1999) Report of a Joint FAO/WHO Expert Consult-
ation Risk Assessment of Microbiological Hazards in
Foods. Geneva: WHO.
Roller Milling Operations See Flour: Roller Milling Operations; Analysis of Wheat Flours; Dietary
Importance
Root Vegetables See Cassava: The Nature of the Tuber; Uses as a Raw Material; Potatoes and Related
Crops: The Root Crop and its Uses; Fruits of the Solanaceae; Processing Potato Tubers; Vegetables of Temperate
Climates: Commercial and Dietary Importance; Cabbage and Related Vegetables; Broccoli-type Brassicas; Leaf
Vegetables; Oriental Brassicas; Carrot, Parsnip, and Beetroot; Swede, Turnip, and Radish; Miscellaneous Root
Crops; Stem and Other Vegetables; Vegetables of Tropical Climates: Commercial and Dietary Importance; Root
Crops of Uplands; Root Crops of Lowlands; Edible Aroids
Roughage See Dietary Fiber: Properties and Sources; Determination; Physiological Effects; Effects of Fiber
on Absorption; Bran; Energy Value
RUM
L Fahrasmane and A Parfait, Institut National de la
Recherche Agronomique, Petit-Bourg, French West
Indies, Guadeloupe
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The term ‘rum’ is used to describe the distillates ori-
ginating from the fermented products of sugarcane
(Saccharum spp.), that is to say fresh juice, molasses
(the thick brown liquid separated from raw sugar at
the end of the sugar manufacturing process), and
syrup obtained from sugarcane juice concentration
(Table 1). The fermented liquid must be distilled
under 96% ethanol by volume (Directive 89/1576/
EEC).
0002Rum production has always been a way to use and
value molasses. Thus, in the French West Indies
islands, de sucrerie rum, obtained from molasses, is
produced from distilleries attached to sugar plants.
However, in the Caribbean French colonies, Guade-
loupe and Martinique and also in Haiti, some small
sugarcane planters who were disadvantaged, during
RUM 5021
the first half of the eighteenth century, by the devel-
opment of central sugar plants and a general reduc-
tion in sugar sales began to distil rum directly from
the fresh juice of their harvest or from the syrup
resulting from the juice concentration. This was
how agricole rum was born. It is produced by inde-
pendent distilleries, outside the sugar industry, using
the raw material from farming.
0003 Nowadays the world rum market is dominated by
the light rums which are produced from molasses,
in modern productive distilleries. Light rum is
marketed white or aged, and drunk as an ingredient
in long drinks. However, traditional rums are still
produced and sold. These are characterized by a
mixed fermentation involving alcohol-producing
yeast strains and cofermenting bacteria. They are
distilled at a lower ethanol content than the light
rum (about 80% by volume) and are richer in aro-
matic compounds. Jamaican type (also known as
heavy rum or grand aro
ˆ
me, is a typical example of
these traditional products.
History and Uses
0004 The British term ‘rum’ referred to the alcoholic
liquors distilled from fermented sugarcane products,
coming from the British colonies of the Caribbean.
They were generally of a better quality than those
produced in the French colonies, where they were
referred to as tafia and guildive. Indeed, rum has
had and still does have various names throughout
the world, including some archaisms, such as kill-
devil, the obsolete word rumbullion, guildive which
is probably a French adaptation of kill-devil, tafia,
grappe, arrack (Mauritius), and gavine (South
Africa). Nowadays, it is rhum in French, aguardiente
and ron (in Spanish-speaking Latin America),
cachac¸a, pinga, aguardente (Brazil), clairin (Haitian
traditional rum, with 30–35% alcohol content),
toaka (Madagascar), and kana (Guinea-Bissau).
0005 Although the cultivation of sugarcane and its trans-
formation into sugar date back to time immemorial,
rum production is more recent; it developed with
sugar production in the Caribbean islands in colonial
plantations in the seventeenth century. Every planta-
tion had its distillery. Rum appeared when the world
sugar production, which was only from sugarcane,
was taken from the Mediterranean area to America.
The increase in sugar production generated important
molasses volumes. The European colonists discovered
that they could take advantage by distilling fermented
washes from the molasses and sugary wastes such as
scum.
0006Among the first writers to mention rum was the
father Du Tertre, who stayed in the French West
Indies from 1640 to 1657. At much the same time,
Robert Ligon in his History of the Island of Barbados
(1657) described the preparation of a fermented
beverage called punch. Father Labat, who arrived
in the West Indies in 1694, described the elabor-
ation of guildive in his Nouveau voyage aux isles de
l’Ame
´
rique (New Journey to the American Islands;
1722).
