Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


potassium secretion by the collecting duct are listed
in Table 5.
0036 In the medullary collecting duct, potassium re-
absorption occurs again. Potassium reabsorption is
accompanied by proton secretion that is mediated
via hydrogen–potassium ion (H
þ
–K
þ
) exchange
pumps in the luminal membrane of intercalated cells
of the medullary collecting duct. The activity of the
H
þ
–K
þ
exchange pump increases with potassium de-
pletion and decreases with potassium loading.
0037 It should be noted that the amount of potassium
excreted in the urine depends on the relative degree of
potassium secretion and reabsorption at the distal
nephron. Moreover, it is clear, therefore, that the net
potassium excretion is tightly linked to sodium re-
absorption and hydrogen ion secretion in the distal
nephron. Generally, renal regulation of sodium and
hydrogen ion balance takes priority over regulation of
potassium balance.
Response to Dietary Potassium Intake
0038 Urinary excretion of a potassium load occurs over
24–48 h. It is not surprising that other factors, which
are rapid and extremely sensitive to a change in po-
tassium intake and output, are involved to maintain
the constancy of the ratio of potassium concentration
between the ICF and ECF.
0039High dietary potassium intake triggers several pro-
tective mechanisms to increase the cellular uptake of
potassium, and to excrete the excessive potassium
load by the colon and kidneys. The increase in cellular
uptake of potassium is mediated through the release
of insulin and catecholamine (Table 4) and probably
mediated through an increase in activity of Na
þ
–K
þ
–
ATPase pumps in skeletal muscle as well. Hyperkale-
mia stimulates aldosterone secretion. This hormone
increases the colonic excretion of potassium directly
as well as indirectly through an increase in Na
þ
–K
þ
–
ATPase pump activity in the basolateral membrane of
the colon.
0040Most of the potassium load is excreted by the
kidneys. Similar to the colon, aldosterone increases
urinary potassium secretion through an increase in
Na
þ
–K
þ
–ATPase pump activity, and by increasing
the potassium channels of the principal cells of the
collecting duct. In addition, a high potassium intake
inhibits sodium reabsorption, and hence water re-
absorption, at the proximal convoluted tubule and
the thick ascending limb of the loop of Henle. Conse-
quently, sodium delivery and urine flow to the distal
nephron increase. Moreover, hyperkalemia decreases
renal ammonia production, which in turn stimulates
aldosterone secretion. All these factors work directly
on the distal nephron to increase potassium secretion
(Table 5). The adapted kidney can excrete up to 20
times more potassium than the baseline.
0041Basically, the adaptation response to a low potas-
sium intake is the reverse of that to a high potassium
diet. The cellular uptake of potassium decreases by a
decrease in the number of Na
þ
–K
þ
–ATPase pumps in
skeletal muscle but not in other tissues like red blood
cells and cardiac muscle. This shifting of potassium
extracellularly from skeletal muscle aims to correct
an extracellular potassium deficit caused by a low
dietary intake. The colon and kidneys also conserve
potassium by decreasing the secretion and increasing
the reabsorption of potassium, respectively.
See also: Potassium: Properties and Determination;
Physiology; Renal Function and Disorders: Kidney:
Structure and Function; Sodium: Properties and
Determination; Physiology; Thirst; Water: Physiology
Further Reading
Halperin ML and Goldstein MB (1999) Fluid, Electrolyte,
and Acid–Base Physiology: A Problem-based Approach.
Philadelphia, PA: W B Saunders.
Koeppen BM and Stanton BA (2001) Renal Physiology.
St. Louis, MO: Mosby.
Kokko JP and Tannen RL (1996) Fluids and Electrolytes.
Philadelphia, PA: W B Saunders.
tbl0005 Table 5 Factors affecting potassium secretion by the collecting
duct
Factors Potassium secretion
Plasma potassium concentration
""
##
Urine flow rate at distal nephron
""
##
Sodium delivered to distal nephron
""
# (<30 mM) #
Aldosterone "
Arginine vasopressin "
Poorly absorbed anions in distal nephron "
pH
Acidemia #
Alkalemia "
Renal ammonia production #
Glucocorticoid "
Catecholamine
b
1
adrenergic agonist #
a
2
adrenergic agonist "
Insulin #
Dietary potassium intake
High "
Low #
Potassium-sparing diuretic #
ELECTROLYTES/Water–Electrolyte Balance 2047

Acid–Base Balance
E W Kun and A W Yu, The Chinese University of Hong
Kong, Hong Kong, China
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Virtually all cellular, tissue, and organ systems are
sensitive to changes in the hydrogen ion (H
þ
) concen-
tration in the body. They function best at an extracel-
lular fluid H
þ
concentration of 35–45 nmol l
1
(each
nmol l
1
equals 10
6
mmol l
1
). This concentration is
extremely low compared with other ions; sodium
(Na
þ
), for example, has a 3 million times greater
concentration. Therefore, H
þ
concentration is com-
monly expressed as pH, a negative logarithm of H
þ
concentration. Maintenance of acid–base balance
in the body is important because H
þ
binds avidly
to proteins and changes their shape and function.
Many essential metabolic, enzymatic, and trans-
membrane transport processes can be jeopardized
because of alterations of the protein structure and
function, resulting in severe organ dysfunction
and clinical consequences. With an increase in body
H
þ
concentration, a patient may experience hypoten-
sion, depressed myocardial contractility, and sensor-
ium. With decreased body H
þ
concentration, the
patient may develop coronary spasm, cardiac arrhy-
thmias, hyperreflexia, muscle spasm, and seizure
(Table 1).
0002 In normal conditions, H
þ
varies little from the
normal value of approximately 40 nmol l
1
, even
though acids and bases are continually being added
to the extracellular fluid. This can be achieved by
three homeostatic mechanisms: (1) intracellular and
extracellular buffers; (2) changing partial pressure of
carbon dioxide in the blood by altering the ventilation
rate; and (3) renal H
þ
excretion. The basic principles
of acid–base physiology and the role of the kidneys
and lungs in acid–base balance will be discussed in
this article.
General Concepts
0003 An acid is defined as a compound capable of donating
an H
þ
, and a base is a compound that is capable
of accepting an H
þ
. Examples of acids in the body
include H
2
CO
3
, HCl, NH
4
þ
and H
2
PO
4
, and
examples of bases in the body are HCO
3
,Cl
,NH
3
and HPO
4
2
.
