Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


.0007 sodium, 135–145 mmol l
1
;
.
0008 potassium, 3.5–5.0 mmol l
1
;
.
0009 calcium, 8.5–10.2 mg dl
1
;
.
0010 magnesium, 1.8–2.4 mg dl
1
.
Analytical Methods
0011 Many methods exist for the assay of electrolytes.
These methods include flame emission spectropho-
tometry (FES), atomic absorption spectrophotometry
(AAS), electrochemical analysis with ion-selective
electrodes (ISE), redox titration, colorimetry, and
fluorescent probes. In the clinical laboratory, the ma-
jority of the analyses are performed by FES, ISE, and
colorimetry. (See Calcium: Properties and Determin-
ation; Sodium: Properties and Determination.)
Flame Emission Spectrophotometry
0012 Flame emission spectrophometry is most commonly
used for the analysis of Na
þ
and K
þ
. When metals
such as Na
þ
and K
þ
are sufficiently heated in a flame,
they emit light of wavelengths characteristic of the
metal. The metal ions absorb heat energy, which
causes electrons to be driven out of their normal
orbital position. These excited metal atoms are said
to be in high-energy states and are very unstable.
The extra energy is given off in the form of light.
When Na
þ
and K
þ
are excited, they emit spectra
with sharp, bright lines at 589 and 768 nm, respect-
ively. Within certain limits, the amount of light given
off by the excited atoms is proportional to the con-
centration of the metal ions in the solution. To pre-
pare a flame emission spectrophotometer for
detecting Na
þ
and K
þ
, standard solutions containing
known concentrations (two levels) of these electro-
lytes are aspirated into the instrument. The digital
readout is then set to the known concentrations of
the fluid being aspirated. When the flame photometer
has been so calibrated, the fluid containing the un-
known concentration is aspirated, and the result indi-
cating the concentration is read from the digital
display.
0013 Another FES-type system, inductively coupled
argon plasma (ICAP), utilizes the principle of atomic
emission spectrophotometry. It is similar to FES,
except that the plasma has a much higher excitation
temperature. This results in lower limits of detection,
a wide dynamic range, and virtual freedom from
chemical interference. It is possible to use ICAP for
the analysis of metals, such as sodium and potassium,
as well as many nonmetals, such as sulfur and boron;
however, Cl
cannot be assayed by using ICAP spec-
trophotometry. Although the principle of ICAP has
been used to develop automated instrumentation,
at present this system has not been used in clinical
laboratories.
Atomic Absorption Spectrophotometry
0014Atomic absorption spectrophotometry is not com-
monly employed in clinical laboratories. As AAS is
much more sensitive than FES, AAS is used as a
reference method for many analytical procedures.
The principle on which AAS is based is similar to
FES. In AAS, however, the flame serves to dissociate
the element from its chemical bonds and place it in a
ground state at which it is capable of absorbing light
of a wavelength specific for the element. The method
involves using hollow cathode lamps containing
the metal of interest. The metal in the lamp is sub-
jected to an electric current, causing it to emit light at
a characteristic wavelength specific to the element in
the lamp. This light passes over a burner to a special
detector that measures emitted intensity.
0015To prepare an AAS for analyzing electrolytes, a
standard solution containing a known concentration
of the metal of interest is aspirated into the burner;
the atoms in this field absorb the light proportional
to their concentration in the fluid. The resulting
decrease in intensity of the beam of light given off
from the hollow cathode lamp is then set on a digital
readout to reflect the known concentration. Once
the instrument has been so calibrated, the unknown
fluid is aspirated, and the electrolyte concentra-
tion can be obtained from the digital display. This
method can be used in the analysis of Na
þ
,K
þ
,
Ca
2þ
, and Mg
2þ
.
Ion-selective Electrodes
0016Potentiometry with ISE is a comparatively recent
development in the electrolyte analytical field. It is
the measurement of the electrical potential difference
between two electrodes, which, in contact with one or
more electrolyte solutions, form an electrochemical
cell. A high-impedance voltmeter measures the elec-
trical potential difference between the two electrodes.
Ion-selective electrodes respond preferentially to a
particular ion species in solution. Some potentiomet-
ric analytical methods use an ion-selective indicator
electrode and a reference electrode. A method de-
veloped by Eastman Kodak and used on their Ekta-
chem analyzer uses two identical ISE mounted in a
single-use disposable slide. The reference fluid con-
taining ions at fixed concentration is applied to the
reference electrode. The sample fluid is simultan-
eously applied to the indicator electrode. The two
solutions are allowed to form a liquid junction in
the bridge portion of the slide. This arrangement
constitutes a concentration cell.
ELECTROLYTES/Analysis 2037

0017 The specific components of the cell vary according
to the ion being tested. Over the physiological range,
the relationship between the measured cell potential
and the logarithm of the molal concentration of the
ion being tested from the fluid is linear. The junction
potential is produced between the reference fluid and
the sample fluid in the bridge portion of the analysis
slide. The magnitude and sign of the junction poten-
tial depend on the total ionic concentration and com-
position of the fluid but are primarily determined by
the Cl
and Na
þ
concentrations. The magnitude of
this term is minimized by using a reference fluid
having ionic concentrations near physiological aver-
ages. Applications of this have been developed for the
analysis of carbon dioxide (CO
2
), Cl
,Na
þ
, and K
þ
.
The results are normally reported in molar units.