0007In those days, rum was a byproduct of the sugar
industry. The manufacturing process was simple: the
fermentable sugar was seldom derived from cane
juice but came from the scum that comprised the
sugary impurities raised during juice boiling, and
molasses rich in sucrose were drained from the
sugar production process. These sugary resources,
diluted – around 100 g l
1
of sugar – in water and/
or distillation waste waters, were spontaneously fer-
mented (Table 2). Then the fermented wash was
single- or double-distilled in a pot still. The manufac-
turing process in use in the seventeenth and eight-
eenth centuries was diverse. The quality of the
product was often poor, or frankly bad, because of
the low microbial quality of the raw materials (scum,
molasses, waste waters), the rudimentary condition
of the fermentation process, and above all the poor
resolving power of the distillation apparatus. Spirits
were often derived from washes inside which acetic,
butyric, and even putrid fermentation had occurred,
and the pot stills were unable to provide the rectifica-
tion to remove undesirable compounds. Moreover,
the alcohol content was weak, often under 42% (by
volume), especially in the products from the French
colonies.
tbl0001 Table 1 Average percentage of components in sugarcane, and
raw materials used in the distillery
Component Cane and distilleries rawmaterial
Cane Juice Syrup HTM Molasses
Water 69.0 78.0 29.0 16.0 20.0
Sugar 16.0 20.0 66.0 77.0 62.0
Nonsugar 3.0 2.0 5.0 7.0 18.0
Fiber 12.0
HTM, high-test molasses.
tbl0002Table 2 Wash components, as a percentage, at the beginning
of the nineteenth century
Component North Caribbean Jamaica
Molasses 10 6
Scum 30 36
Slops 20 50
Water 40 8
5022 RUM

0008 The phylloxera crisis in the nineteenth century had
involved a high demand in alcoholic products includ-
ing those from the rum distillery. In the meantime
there was development of industrial processes with
rational management and mass production. Pasteur’s
works on fermentation allowed microbial control in
the washes. This evolution and new knowledge were
applied in rum production where there was a chem-
ical comprehension of distillery products and pursuit
of productivity. During the second part of the nine-
teenth century appeared an untraditional process,
which featured microbial control and bacteria elim-
ination in the washes, a plant with voluminous
fermentation vats, and a multistage column for distil-
lation which involved a high level of rectification of
the distillates.
0009 Rum was first a drink for slaves and seamen. It was
considered as a coarse drink, not appropriate for the
palates of the upper classes. Admiral Penn, after he
took Jamaica in 1655, instituted the daily rum ration
distribution to his sailors. Admiral Vernon, who was
nicknamed Old Grog – grog is a mixture of one
portion of rum, two portions of water and some
lemon juice – replaced the Admiral Penn ration,
with grog in 1731. Rum was distributed daily to
sailors of the British Royal Navy until recent times.
In the West Indies the British blended rum with
various foodstuffs, including tea, sugar, lemon, and
cinnamon and named the cocktails punch. In the
eighteenth century there were many rum liquors
known by the name island liquor.
0010 The British encouraged rum production from the
beginning, because it was favorable for traffic with
the North American Indians, and the slave trade on
the African coast. Whisky production was low at that
time. Barbados, Jamaica, and New England were
colonies where rum production was developed. In
1765, two distilleries were situated in Liverpool,
where they produced rum to supply ships bound for
Africa.
0011 From the eighteenth century, rum-based drinks
became numerous. An important feature of rum util-
ization is its ready association with fruit aroma and
vegetable extracts in the mixing of punch, cocktails,
planteurs and, more recently, rum and cola; some
mixes are sold ready-to-use. Rum has the distinction
of being an excellent basic ingredient for composite
drinks. The earliest English terms, punch and grog,
are still largely used, although the beverages are
rather different from those in the eighteenth century.
Nowadays one can find, for example:
.
0012 curac¸ao from the Dutch West Indies, produced
after soaking orange peel in rum: shrub is very
similar to curac¸ao
.
0013cocktails which are mixtures of rum with other
liquids, mainly tropical fruit juices and spices:
punch and planteur from the French West Indies
are similar
.
0014Alexandra is a sophisticated mixture of aged rum
and cream
.
0015in Brazil there is a variety of Batida, which is a
mixture of fruit and cachac¸a
.
0016daı
¨
quiri or daiquiri was created in 1898 by an
American in Cuba. Originally it was a light rum
like Bacardi mixed with lemon juice
.
0017Cuba libre, one of the most popular drinks in
North America, is a mixture of Coca-Cola and rum
Another very interesting feature of rum which is not
often emphasized is its use in eaten products, confec-
tioneries, icecreams, chocolate, and foods. For
example, in France, more than 40% of marketed
rum is used as an aromatic resource, in confectionery.