0004 Food and cellular metabolism produces more acid
than base. Base is lost in the feces daily. The net effect
is the addition of acid to the body fluids. To maintain
acid–base balance, acid must be excreted from the
body at a rate equivalent to its addition. Acidosis (a
disease process that tends to decrease the pH) results
if acid addition exceeds excretion. Conversely, alkal-
osis (a disease process that tends to increase the pH)
results if acid excretion exceeds addition. Systemic
pH can still be maintained within a normal range at
acidosis or alkalosis because of compensatory mech-
anisms, which are discussed in the section below. If
these conditions or processes are left unopposed, the
pH will increase or decrease from the normal range,
and result in acidemia (pH < 7.35) or alkalemia
(pH > 7.45). Metabolic acidosis results from a pri-
mary reduction in plasma bicarbonate concentration
and respiratory acidosis results from a primary in-
crease in CO
2
partial pressure (Pco
2
). Metabolic al-
kalosis is a result of a primary decrease in plasma
bicarbonate concentration and respiratory alkalosis
is a result of a primary decrease in Pco
2
. An increase
in Pco
2
secondary to compensation for metabolic
alkalosis is not called respiratory acidosis; a second-
ary decrease in Pco
2
in response to metabolic acidosis
is not called respiratory alkalosis.
Acid and Alkali Generation
0005The major constituents of the human diet are carbo-
hydrates, fats, and proteins. In normal conditions,
15 000 mmol CO
2
is generated from the metabolism
of carbohydrates and fats. Although not an acid, this
CO
2
will combine with H
2
O to form H
2
CO
3
; there-
fore, it is termed a volatile acid, reflecting the fact that
it has the potential to generate H
þ
after hydration
with H
2
O. An accumulation of this endogenously
produced CO
2
will result in respiratory acidosis
(Table 2). Fortunately, this large quantity of CO
2
is eliminated by alveolar ventilation, and acid–base
balance is maintained.
0006However, nonvolatile acids (mostly sulfuric acid)
are primarily generated from the metabolism of pro-
teins. For example, oxidation of the sulfur-containing
amino acids cysteine and methionine yields sulfuric
acid (H
2
SO
4
), whereas metabolism of lysine, argin-
ine, and histidine generates hydrochloric acid (HCl).
tbl0001Table 1 Clinical manifestations of an acid–base imbalance
Increased acidity (pH < 7.20) Increasedalkalinity (pH > 7. 5 5)
Hypotension Perioral and extremity paresthesias
Depressed myocardial
contractility
Muscle spasms
Hyperreflexia
Mental confusion Seizures
Coronary spasm
Cardiac arrhythmias
2048 ELECTROLYTES/Acid–Base Balance

Part of this nonvolatile acid is neutralized by the
bicarbonate (HCO
3
) generated through metabolism
of the amino acids, such as aspartate and glutamate,
and certain organic anions, such as citrate. The net
amount of nonvolatile acid produced depends on diet-
ary intake. Ingestion of a meal with a large amount of
meat will result in more acid production, whereas a
vegetarian meal can result in less acid generation. In
general, approximately 1 mmol kg
1
body weight of
nonvolatile acid is added to the body each day (50–
100 mmol day
1
for adults) on a typical diet. The
nonvolatile acids are buffered initially, followed by
renal excretion of the H
þ
.
0007 In abnormal clinical conditions, e.g., diabetes mel-
litus and hypoxia, whereby carbohydrates and fats
are incompletely metabolized to CO
2
and H
2
O,
other nonvolatile acids are produced (Table 2). In
diabetes mellitus, when insulin levels are abnormally
low, fatty acids are oxidized to yield energy. This
leads to the production of acetone and two ketoacids
(b-hydroxybutyric and acetoacetic acid). During hyp-
oxia, oxygen delivery to cells is inadequate, glucose is
metabolized anaerobically to pyruvate and then to
lactate and an H
þ
(one per lactate). Therefore,
normal individuals performing vigorous exercise, pa-
tients with reduced cardiac output (heart failure), and
those with hypotension (shock) usually have higher
lactic acid levels. The acid produced will combine
with HCO
3
and lower the plasma HCO
3
concentra-
tion producing metabolic acidosis:
H
þ
þ HCO
3
! H
2
CO
3
0008 With an increased production of acid, the kidneys
may increase excretion of H
þ
and maintain the
acid–base balance. However, in the presence of renal
diseases (renal failure or renal tubular acidosis),
endogenous acid production or ingestion of acid-
producing substances, e.g., aspirin (salicylic acid),
methanol, and ethylene glycol, may exceed the cap-
acity of the buffer system and renal excretion of H
þ
.
This will result in metabolic acidosis (Table 2).
0009The loss of HCO
3
from the body is also equivalent
to the addition of acid and produces metabolic
acidosis. This is commonly associated with gastro-
intestinal or renal loss of bicarbonate (Table 2).
0010Whereas the stomach is an acid-secreting organ,
the gastrointestinal tract distal to the stomach is
bicarbonate-secreting. The small bowel has a daily
secretory volume of 600–700 ml and this may be
markedly increased if the small bowel is diseased.
The biliary system secretes about 1 l of fluid per day
containing an HCO
3
concentration of 60 mmol l
1
,
whereas the pancreas secretes 2 l day
1
with an
HCO
3
concentration reaching 120 mmol l
1
. Most
of the secretions are reabsorbed, but with diarrhea
and external drainage of pancreatic, biliary, or small-
bowel juice (external fistulae), the loss of HCO
3
-rich
fluid results (Table 2).
0011As mentioned above, alkali can also be produced
by the metabolism of certain amino acids but it is
neutralized by the larger amount of dietary acid pro-
duced. A net alkali excess can only occur when there
is exogenous alkali ingestion, primary increase in
ventilation with lowering of Pco
2
(respiratory alkal-
osis), and excessive H
þ
loss from the stomach or
kidneys (metabolic alkalosis). Milk-alkali syndrome
is seen in patients with gastric distress who consume
large amounts of milk and antacids containing cal-
cium carbonate and sodium bicarbonate. Suppression
of parathyroid hormone secretion caused by hyper-
calcemia from absorbed calcium contributes to
failure to excrete the alkali load. Chronically, nephro-
calcinosis and renal failure develop, which further
reduces the excretion of the absorbed alkali, causing
metabolic alkalosis (Table 3).