0018 Other methods employing ISE technology use elec-
trodes of a nondisposable variety. Glass electrodes are
made from specially formulated glass with added
oxides of various metals. For example, the most prac-
tical sodium electrode is made of specialized glass,
selective for Na
þ
. A solid-state electrode employing
a homogeneous membrane of silver chloride is used
for Cl
analysis of sweat by direct measurement from
the skin surface. A liquid ion-exchange electrode with
an ion-selective carrier embedded in a polyvinylchlor-
ide matrix is used for chloride analysis of body fluids.
A liquid ion-exchange membrane electrode, incorpor-
ating the antibiotic valinomycin as the K
þ
binder, is
the most selective for potassium. A gas electrode is
available for total CO
2
(HCO
3
) analysis. This
involves a combination of a glass pH electrode and
a reference electrode in contact with a weakly buf-
fered electrolyte solution behind a membrane perme-
able to CO
2
gas. When the CO
2
gas is forcibly
released from the sample, it diffuses through the
membrane and reacts with water in a buffer solution,
resulting in a change in the hydrogen ion activity of
the buffer. The pH electrode senses the change in
hydrogen ion activity, which is an indication of the
concentration of the CO
2
present in the sample fluid.
Ion-selective electrodes have been incorporated into
many automated systems used in clinical laboratories
for assaying the ‘electrolyte profile.’
Redox Titration
0019 Redox titration is characterized by the transfer of
electrons from one substance to another with an end
point determined calorimetrically or potentiometri-
cally. This method is used for the determination of
Ca
2þ
. Calcium is precipitated as calcium oxalate,
which is treated with sulfuric acid. The oxalic acid
formed is redox-titrated with potassium permangan-
ate (oxidant). This method is time-consuming and is
not used in clinical laboratories.
Colorimetry
0020In clinical laboratories, colorimetry is commonly
employed for the analysis of Ca
2þ
and Mg
2þ
. The
analysis is based on reacting the metal with an indica-
tor dye to form a colored complex. The density of
the resulting complex is related to the concentration
of the metal and can be measured spectrophotome-
trically. A common dye used for assaying total
calcium inbodyfluid iso-cresolphthaleincomplexone.
This reagent contains other compounds (8-hydroxy-
quinoline, urea, and ethanol) which serve to mask
interferences and enhance the reaction. Magnesium
is commonly determined by complexing Mg
2þ
with
a Calmagite reagent, resulting in another chromo-
phore which can also be measured spectrophotome-
trically. (See Spectroscopy: Visible Spectroscopy and
Colorimetry.)
Fluorescent Probes
0021The assay of intracellular levels of Ca
2þ
can be con-
ducted in living cells by the use of fluorescent probes.
These dyes are introduced into the cell as an ester
derivative which is nonpolar and thus freely diffusible
across cell membranes. Once within the cell, the com-
pounds are hydrolyzed by nonspecific esterases pre-
sent in the cytosol. The resulting cation is trapped
within the cell owing to its inability to traverse the
plasma membrane. Thus, the cation gradually accu-
mulates intracellularly. When excited at a given wave-
length, its fluorescence reflects the concentration of
free cytosolic calcium.
See also: Calcium: Properties and Determination;
Physiology; Coenzymes; Magnesium; pH – Principles
and Measurement; Potassium: Physiology; Sodium:
Properties and Determination; Physiology;
Spectroscopy: Visible Spectroscopy and Colorimetry
Further Reading
Anon. (1986) Test methodologies. Potentiometric method-
ology, section 2. In: Ektachem Clinical Chemistry
Products Manual. Rochester, NY: Eastman Kodak.
Blick KE and Liles SM (1985) Principles of Clinical
Chemistry. New York: John Wiley.
Henry JB (1984) Clinical Diagnosis and Management by
Laboratory Methods, 17th edn. Philadelphia, PA: WB
Saunders.
Parker SP (ed.) (1989) McGraw Hill Dictionary of Scientific
and Technical Terms. New York: McGraw-Hill.
Tietz NW, Pruden EL and Sigaard-Anderson O (1987) Elec-
trolytes, blood gases and acid–base balance. In: Tietz
NW (ed.) Fundamentals of Clinical Chemistry, 3rd edn.
Philadelphia, PA: WB Saunders.
Tsien RY (1989) Fluorescent probes of cell signalling.
Annual Review of Neuroscience 12: 227–253.
2038 ELECTROLYTES/Analysis
Water–Electrolyte Balance
Y L Cheng and A W Yu, The Chinese University of
Hong Kong, Hong Kong, China
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 The water–electrolyte balance is regulated tightly in
the human body for the normal function of cells and
organs. The kidneys have a critical role in regulating
the composition of water and several important inor-
ganic ions (sodium, potassium, chloride, bicarbonate,
hydrogen ion, calcium, and phosphate) in the body
and thus homeostasis. This is maintained by an
appropriate balance between the excretion of these
electrolytes by the kidneys and the daily intake.
Hypernatremia and hyponatremia (excess and inad-
equate plasma sodium), hyperkalemia and hypokale-
mia (excess and inadequate plasma potassium) are
the most common electrolyte disorders and result
from an imbalance between intake and excretion of
water and these electrolytes. This article is a consider-
ation of the interactions of hormones and the auto-
nomic nervous system on renal tubules in maintaining
the water–electrolyte balance.
Water Balance
Water Content in the Body
0002 Water is the most abundant component in the human
body. Total body water is about 50% of body weight
for normal adult women and about 60% of body
weight for normal adult men. Fat has far less water
than other body tissues. Consequently, women, obese
people and the elderly contain less water in propor-
tion to their body weight because, generally, they
have proportionately more body fat.