Baba (sponge-cake) is one of the best-known confec-
tioneries containing rum. Traditional rum and heavy
rum are used to flavor various foods because both are
rich in aromatic compound and can endure physical
hard treatment, such as the high temperature encoun-
tered in food processing. Light rum, on the other
hand, is poor in aromatic compounds and is not
suitable as a flavoring agent in foodstuffs.
Manufacturing Process
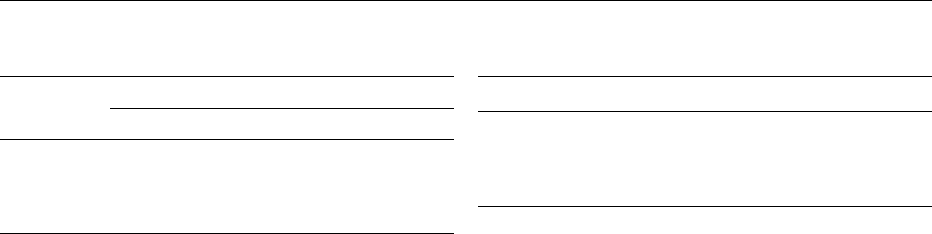
0018The first stage of rum elaboration is preparation of
the mash. The goal is to favor the alcoholic fermenta-
tion of sugars by yeasts, either occurring naturally or
introduced in the fermentation media by the distiller.
During fermentation there is also a production of heat
which in small vats is quickly dissipated, but in larger
vats may raise the temperature such that it kills the
yeast. When fermentation is finished, the mash is
distilled either in a pot still in continuous distillation
columns, or in a multistage distillation column
(Figure 1). This operation makes it possible to separ-
ate from the fermented media the volatile compon-
ents and concentrate them in the distillate. The
resulting raw rum is at a higher percentage in ethanol
(around 80%) than the marketed products, so it is
diluted before sale. It contains aromatic volatile
compounds which distil together with the ethanol.
These congeners are generally in small quantities
(0.0001–0.001% of the ethanol, in weight).
Depending on their proportion, they add significantly
to the taste and aroma of the raw rum.
0019More than 95% of the rum produced in the world
is derived from molasses and high-test molasses
(HTM). Molasses is rich in sugar, and has a density
comparable to honey: 1.4. It also contains nonsugar
RUM 5023
compounds such as minerals, organic acids, phenols,
and others chemicals generated by the thermal treat-
ment during sugar manufacturing.
0020 Rum from sugarcane juice is a special feature of
traditional production in the French West Indies.
0021 Before the eighteenth century, the essential elem-
ents of rum production were:
.
0022a mash comprising molasses and scum as the
source of sugar, which was diluted with waste
water (slops) and/or water in varying proportions
(Table 2)
.
0023in this mixture sugars were spontaneously fer-
mented over a 7–15-day period by the abundant
flora of Schizosaccharomyces yeast and various
bacteria. Often, the molasses were introduced repe-
teadly, three times, to avoid fermentation failure
.
0024the fermented washes were distilled in a pot still
(Figure 1a) with double distillation. The kettle was
manufactured of red copper, and a tap at the
bottom was used to drain the spent liquor. The
still head was also of red copper, sometimes tin-
plated. The condenser was made from copper or
pewter.
The rums obtained were between 41 and 45% etha-
nol content by volume. Developments in distillation
engineering and microbiology were destined to
improve these early processes dramatically.
0025During the eighteenth century, distillation equip-
ment evolved, with the use of the retort, making it
possible to obtain marketable spirit from a pot still
without double distillation. In some devices the distil-
lates were around 80% ethanol content. The continu-
ous single distillation column (Figure 1b) was
developed in the nineteenth century, in order to in-
crease production. The distillates were also more
consistent and the raw rums contained up to 90%
ethanol – sometimes even 95%. The Creole, the
Savalles, and the Barbet columns were typical of this
period; the Barbet columns produced very aromatic
distillates.
0026Multicolumn devices appeared during the nine-
teenth century (Figure 1c) and are used extensively
today. The Coffey still was introduced for the distilla-
tion of grain whisky in Scotland and used largely
in the British colonies. It produces distillates with
84–85% ethanol content. It initially comprised two
columns: the analyzing column and the rectifying
column, as used for grain whisky distillation. Today,
multicolumn stills with three stages (beer, purifying,
and rectifying columns) or even four columns are
used for rum distillation.
0027The other great advance in rum production in the
twentieth century concerned microbiology. In the
early days, the spontaneous flora was used, and was
often initiated with a natural starter by using a piece
of wool soaked in washes and then carefully dried
and kept to be introduced into the new fermentations.