The Buffering System
0012One of the major ways in which large changes in H
þ
concentration are prevented is by buffering. A ‘buffer’
tbl0002 Table 2 Generation of acid
Volatile acid ^ respiratory acidosis
CO
2
production (cellular metabolism)
Nonvolatile acids ^ metabolic acidosis
Dietary protein and phospholipid metabolism (sulfuric acid,
phosphoric acid)
Diabetes mellitus (b-hydroxybutyric acid and acetoacetic acid)
Hypoxia (lactic acid)
Decreased tissue perfusion, e.g., vigorous exercise, heart
failure, shock
Decreased renal H
þ
excretion (e.g., renal failure, renal tubular
acidosis)
Loss of HCO
3
Gastrointestinal losses in stool, e.g., diarrhea, external fistulae
Urinary losses of HCO
3
, e.g., proximal renal tubular acidosis,
drug (acetazolamide)
tbl0003Table 3 Generation of alkali
Volatile alkali ^ respiratory alkalosis
CO
2
removal (hyperventilation)
Nonvolatile alkali ^ metabolic alkalosis
Loss of acid
Gastrointestinal losses in gastric fluid, e.g., vomiting, gastric
suction
Urinary losses of H
þ
: diuretics, licorice excess,
hyperaldosteronism, Bartter’s syndrome, Cushing’s syndrome
Exogenous alkali loads
Milk-alkali syndrome
Sodium bicarbonate ingestion
ELECTROLYTES/Acid–Base Balance 2049

is a pair of substances that can donate or accept an
H
þ
to moderate changes in H
þ
concentration or pH.
This is the most immediate mechanism of defense
against changes in physiological pH. The major
buffers in the body are H
2
CO
3
-HCO
3
(pK
a
6.1),
H
2
PO
4
-HPO
4
2
(pK
a
6.8), and H-protein
. The dis-
sociation constant (pK
a
) and pH determine whether a
buffer pair will bind or release the H
þ
in the solution.
When the pH is 1.0 unit below the pK
a
,H
þ
will bind
to the buffer pair, whereas at 1.0 unit above the pK
a
,
H
þ
will release.
0013 Bicarbonate/carbon dioxide is the most important
extracellular buffer in the body. This buffer system
can be described by the following reactions:
Carbonic anhydrase
CO
2
$ CO
2
þ H
2
O $ H
2
CO
3
$ H
þ
þ HCO
3
ðgaseousÞðaqueousÞ
40 mmHg ð5:3kPaÞ 1:2 mmol l
1
24 mmol l
1
ð1Þ
The hydration/dehydration of CO
2
is the rate-
limiting step and is enhanced by the enzyme carbonic
anhydrase (CA). When H
2
CO
3
is formed, it is ionized
to H
þ
and HCO
3
virtually instantaneously. The rela-
tionship of pH, HCO
3
,andPco
2
is described by the
Henderson–Hasselbalch equation:
pH ¼ pK
a
þ logf½HCO
3
½0: 03 PCO
2
g
where pK
a
¼ 6.1, HCO
3
is in mmol l
1
,andPco
2
is
in mmHg.
0014 When nonvolatile acid is added to the body fluids,
the reaction of eqn (1) is driven to the left. HCO
3
is
consumed during the buffering and plasma HCO
3
concentration is reduced. In contrast, when acid is
removed from the body, the reaction is driven to the
right. More HCO
3
is produced from the dissociation
of H
2
CO
3
(carbonic acid) and HCO
3
concentration
increases. With a normal plasma HCO
3
concentra-
tion of 24 mmol l
1
and an extracellular fluid volume
of 14 l, this HCO
3
buffer system can potentially
buffer 340 mmol acid. However, for optimal function
of this system, regeneration and maintenance of
normal HCO
3
concentration by the lungs and
kidneys are required.
0015 In addition, the bones can release buffer com-
pounds such as NaHCO
3
, K HCO
3
, CaCO
3
and Ca
HPO
4
into extracellular fluid when stimulated by a
decrease in the plasma HCO
3
concentration. It has
been estimated that as much as 40% of the buffering
of acute acid load takes place in the bones. The role of
bone buffers is even greater in chronic acidosis, such
as that seen in chronic renal failure. One of the con-
sequences is that acid loading directly increases Ca
2þ
release from the bones and urinary Ca
2þ
excretion,
promoting stone formation. An inappropriately high-
protein diet may increase acid load (a normal diet
produces about 70 mmol acid per day) and promote
calcium stone formation. In patients with end-stage
renal disease, a disorder associated with progressive
acid retention caused by impaired urinary acid excre-
tion, there is a gradual reduction in bone calcium
stores.
0016Plasma phosphate and protein also act as extra-
cellular buffers but they are less important quantita-
tively (plasma phosphate concentration is 1 mmol l
1
versus 24 mmol l
1
HCO
3
).
H
þ
þ HPO
2
4
$ H
2
PO
4
ð2Þ
H
þ
þ protein
$ H-protein ð3Þ
However, intracellularly, phosphate and protein
are important buffers besides HCO
3
. In metabolic
acidosis, about 43% of buffering occurs in the extra-
cellular fluid and 57% of buffering is mediated by
intracellular buffering. Whereas extracellular H
þ
is
buffered immediately, buffering by intracellular
buffers takes about 2–4 h. This is the time required
for H
þ
to diffuse into interstitial spaces and enter
cells, which occurs more slowly.
Respiratory Control of Acid–Base Balance
0017Alveolar ventilation provides oxygen for oxidative
metabolism and eliminates the CO
2
produced by
these metabolic processes in order to maintain Pco
2
at about 5.3 kPa (40 mmHg). Approximately 15 000
mmol CO
2
is produced daily and eliminated by the
lungs. The stimulation to ventilation is located in the
chemoreceptors in the brainstem respiratory center
(medulla oblongata) and to a lesser extent in the ca-
rotid bodies located near the bifurcation of the carotid
arteries. The chemoreceptors are sensitive to changes
in the cerebral interstitial pH. With increased CO
2
in
the blood, cerebral interstitial pH will decrease and
result in the stimulation of ventilation that will return
Pco
2
to normal. In contrast, interstitial pH will in-
crease with decreased plasma CO
2
, resulting in venti-
lation inhibition (Figure 1).