0003 Body water is distributed in two major compart-
ments that are separated by cell membranes: the intra-
cellular fluid (ICF) and the extracellular fluid (ECF).
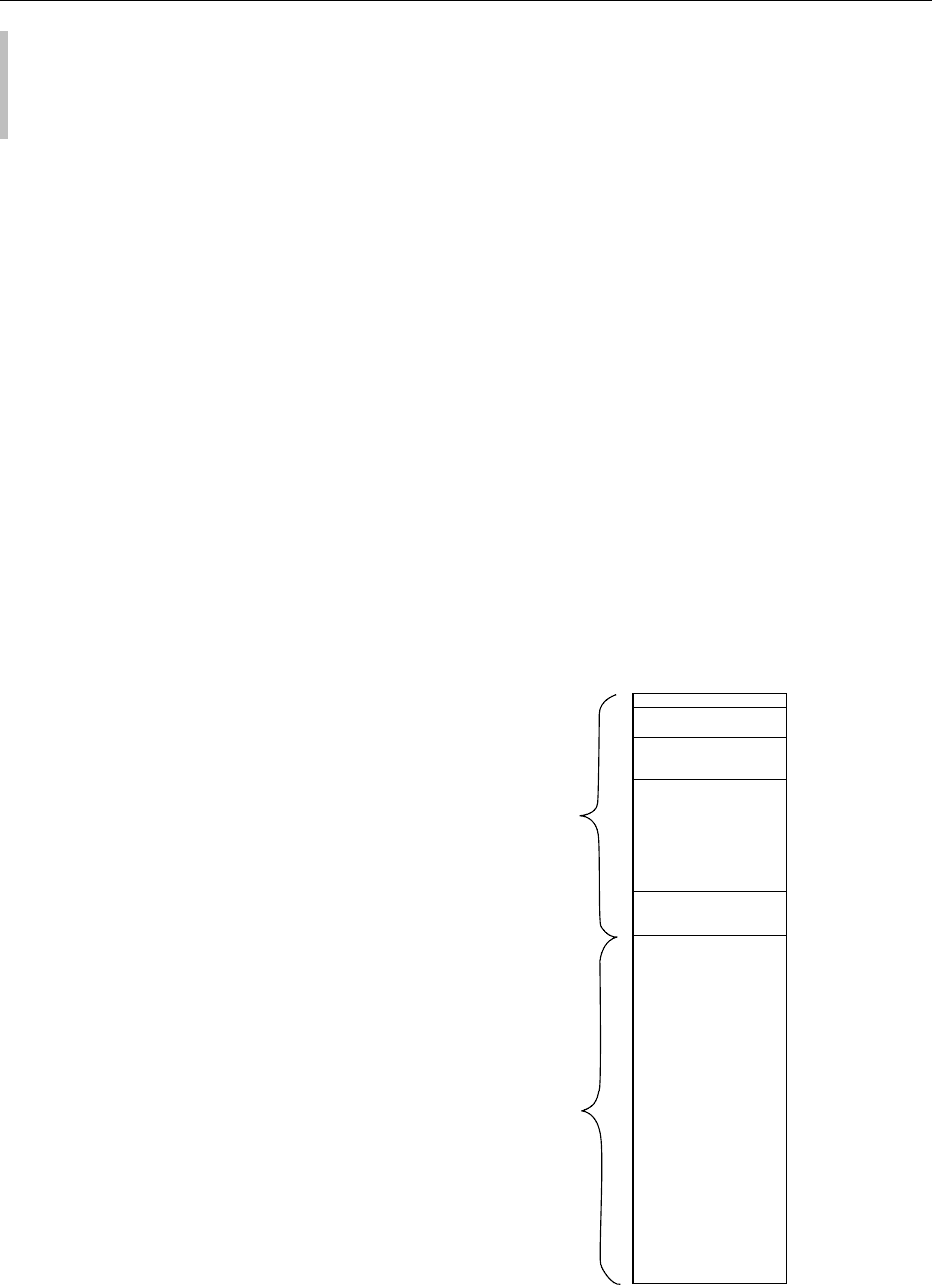
Approximately two-thirds of the total body water is
in the ICF and one-third in the ECF. The distribution
of total body water in each compartment is shown in
Figure 1.
0004 Water distribution in the ICF and ECF depends on
the number of particles (electrolytes and macromol-
ecules) confined in these compartments. The con-
centration of an individual substance in the two
compartments may not be equal because the cell
membrane has different permeabilities and channels
for different substances. The concentration of a
substance in a solution can be expressed as osmolality.
Some substances, such as urea and alcohol, freely
cross most cell membranes. ‘Effective osmolality,’ or
tonicity, refers to the osmolality that contributes
to non-freely permeable substances. Water will move
across the cell membrane along the osmotic
gradient, i.e., from a compartment with a low effective
osmolality to a compartment with a high effective
osmolality, until the tonicity is equal on both sides of
the membrane. The normal plasma osmolality is
about 285–295 mosmoles (mOsm) per kilogram of
water.
Water Intake and Water Output
0005In the steady state, water intake must be equal to
water output. For a normal adult, the usual water
intake is about 1.5–2.5 l per day (which includes
ingested water and water contained in foods), and
the endogenous water production by oxidative me-
tabolism is about 0.5 l per day. Water loss is through
urine (about 1–2 l per day), ‘insensible water loss’ by
evaporation of water through skin and lungs (about
1 l per day), and stool excretion (about 0.1–0.2 l per
day). Urine is the only route by which water excretion
Transcellular water
Bone matrix
Dense connective
tissue
Interstitium
Plasma
1.5%
4.5%
4.5%
11.5%
3.0%
Extracellular
compartment
25%
Intracellular
compartment
36%
fig0001Figure 1 Distribution of body water in typical adult men,
expressed as a percentage of body weight.