After Pasteur’s studies of wine and beer fermenta-
tions, some researchers considered replacing the
spontaneous or natural starter by pure fermentation
using selected yeast strains. The aim was to improve
1 Cucurbit
2 Still head
3 Retort
4 Coil
5 Condenser
6 Cooling
liquid
7 Testtube
8 Hearth
2
3
1
8
5
7
4
6
(a)
Condenser
Distillate: raw rum
Fermented wash
Slops
(b)
(c)
Heads
Raw light rum
or alcohol
Fusel
oil
Slops Water Water
Fermented
wash
Beer column Rectifying
column
Purifying
column
fig0001 Figure 1 Rum distillation devices. (a) Intermittent pot still;
(b) continuous single distillation column; (c) multistage column.
5024 RUM
productivity and avoid bacterial contamination, since
it was thought by some that the bacterial flora was
detrimental to the flavor of the products. On the
other hand some microbiologists, such as Allan and
Ashby, believed that bacteria played an important
part in producing the rum bouquet.
0028 The application of pure fermentation involved
modifications in fermentation management, in par-
ticular growth of starter cultures with selected yeast
strains, and the use of antiseptics to limit bacterial
presence and activity. The major change was fermen-
tation by Saccharomyces yeast strains rather than
Schizosaccaromyces. The result was an increase in
yield but the quality of the products fell, through an
increase in chemical neutrality. Roques, in 1927, was
commissioned by the French Ministry of Agriculture
to carry out a study which concluded that ‘rums
produced from pure and rapid fermentations are
characterized by low levels of acid and ester, as well
as relatively high contents in higher alcohols’. Most
traditional rum producers consequently gave up the
use of selected yeasts and decided that ‘wild’ fermen-
tations gave the best results, producing rums with
richer flavor.
0029 At that time, which was the Prohibition period,
light rum production was expanding in the greater
West Indies and in the English-speaking lesser West
Indies. Here rum was produced using modern plants
and pure culture yeast. Under these conditions fer-
mentations are rapid, the distillation is carried out
with multistage devices, and the resulting distillates
are low in aromatic compounds. These light rums
formed the basis of the modern rum industry. How-
ever, traditional aromatic rums are still produced in
the French West Indies and in Jamaica.
0030 The distillery products are sold white (without
aging), or after aging, generally in oak vats for many
months.
Typology and Raw Rum Treatments
0031 Rums, like many others alcoholic beverages, are often
classified and labeled according to their geographical
area or country of origin. However, this geographical
classification does not necessarily correspond to the
organoleptic properties of the spirit and, indeed,
the US Federal Administration does not correlate the
geographical designation with rum type.
0032 From the analytical and sensory viewpoints, rums
are most often described according to their aromatic
intensity. This property largely relates to the micro-
bial status of the fermentation and distillation equip-
ment (Table 3).
0033 Rum can be divided into four categories (Table 4):
light, heavy, de sucreride, and agricole.
0034Light rum, of which Bacardi is the market leader, is
the result of improvements in distillation engineering
and fermentation flora control. Fermentation is rapid
– 12–20 h duration – and uses cultured yeast with
minimization of the bacterial flora by various
means. In Cuba, rum with lightened features
appeared around 1870. A gold medal was awarded
to Don Facundo Bacardi, in 1876, at the international
exhibition of Philadelphia for his light products. Simi-
larly, a light rum or common was manufactured in
Jamaica in 1882. In the French West Indies, trad-
itional producers were not satisfied by the aromatic
profile of the light rums and by the 1930s, they
returned to traditional production processes. In
modern light rum, the content of compounds other
than ethanol (not alcohol: NA) is often under 60 g
impurities per hectolitre (hl) of pure alcohol.
0035Traditional rums are a specialty of the French over-
seas territories: Guadeloupe, Martinique, and La Re
´
-
union. While part of this production is exported, the
traditional products from Haiti and Brazil are solely
consumed locally. The raw materials for traditional
rums may be cane juice or molasses, giving respect-
ively agricole rum and de sucrerie rum. The starting
gravity is around 1035 for media from sugarcane
juice, and 1060 for those from molasses. The produc-
tion is on a cottage industry basis with small plants,
because productivity is not a major goal. Length of
fermentation is 20–48 h and features the presence
and activity of a spontaneous bacterial flora that
contributes to the aromatic properties of the product.
The ethanol content of the final fermentation media
is about between 3.5 and 8% by volume and the
tbl0003Table 3 Basic rum types and processing features
Raw material
Molasses Cane juice
PF, MC MF, PD, SC MF, SC MF, SC
Light Heavy de sucrerie Agricole
PF, pure fermentation (yeast only);
MC, multistage column distillation;
MF, mixed fermentation (bacteria and yeast);
PD, pot still distillation;
SC, single-column distillation.
tbl0004Table 4 Basic rum types: appellation, aspect, and post-
distillation treatments
Not traditional Traditional
W, A, SC W W, A, SC W, A, SC
Light Heavy de sucrerie Agricole
W, white, a, aged; SC, straw-colored.
RUM 5025