0018Alveolar ventilation is also affected by metabolic
acid–base imbalance. Acidemia caused by metabolic
acidosis can increase minute ventilation from the
normal of approximately 5 l min
1
to greater than
30 l min
1
as the arterial pH decreases from 7.40 to
7.00. The central chemoreceptors are relatively insu-
lated by the blood–brain barrier and only CO
2
, but
not HCO
3
, rapidly crosses the blood–brain barrier.
Hence, the effect on cerebral interstitial pH is slower,
and a full ventilation response to nonvolatile acid or a
2050 ELECTROLYTES/Acid–Base Balance

decrease in plasma HCO
3
may only be achieved in
12–24 h. The Pco
2
will decrease by 0.16 kPa for
every mmol l
1
reduction of HCO
3
concentration.
Conversely, ventilation decreases with metabolic al-
kalosis with a consequent increase in Pco
2
(0.08 kPa
per every mmol l
1
increase in HCO
3
), lowering the
pH towards normal.
0019 An acid–base imbalance can also be initiated by a
change in carbon dioxide tension of body fluids. A
primary increase in Pco
2
(primary hypercapnia),
Pco
2
> 6.0 kPa, results in respiratory acidosis. This
needs to be distinguished from the secondary hyper-
capnia that results from the correction of metabolic
alkalosis. Hypercapnia (respiratory acidosis) could
develop from increased CO
2
production, decreased
CO
2
excretion, or both (Table 4).
0020 The administration of large carbohydrate loads
(greater than 2000 kcal day
1
) and parenteral nutri-
tion to critically ill patients may increase CO
2
pro-
duction. This is rarely the sole cause of hypercapnia.
Increased CO
2
decreases cerebral interstitial pH,
resulting in an increment in respiratory drive, and
the overproduction of CO
2
is usually matched by
increased excretion and the generation of respiratory
acidosis is prevented. Patients with a marked limita-
tion in respiratory reserve (e.g., chronic obstructive
lung disease) and those receiving constant mechanical
ventilation may not be able to match the CO
2
pro-
duction with its removal, resulting in respiratory
acidosis (Table 4). Therefore, it is recommended
that patients with chronic obstructive airway disease
avoid a high-carbohydrate diet to avert further
retention of CO
2
.
0021 However, excessive alveolar ventilation relative to
the prevailing carbon dioxide production results in
primary hypocapnia and respiratory alkalosis. This is
usually attributed to hyperventilation as a result of
increased ventilatory drive, hypoxia, and maladjusted
mechanical ventilators (Table 5).
0022The body responds to hypercapnia and hypocapnia
in two phases (acute and chronic). The acute phase
takes about 5–10 min to complete. If hyper/hypocap-
nia persists (chronic phase), adaptation requires a few
days to complete.
0023In acute hypercapnia, CO
2
diffuses into the cells,
forming carbonic acid which is buffered by intracel-
lular buffers (such as phosphate or hemoglobulin),
resulting in an increase of plasma bicarbonate con-
centration (Figure 2). On average, plasma bicarbon-
ate concentration increases by 0.8 mmol l
1
for each
kPa acute increase in Pco
2
. Mild increases of Na
þ
and K
þ
are also observed in addition to the increment
in plasma bicarbonate concentration.
tbl0004Table 4 Causes of primary respiratory acidosis
Increased CO
2
production
High-carbohydrate diet with constant mechanical ventilation
CO
2
insufflation during endoscopic procedures
Decreased CO
2
removal
Pulmonary disease
Asthma
Chronic obstruction airway disease
Pulmonary fibrosis
Mechanical ventilatory defect
Pneumothorax
Hemothorax/hydrothorax
Adult respiratory distress syndrome
Muscular or neuromuscular diseases (e.g., Guillain–Barre
´
syndrome, amyotrophic lateral sclerosis, multiple sclerosis)
Defect in respiratory drive
Brainstem infarct
Sleep apnea
Drugs (opiates and sedatives)
Other
Cardiac arrest
Shock
Severe pulmonary edema
Massive pulmonary embolus
Increase Decrease
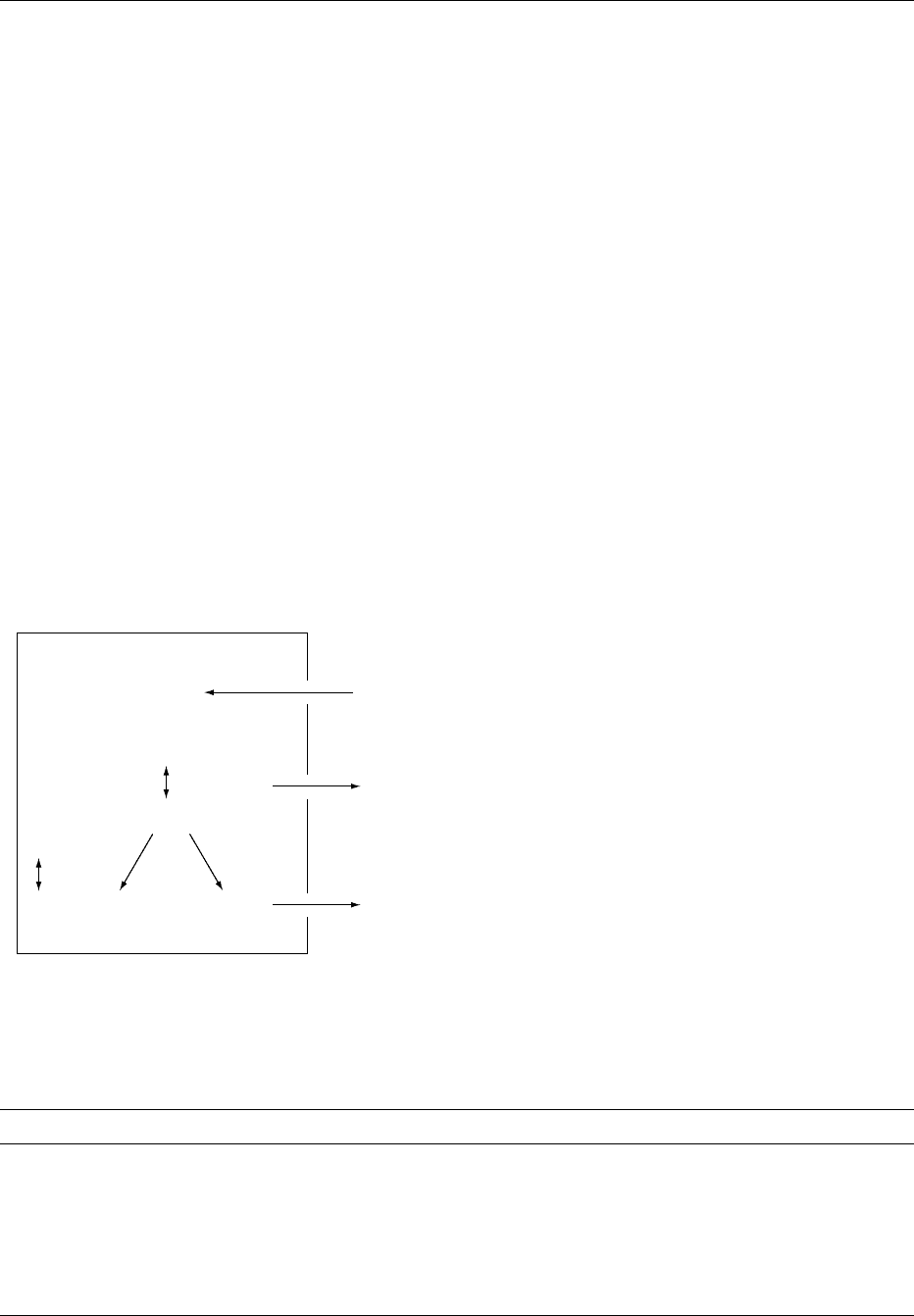
IncreaseDecrease
Ventilation
Chemoreceptor
CSF
interstitial
pH
Metabolic or respiratory
acidosis
Metabolic or respiratory
alkalosis
fig0001 Figure 1 Respiratory control of acid–base balance. CSF, cere-
brospinal fluid.