ELECTROLYTES/Water–Electrolyte Balance 2039
can be closely regulated. Apart from water intake,
urine output also relates directly to the solute excre-
tion, and it cannot be reduced below a certain min-
imal value required to carry the solute load. This is
known as ‘obligatory kidney water loss.’ In order
to produce a maximally concentrated urine (urine
osmolality approximately 1200 mOsm kg
1
), for in-
stance, a daily solute load of 600 mOsm requires
a minimal obligatory urine output of 0.5 l per day
(600 mOsm divided by 1200 mOsm kg
1
) to excrete
the solute.
Regulation of Water Balance
0006 Water balance is the result of the interaction of thirst
and arginine vasopressin (AVP), which is also termed
antidiuretic hormone, to maintain a stable plasma
tonicity. The sensation of thirst promotes water
intake, and AVP regulates urinary water excretion.
AVP is a nine-amino acid polypeptide that is synthe-
sized in the hypothalamus and stored in the posterior
pituitary. Upon stimulation, AVP is released into the
blood circulation. It then binds to the receptor
(known as AVP receptor 2) on the basolateral mem-
brane, opens up the water channel (known as the
aquaporin-2 channel) in the apical membrane of the
principal cells of the collecting ducts in the kidneys,
and increases the water permeability. The medullary
interstitium surrounding the collecting ducts is hyper-
tonic with an osmolality up to 1200 mOsm kg
1
.
When the water channels are open, water is re-
absorbed along the osmotic gradient. In the absence
of AVP, the kidneys excrete a large amount of hypo-
osmotic fluid rapidly. Urine osmolality reflects the
kidneys’ ability to dilute (removal of water) or con-
centrate (conservation of water) the urine. In a
normal adult, the urine osmolality varies from 80 to
1200 mOsm kg
1
.
0007 Thirst is more important than the AVP mechanism.
In the absence of AVP secretion (e.g., patient with
cranial diabetes insipidus), urinary water loss will
increase. If the thirst mechanism is intact, water bal-
ance can still be maintained by an increase in water
intake. However, patients with a defective thirst
mechanism will be predisposed to dehydration, even
if the AVP mechanism is normal.
Control of Thirst and AVP Secretion
0008 Both osmotic and nonosmotic factors are involved in
the regulation of thirst and AVP release. Amongst
these, the osmotic factor (i.e., plasma tonicity) is
more important. The factors and their effects are
summarized in Tables 1 and 2.
0009 The osmolality sensor (osmoreceptor), the thirst
center, and the AVP secretory cells are located in the
hypothalamus. The sensor is a group of specialized
cells that is remarkably sensitive to the changes of
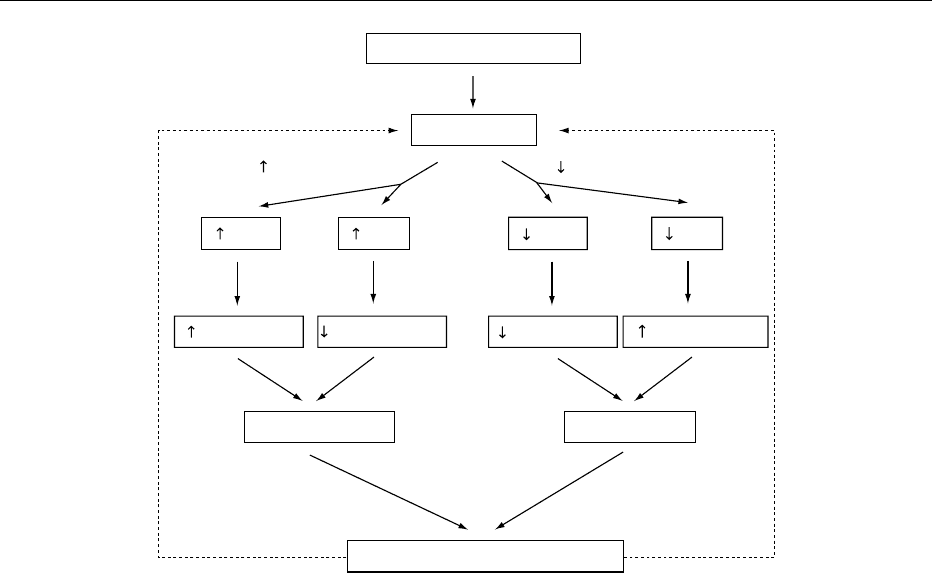
tonicity in the ECF. When there is a water deficit
(Figure 2), plasma tonicity will increase. The osmo-
receptors will send a signal to both the thirst center
and the AVP secretory cells. Intake of water will be
increased, and the kidneys will retain water via the
action of released AVP.
0010Conversely, when there is excessive water (Figure 2),
plasma tonicity will decrease. Both the thirst center
and secretion of AVP will be inhibited to promote net
urinary water loss. These responses act as a feedback
loop to keep the plasma tonicity and, hence, the body
water content within tight limits.