tbl0005Table 5 Causes of primary respiratory alkalosis
Increased central nervous system respiratory drive
Anxiety
Brain infarction, trauma
Sepsis and fever
Drugs (salicylate, nicotine, doxapram)
Tissue hypoxia
Severe anemia
High altitude
Cyanotic heart disease
Pneumonia
Iatrogenic
Increased mechanical ventilation
ELECTROLYTES/Acid–Base Balance 2051
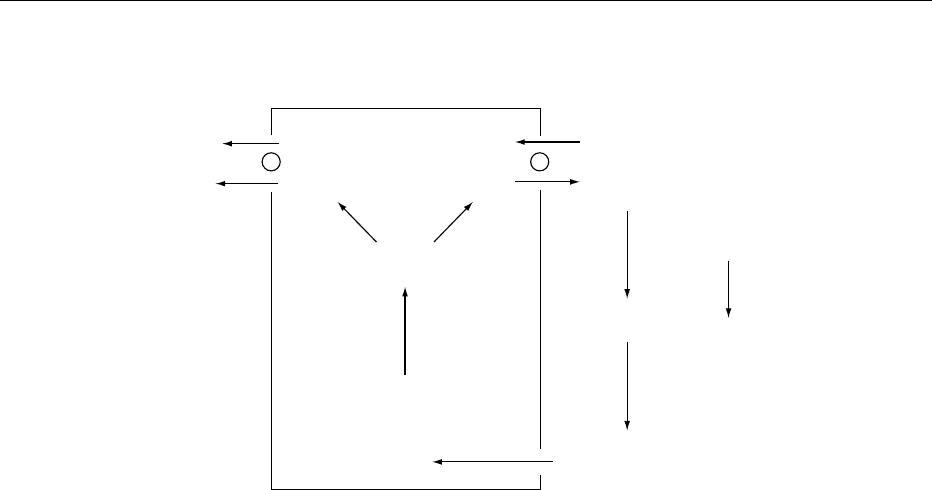
0024 If hypercapnia persists, an adaptive increment in
plasma bicarbonate concentration is markedly ampli-
fied as a result of increasing renal acid excretion,
generating new bicarbonate that is returned to the
blood. Plasma bicarbonate increases by 3.0 mmol l
1
with each kPa chronic increase in Pco
2
. The renal
response usually takes 1–2 days to develop fully.
0025 In contrast, adaptation to hypocapnia is associated
with an immediate decrement in plasma bicarbonate
of 1.5 mmol l
1
for each kPa acute decrease in Pco
2
.
With persisting hypocapnia, renal adaptation re-
quires 3–4 days to complete. This results in a larger
decrement in plasma bicarbonate (3.8 mmol l
1
for
each kPa of chronic decrease in Pco
2
). The relative
changes of Pco
2
, HCO
3
, and pH in various acid–base
disorders are summarized in Table 6.
Renal Control of Acid–Base Balance
0026 Despite the efficacy of respiratory and buffering
systems in maintaining the acid–base balance, the
ventilation rate is limited, and the body buffers even-
tually deplete if the dietary acid load (50–100 mmol
H
þ
) is not excreted in the urine. The kidneys have the
important role of regulating the systemic bicarbonate
concentration and defending against acid–base im-
balance. The kidneys make appropriate adjustments
in the secretion of HCO
3
and H
þ
. The renal response
requires several days to complete. There are two com-
ponents in this regulatory process: (1) reabsorption of
filtered HCO
3
, and (2) generation of bicarbonate by
net H
þ
excretion.
Reabsorption of Filtered Bicarbonate
0027The glomeruli filter about 4300 mmol bicarbonate
per day. About 80% of the filtered bicarbonate is
reabsorbed in the proximal tubules, whereas the re-
mainder is reabsorbed in the distal segments. CA has
a central role in reabsorption of HCO
3
. The filtered
HCO
3
generates carbonic acid (H
2
CO
3
) by combin-
ing with the excreted H
þ
. CA, located in the brush
border of the proximal tubular cells, rapidly dissoci-
ates H
2
CO
3
into water and CO
2
.CO
2
then diffuses
into the tubular cells where it combines with the OH
from H
2
O, forming HCO
3
and H
þ
.H
þ
is excreted by
Na
þ
-H
þ
antiporters and the HCO
3
leaves the cells
and enters the peritubular capillary blood by Na
þ
–3
HCO
3
cotransporter in the basolateral membrane.
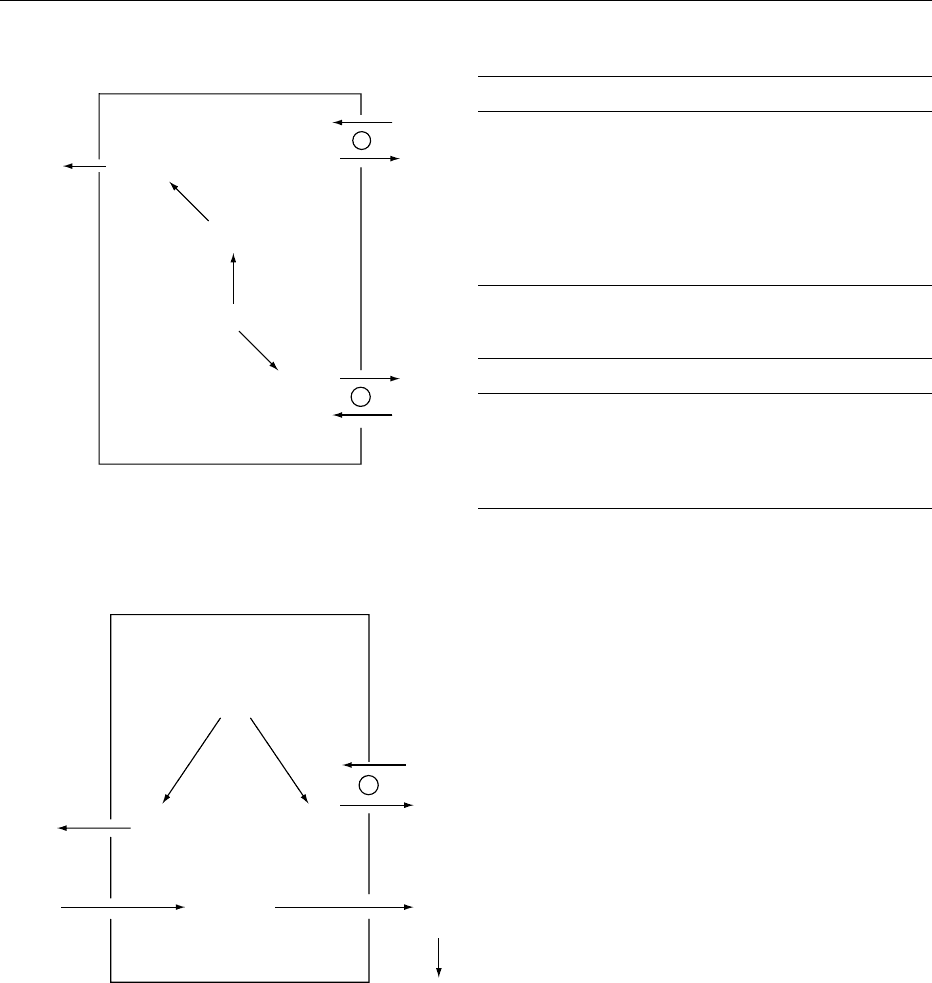
The tubular fluid pH decreases from 7.40 in the
filtrate to about 6.70 by the end of the proximal
convoluted tubule (Figure 3).
Renal Tubular H
þ
Excretion
0028The above process reclaims the filtered bicarbonate
but does not participate in the excretion of the dietary
acid load. The H
þ
requires combination with buffers
or the formation of ammonium for excretion. Weak
acids (titratable acids) filtered from the glomeruli,
mostly monobasic phosphate (HPO
4
2
), act as import-
ant buffers in the tubular fluid (Figure 3). This can
account for the excretion of 10–40 mmol H
þ
per day.
Buffering by phosphate and, to a lesser extent, other
buffers such as urate and creatinine is called titratable
acidity. In the presence of an acid load, the amount
of titratable acidity cannot be easily increased.
tbl0006 Table 6 Changes of pH, PCO
2
(kPa) and HCO
3
(mmol l
1
) in simple acid–base
Disorder pH P
CO
2
HCO
3
Expectedchanges
Metabolic acidosis ## # DP
CO
2
¼ 0.16 D [HCO
3
]
Metabolic alkalosis "" " DP
CO
2
¼ 0.08 D [HCO
3
]
Respiratory acidosis #" "
Acute D [HCO
3
] ¼ 0.8 DPCO
2
Chronic D [HCO
3
] ¼ 3.0 DPCO
2
Respiratory alkalosis "# #
Acute D [HCO
3
] ¼ 1.5 DPCO
2
Chronic D [HCO
3
] ¼ 3.8 DPCO
2
Intracellular Extracellular
CO
2
H
2
O
H
2
CO
3
CO
2
+
Buf
−
HBuf
H
+
Na
+
/K
+
+
HCO
3
−
HCO
3
−
fig0002 Figure 2 Intracellular buffering response to respiratory acid-
osis. Buf
, buffer.
2052 ELECTROLYTES/Acid–Base Balance
Ammonium excretion constitutes the major adaptive
response to an acid load.
Ammonium Excretion
0029 The excretion of H
þ
as ammonium (NH
4
þ
)isanim-
portant step in acid–base regulation because the rate
of NH
4
þ
production and excretion can be regulated in
response to the physiological needs. NH
4
þ
excretion in
the urine represents acid excretion and generation of
new bicarbonate by the kidneys.
H
þ
þ NH
3
$ NH
þ
4
The normal rate of NH
4
þ
excretion in the urine is
about 30–40 mmol day
1
. It can be increased to
> 300 mmol day
1
after a maximum acid load.
0030 NH
4
þ
production in the kidneys takes place pre-
dominantly in the proximal tubules. Glutamine
(primarily from the liver) is the major precursor of
ammonia in the kidneys. Glutamine is metabolized in
the kidneys to glutamate and NH
4
þ
.
Glutamine ! 2NH
þ
4
þ glutamate
!
2NH
þ
4
þ -ketoglutarate
2
The metabolism of a-ketoglutarate generates two
HCO
3
that are returned to the systemic circulation.
0031 In the proximal tubules, NH
4
þ
is secreted into the
tubular lumen by Na
þ
-H
þ
antiporter with NH
4
þ
sub-
stituting the H
þ
, and the HCO
3
enters the blood
(Figure 4a). Some secreted NH
4
þ
is reabsorbed in the
ascending limbs of loops of Henle into the medullary
interstitium where it exists as both NH
4
þ
and NH
3
.In
the collecting tubules, lipid-soluble ammonia (NH
3
)
passively diffuses into the lumen and combines with
secreted H
þ
to form NH
4
þ
(Figure 4b). Cationic NH
4
þ
is lipid-insoluble and excreted in the urine because
back-diffusion into the cells cannot occur.