Kidneys and Water Balance
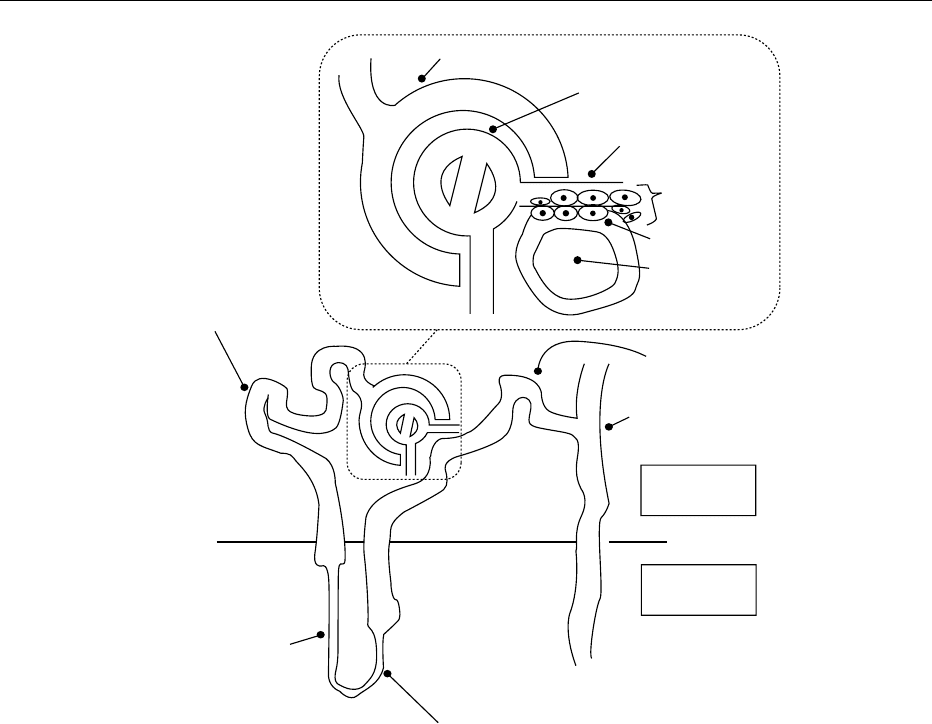
0011A nephron is the functional unit of the kidneys. Each
kidney contains approximately one million such units.
A nephron is composed of a glomerulus and a renal
tubule (Figure 3). The renal tubule is subdivided fur-
ther into the proximal convoluted tubule, the loop
tbl0001Table 1 Control of thirst
Factors Stimulation Inhibition
Osmotic " Tonicity # Tonicity
Nonosmotic # ECF volume " ECF volume
Congestive heart failure Dopamine antagonists
Hormones/drugs
Angiotensin II
Catecholamines
Parasympathomimetic
drugs
tbl0002Table 2 Control of arginine vasopressin release
Factors Stimulation Inhibition
Osmotic " Tonicity # Tonicity
Nonosmotic # ECF volume " ECF volume
# Blood pressure " Blood pressure
Nausea/pain/anxiety Hormones/drugs
Congestive heart
failure
Dopamine
antagonists
Liver disease Ethanol
Hypoglycemia Norepinephrine
Adrenal insufficiency
Hormones/drugs
Angiotensin II
General anesthetics
Barbiturates
Chlorpropamide
Clofibrate
Cyclophosphamide
Nicotine
Vincristine
2040 ELECTROLYTES/Water–Electrolyte Balance
of Henle, the distal convoluted tubule, and the col-
lecting duct.
0012 The glomeruli filter about 180 l of fluid (which
contains water, electrolytes, and glucose) each day.
Up to 90–99% of the filtered water is reabsorbed by
the renal tubules. It is reabsorbed passively in the
proximal convoluted tubules (60–70% of the filtered
water) and the descending limbs of the loops of Henle,
with down osmotic gradients created by the active
transport of sodium and chloride out of the lumina.
Water is not permeable in the ascending limb of the
loop of Henle and the distal convoluted tubule. The
final urine volume is determined by the action of AVP
on the collecting ducts.
Sodium Balance
Sodium Content in the Body
0013 For a normal adult, total body sodium content is
about 55–60 mmol per kilogram of body weight.
The distribution of sodium in different compartments
of the body is shown in Figure 4.
0014 Sodium is the major cation in the ECF. Its concen-
tration is about 135–145 mmol l
1
and 3 mmol l
1
in
the ECF and ICF, respectively. The high sodium con-
tent in the ECF is maintained by sodium–potassium–
adenosine triphosphatase (Na
þ
–K
þ
–ATPase) pumps.
The Na
þ
–K
þ
–ATPase pump is located in the cell
membrane, and it hydrolyzes a molecule of adeno-
sine triphosphate to release energy to pump three
sodium molecules out of the cells (from ICF to
ECF) and to transport two potassium molecules
into the cells (from ECF to ICF). In normal circum-
stances, sodium and its accompanying anions
(mainly chloride) contribute to more than 90% of
the effective osmolality in the ECF. As noted previ-
ously, effective osmolality determines the volume in
the corresponding compartment. Therefore, sodium
is the major determinant of the ECF volume. An
excess in total body sodium content leads to expan-
sion of the ECF volume, and a deficit in total body
sodium content is associated with contraction of the
ECF volume. A stable ECF volume is essential to
maintain tissue perfusion, because the plasma
volume (one of the compartments in ECF) is directly
proportional to the ECF volume.