Regulation of Renal Bicarbonate Reabsorption and
Acid Excretion
0032A variety of factors, such as intracellular pH, effective
circulating volume, changes in plasma potassium
concentration, as well as several hormones, may
affect bicarbonate reabsorption and acid secretion,
leading to alkalosis or acidosis (Table 7).
0033Decreases in intracellular pH, as a result of meta-
bolic acidosis (increased H
þ
) or respiratory acidosis
(increased Pco
2
), will increase the availability of
H
þ
for excretion. At increased acid load or meta-
bolic acidosis, plasma bicarbonate concentration is
decreased. A greater concentration gradient allows
HCO
3
to diffuse out of the tubular cells. A decrease
in intracellular HCO
3
will lower intracellular pH
and provide a signal to increase HCO
3
reabsorption
and H
þ
secretion. In respiratory acidosis or alkal-
osis, changes in intracellular pH are mediated by
the diffusion of lipid-soluble CO
2
into or out of
the cells.
0034The effective volume status can influence bicarbon-
ate reabsorption. Decreases in the effective circu-
lating volume increase bicarbonate reabsorption,
whereas increases in the effective circulating volume
Proximal
tubular cell
Tubular
lumen
Capillary
blood
3HCO
3
H
2
CO
3
CA
CA
H
2
O
+
CO
2
CO
2
+ H
2
O
Na
+
Na
+
H
+
H
2
CO
3
3HCO
3
−
H
+
+ HCO
3
−
−
H
+
+ HPO
4
2−
H
2
PO
4
−
fig0003 Figure 3 Bicarbonate reabsorption and acid excretion in the proximal tubule. CA, carbonic anhydrase.
ELECTROLYTES/Acid–Base Balance 2053
inhibit proximal bicarbonate reabsorption. This is
clinically important in patients with volume-depleted
metabolic alkalosis. The inability to excrete excess
HCO
3
prevents resolution of metabolic alkalosis. In
this situation, Na
þ
reabsorption (together with
HCO
3
) prevents further volume depletion at the
expense of systemic pH.
0035The plasma potassium concentration varies in-
versely with both HCO
3
reabsorption and H
þ
excre-
tion. With potassium loss, hypokalemia causes a
transcellular shift of potassium out of the cells
(which contain approximately 98% of total body
potassium). To maintain electroneutrality, Na
þ
and
H
þ
move into the cells. Intracellular acidosis results,
stimulating HCO
3
reabsorption and H
þ
excretion.
0036Several hormones also influence bicarbonate re-
absorption and H
þ
excretion. Angiotensin II stimu-
lates bicarbonate reabsorption in the early proximal
tubules by increasing the activity of both the Na
þ
-H
þ
antiporters and the basolateral Na
þ
-3HCO
3
co-
transporters. Aldosterone, however, acts on distal
nephrons and promotes H
þ
excretion in the collecting
tubules.
0037The parathyroid hormone inhibits proximal HCO
3
reabsorption by inhibiting Na
þ
-H
þ
antiporters in lu-
minal membranes and Na
þ
-3HCO
3
cotransporters in
basolateral membranes. The physiological import-
ance is unknown because the extra HCO
3
delivered
out of the proximal tubules is mostly reabsorbed in
the distal tubules. However, patients with primary
hyperparathyroidism and hypercalcemia tend to
have metabolic acidosis.
Summary
0038In summary, the pH of body fluids is maintained
within a narrow limit by the coordinated function of
the buffers, lungs, and the kidneys. Volatile and non-
volatile acids together with other acids and alkali
ingested must be excreted to maintain acid–base
Na
+
2NH
4
2HCO
3
Glutamine
α-Ketoglutarate
2−
Na
+
H
+
H
+
Capillary
blood
Proximal
tubular cell
Tubular
lumen
−
2HCO
3
−
+
2NH
4
+
(a)
Medullary
interstitium
Collecting
tubular cell
Tubular
lumen
CO
2
HCO
3
H
2
O
CA
H
+
H
+
K
+
(Blood)
NH
3
NH
3
NH
3
NH
4
+
+
−
+
(b)
fig0004 Figure 4 Acid excretion as ammonium in (a) the proximal
tubule; (b) the collecting tubule. CA, carbonic anhydrase.
tbl0007Table 7 Factors affecting renal HCO
3
reabsorption and/or H
þ
excretion
Increase Decrease
Metabolic acidosis Respiratory alkalosis
Metabolic alkalosis with volume
depletion
Volume expansion
Hyperkalemia
Respiratory acidosis Parathyroid hormone
Volume depletion
Hypokalemia
Angiotensin II
Aldosterone
tbl0008Table 8 Sequential event in response to an acid or alkali load
Duration
Extracellular HCO
3
buffering Immediate
Respiratory response by changing P
CO
2
Minutes to hours
Intracellular and bone buffering 2–4 h
Adjustment in renal HCO
3
reabsorption
and H
þ
excretion
24 h to few days
2054 ELECTROLYTES/Acid–Base Balance

balance. The sequential responses to acid or alkali
load are depicted in Table 8. The kidney is the ultim-
ate organ to control acid–base balance by adjusting
the amount of H
þ
excretion. With kidney failure,
acid–base balance cannot be maintained despite the
presence of other intact buffering systems.
See also: Acids: Properties and Determination; Natural
Acids and Acidulants; Amino Acids: Metabolism;
Diabetes Mellitus: Secondary Complications; Glucose:
Function and Metabolism; pH – Principles and
Measurement; Phosphorus: Physiology; Renal
Function and Disorders: Kidney: Structure and Function
Further Reading
Druml W (2001) Nutritional management of acute renal
failure. American Journal of Kidney Disease 37 (Suppl
2): S89–S94.
Halperin ML and Goldstein MB (1999) Fluid, Electrolyte,
and Acid–Base Physiology: A Problem-Based Approach,
3rd edn. Philadelphia: W.B. Saunders.
Henger A, Tutt P, Riesen WF, Hulter HN and Krapf R
(2000) Acid–base and endocrine effects of aldosterone
and angiotensin II inhibition in metabolic acidosis in
human patients. Journal of Laboratory and Clinical
Medicine 136: 379–389.
Kellum JA (2000) Determinants of blood pH in health and
disease. Critical Care 4: 6–14.