Sodium Intake and Excretion
0015A typical Western diet contains approximately
150 mmol of sodium per day. However, social and
cultural differences in dietary habits can have a sig-
nificant effect on sodium intake. Amounts of sodium
can be up to 300 mmol per day in some areas in Japan
to less than 30 mmol per day in the New Guinea
Highlands. In normal health, the amount of sodium
Change of plasma tonicity
Osmoreceptors
Thirst
Plasma tonicity Plasma tonicity
Water intake
ThirstAVP AVP
Water retention
Normalization of plasma tonicity
Water loss
Water excretion Water intake Water excretion
Feedback loop Feedback loop
fig0002 Figure 2 Pathway of water conservation/excretion. AVP, arginine vasopressin.
ELECTROLYTES/Water–Electrolyte Balance 2041
excretion in the stool (about 5 mmol per day) and
sweat (about 10 mmol per day) is small and cannot
be regulated. Urine is the only route by which sodium
excretion can be precisely regulated to match the
sodium intake. Depending on the dietary intake and
the status of body sodium content, urinary sodium
loss can range from less than 1 mmol in a state of
sodium deficit, to more than 500 mmol per day in a
state of sodium excess. Thus, the kidneys have an
important role in maintaining sodium balance and
ensuring a stable ECF volume.
Kidneys and Sodium Balance
0016 In people with normal kidney function, the glomeruli
filter about 25 000 mmol sodium each day. This
amount is approximately six times the total body
sodium. Consequently, control of sodium reabsorp-
tion is important in maintaining sodium homeostasis.
As shown in Table 3, most of the filtered sodium
(60–70%) is reabsorbed at the proximal convoluted
tubules. Twenty to twenty-five per cent is reabsorbed
at the thick ascending limbs of the loops of Henle,
and another 10–15% is reabsorbed at the distal
nephrons (distal convoluted tubules and collecting
ducts).
0017Although the distal nephron is responsible for a
relatively small amount of sodium recovery, this seg-
ment of renal tubule is important for fine-tuning
sodium reabsorption.
0018There are two pathways by which sodium is re-
absorbed from the lumen to the renal tubular cells:
the paracellular pathway and the transporters at the
luminal membrane (Figure 5).
0019The paracellular pathway refers to channels be-
tween renal tubular cells. Sodium is freely permeable
in these channels in the proximal convoluted tubule
and the thick ascending limb of the loop of Henle, but
not in the distal nephron. The transporters at the
luminal membrane are different in various segments
of the renal tubule (Table 3). There are generally three
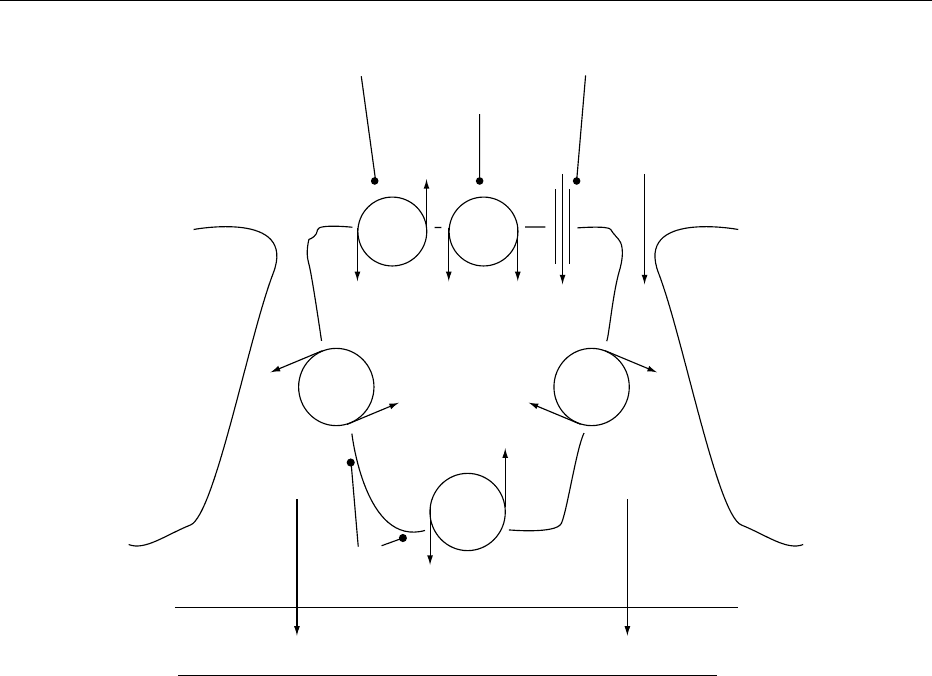
Bowmans capsule
Glomerulus
Afferent arteriole
Juxtaglomerular
apparatus
Macula densa
Thick ascending
limb of loop
of Henle
Distal
convoluted tubule
Collecting duct
Cortex of
kidney
Medulla of
kidney
Ascending limb of
loop of Henle
Descending limb of
loop of Henle
Proximal
convoluted tubule
fig0003 Figure 3 Nephron and juxtaglomerular apparatus.
2042 ELECTROLYTES/Water–Electrolyte Balance
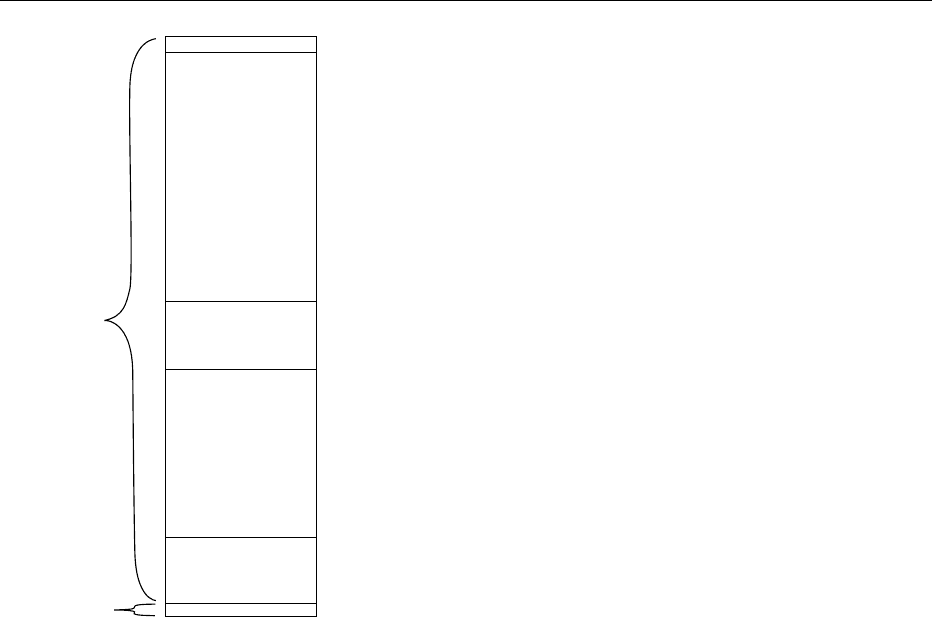
classes of transporters, namely sodium exchanger,
sodium cotransporter, and specific sodium channel.
The sodium exchanger moves sodium into the cell in
exchange for another cation (for instance, potassium
and hydrogen ion) moving out of the cell. The sodium
cotransporter couples sodium entry with the entry of
other solutes, such as amino acids, glucose, phos-
phate, potassium, and chloride. The specific sodium
channel is present in the principal cells of the cortical
and medullary collecting duct (the intercalated cell is
the other cell type in the collecting duct that is in-
volved in acid or bicarbonate secretion and potassium
reabsorption). Under the effect of aldosterone, the
channel opens and allows passage of sodium ions
only.
0020 The pathways share the same mechanism for
sodium transport (Figure 5). There are Na
þ
–K
þ
–
ATPase pumps that operate across the base of renal
tubular cells and also across their lateral walls into
the intercellular spaces (also known as the basolateral
membrane). The pumps pump out sodium and keep
the intracellular sodium at a low level, which then
provides a steep electrochemical gradient favoring
reabsorption of sodium from the lumen, through the
various pathways (paracellular pathway and sodium
transporters), into the cells. Having entered the renal
tubular cell, the sodium then moves across the
basolateral membrane into the peritubular space
by the Na
þ
–K
þ
–ATPase pumps. The sodium then
enters the peritubular capillary and returns back to
the ECF.
Regulation of Urinary Sodium Excretion
0021Numerous mechanisms have been implicated in the
regulation of sodium balance and maintenance of a
stable ECF. It is unclear whether there are any other
mechanisms that have not been discovered yet. The
identified mechanisms are overlapped and extensively
interrelated. Failure of one of the mechanisms does
not result in significant long-term effects. This ar-
rangement is important to maintain stability of the
ECF volume. The more constant the ECF volume,
the more the body will be protected from changes
in the external environment.
0022The sensing mechanisms include: (1) mechano-
receptors in the walls of large blood vessels and
cardiac chambers (detecting changes in vascular dis-
tension); (2) chemoreceptors in the renal tubule
(macula densa, responding to an alternation in lu-
minal sodium and chloride ions concentration); (3)
volume receptors in the central nervous system (sen-
sitive to a change in sodium level in the cerebrospinal
fluid); and (4) peripheral tissue receptors (monitoring
the adequacy of tissue perfusion). The sensors feed
back signals to several mediating mechanisms, and
the kidneys are then ‘informed’ by renal nerves, hor-
mones, and physical factors to retain or excrete
sodium in the urine.
0023Factors that affect sodium reabsorption by the
renal tubule are listed in Table 3. The major mediat-
ing mechanisms are described below.
0024-Tubuloglomerular feedback The thick ascending
limb of the loop of Henle has a cortical segment that
closely relates to the afferent arteriole of the parent
glomerulus. The cells in this particular segment of the
renal tubule constitute the macula densa. The macula
densa, the corresponding afferent arteriole, and the
nearby extraglomerular mesangium are collectively
called the juxtaglomerular apparatus (Figure 3).
Changes in the delivery and composition of the fluid
flowing past the macula densa elicit changes in
glomerular filtration of the same nephron via the inter-
action of angiotensin II and nitric oxide. For instance,
an increase in the delivery of fluid to the macula densa
or a low sodium and chloride concentration in the
fluid will stimulate the juxtaglomerular apparatus.
The renin–angiotensin system is then activated to
reduce urinary sodium excretion.