Khanna A and Kurtzman NA (2001) Metabolic alkalosis.
Respiratory Care 46: 354–365.
Koeppen BM and Stanton BA (2001) Renal Physiology, 3rd
edn. St Louis: Mosby.
Kraut JA (2000) Disturbances of acid–base balance and
bone disease in endstage renal disease. Seminars in
Dialysis 13: 261–266.
Kraut JA and Madias NE (2001) Approach to patients with
acid–base disorders. Respiratory Care 46: 392–403.
Oh MS (2000) New perspectives on acid–base balance.
Seminars in Dialysis 13: 212–219.
Remer T (2000) Influence of diet on acid–base balance.
Seminars in Dialysis 13: 221–226.
Rose BD and Post TW (2001) Clinical Physiology of Acid–
Base and Electrolyte Disorders, 5th edn. New York:
McGraw-Hill.
Silver RB, Breton S and Brown D (2000) Potassium deple-
tion increases proton pump (H
þ
-ATPase) activity in
intercalated cells of cortical collecting duct. American
Journal of Physiology and Renal Physiology 279: F195–
F202.
Sugiura S, Inagaki K, Noda Y, Nagai T and Nabeshima T
(2000) Acid load during total parenteral nutrition: com-
parison of hydrochloric acid and acetic acid on plasma
acid–base balance. Nutrition 16: 260–263.
ELECTROPHORESIS
R J Fritsch, Kraft General Foods Research and
Development Inc., Munich, Germany
I Krause, Technical University Munich,
Weihenstephan, Germany
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Background
0001 Electrophoretic techniques are among the premier
methods for the separation and analysis of proteins
in foods. This article reviews the basic principles of
analytical electrophoresis and the most important
separation and detection modes, including the use of
immunoreagents, and applications to food analysis/
authentication. (See Protein: Determination and
Characterization.)
Principles
0002 Electrophoresis is a general term that describes the
migration and separation of charged particles (ions)
under the influence of an electric field. An electro-
phoretic system consists of two electrodes of opposite
charge (anode, cathode), connected by a conducting
medium called an electrolyte. The separation effect
on the ionic particles results from differences in their
velocity (v), which is the product of the particle’s
mobility (m) and the field strength (E):
v ¼ mE: ð1Þ
The mobility (m) of an ionic particle is determined by
particle size, shape, and charge, and the temperature
during the separation, and is constant under defined
electrophoretic conditions.
0003Electrophoretic conditions are characterized by the
electrical parameters (current, voltage, power), and
factors such as ionic strength, pH value, viscosity,
pore size, etc., which describe the medium in which
the particles are moving.
0004The removal of heat generated by the passage of
electric current is one of the major problems in most
forms of electrophoresis. Any temperature difference
causes variations in the rates of migration through the
ELECTROPHORESIS 2055

medium, resulting in distortion in the bands of separ-
ated molecules. Clearly, it would be ideal if electro-
phoretic analyses could be carried out at a constant
temperature.
0005 The different separation modes and their basic
characteristics are summarized in Table 1; a more
detailed discussion follows in subsequent sections.
Formats
0006 Generally, all modes of electrophoresis can be carried
out either in ‘free solution,’ in which no anticon-
vective stabilizers are used (see capillary zone
electrophoresis), or in a ‘support medium,’ where
anticonvective support matrices suppress the ther-
mally driven convection currents and diffusion in
the electrophoretic medium.
0007 In supporting media, the mobility and sharpness of
separations can be influenced by additional factors.
These include adsorption and ion-exchange effects
with the matrix, inhomogeneities within the support
matrix, and electroendosmosis. Furthermore, support
media offer visualization of separated zones in a con-
ventional format where strips, foils, and slabs can be
easily stained, destained, and manipulated in ways
not possible for free solutions.
0008 The most commonly practised formats and tech-
niques of electrophoresis in food analysis are listed in
Table 2. Gels made from polyacrylamide and agarose
are the supporting media of choice today. The use
of cellulose acetate strips is popular for routine
screening work where the ease of handling, commer-
cial availability of ready-to-use material, and rapidity
are relevant attributes. Paper and thin-layer electro-
phoresis (e.g., on silica gel) are successfully used for
the analysis of high-molecular-weight polysacchar-
ides and lipopolysaccharides.
Agarose Gel
0009Agarose is a highly purified polysaccharide derived
from agar, a natural product of red seaweed. Com-
mercially available agarose materials show different,
well-characterized levels of electroendosmosis, due
to the presence of sulfate and carboxyl groups in
agar. Although electrophoresis on agarose gel has
been eclipsed by the use of polyacrylamide in the
analysis of most proteins and glycoproteins, it
remains invaluable in applications where a very
large pore size and hence nonrestrictive gel is re-
quired, e.g., in:
.
0010immunoelectrophoretic procedures, especially
those relying on an immunodiffusion step (immu-
noprinting and immunofixation);
.
0011the separation of very large molecules with an aver-
age hydrodynamic radius above 5–10 nm, such as
tbl0001 Table 1 Modes of electrophoresis and basic characteristics of systems
Mode Characteristics
Zone electrophoresis Continuous electrolyte systems, continuous pH and ionic strength, sieving effect possible
depending on support medium
Isotachophoresis Discontinuous electrolyte system, concentrating effect, migration at same velocity
Isoelectric focusing Continuous electrolyte system, stable and linear pH gradient, no molecular sieving effect
tbl0002 Table 2 Important electrophoretic techniques and types of detection in food analysis
Format Electrophoretic technique Type of detection
In free solution Capillary zone electrophoresis On-line electrical
Capillary isotachophoresis On-line optical
On-line thermal
In nonsieving medium Zone electrophoresis in agarose Staining for proteins
a
:
Isoelectric focusing in agarose Coomassie Blue, Amido Black
silver
colloidal gold
In sieving medium Zone electrophoresis in polyacrylamide: Localization of specific constituents
a
:
in homogeneous gel and buffer system assays for enzyme activity
in multiphasic gel and buffer system glyco-, lipo-, phosphoproteins
with pore size gradients immunofixation, immunoprinting
in presence of SDS detergent
Isoelectric focusing in polyacrylamide Blotting on immobilized matrix
a
:
normal staining methods
immunoblotting/enzyme labelling
a
These types of detection are applicable to both the sieving and nonsieving media.
2056 ELECTROPHORESIS