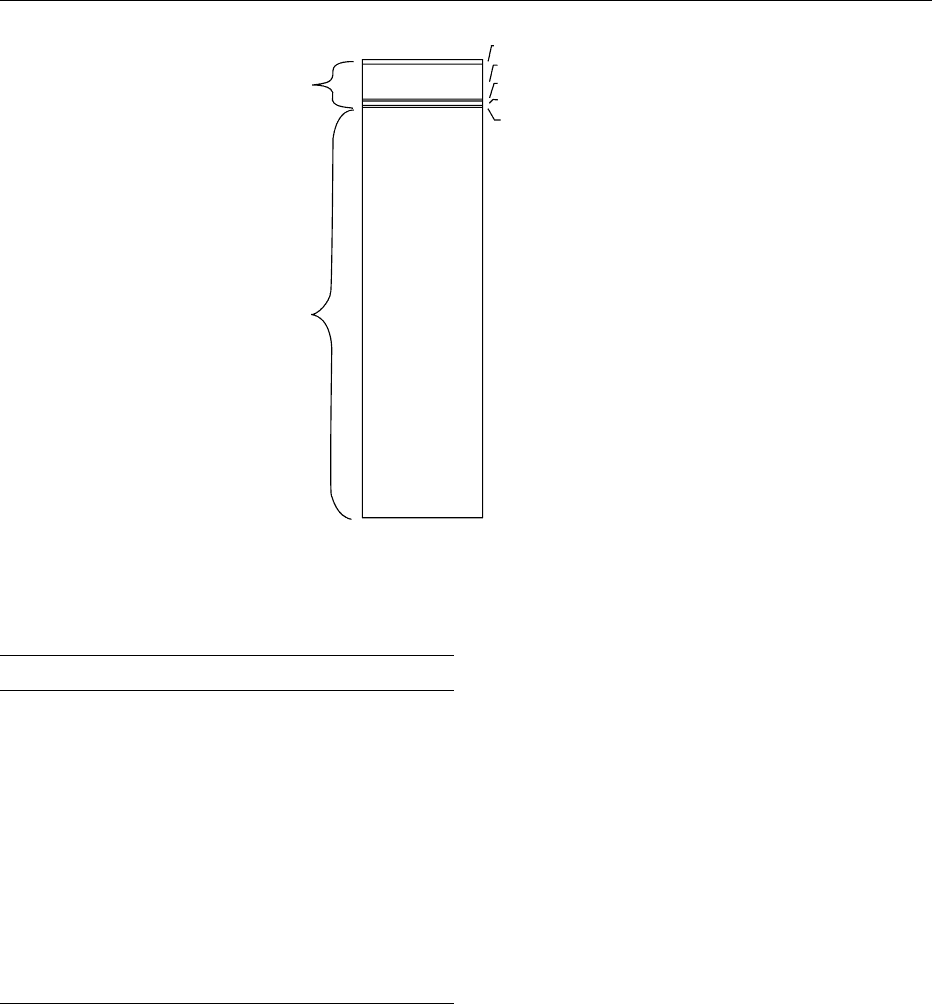
2.6%
43.1%
11.7%
11.2%
29.0%
Extracellular
compartment
97.6 %
Intracellular
compartment
2.4 %
Transcellular
water
Bone matrix
Dense connective
tissue
Interstitium
Plasma
fig0004 Figure 4 Distribution of body sodium, expressed as a percent-
age of total body sodium content.
ELECTROLYTES/Water–Electrolyte Balance 2043

0025 -Renin–angiotensin system and aldosterone When
there is sodium depletion or a reduction in kidney
perfusion (for instance, secondary to a low ECF
volume), the juxtaglomerular apparatus releases an
enzyme, called renin, in the circulation. Renin then
acts on angiotensinogen to release angiotensin I. The
latter is converted to angiotensin II under the action
of the angiotensin-converting enzyme. Angiotensin II
stimulates the release of aldosterone, a hormone
produced by zona glomerulosa cells of the adrenal
cortex.
0026 Angiotensin II reduces urinary sodium excretion by
constricting the glomerular arterioles and decreasing
the volume of glomerular filtrate, by enhancing
sodium reabsorption at the proximal convoluted
tubule, and by increasing the responsiveness of tubu-
loglomerular feedback to a given concentration of
sodium and chloride ions at the macula densa. Aldos-
terone enhances sodium reabsorption by opening up
the specific sodium channels at the collecting ducts,
and by stimulating the action and synthesis of Na
þ
–
K
þ
–ATPase pumps at the basolateral membrane of
renal tubular cells.
0027-Sympathetic nervous system The kidneys receive a
rich supply of sympathetic nerves. There is evidence
that sodium and volume depletion activate renal
sympathetic nerves. The kidneys then conserve
sodium by constricting the glomerular arterioles, by
increasing sodium reabsorption at the proximal con-
voluted tubules, by increasing renin release from jux-
taglomerular apparatus, and by interacting with AVP
and atrial natriuretic peptide (ANP).
0028-Atrial natriuretic peptide Atrial natriuretic peptide
is a peptide hormone and is synthesized by atrial
myocytes in cardiac atria. When the ECF volume is
expanded, the atrial wall distends, and ANP is re-
leased. ANP enhances sodium excretion by inhibiting
sodium reabsorption at the collecting duct.
Response to Dietary Sodium Intake
0029Although the relative importance of the different
regulatory mechanisms is not well established, many
studies suggest that the renin–angiotensin system,
aldosterone, and the sympathetic nervous system
appear to be more important in conserving sodium
tbl0003 Table 3 Tubular sodium absorption
Sodiumreabsorption pathways
Renal tubule Amount of
reabsorbed
sodium (%)
Paracellular
pathways
Luminal transporters Factorsthat stimulateluminal
transporter to enhance sodium
reabsorption
Factors that inhibit luminal
transporter to decrease
sodium reabsorption
Proximal convoluted tubule 60–70% Presence Sodium–hydrogen
ion exchanger
a-Adrenergic agonist
a
Dopamine
a
Angiotensin II
a
Parathyroid hormone
a
Thyroid hormone
a
Sodium–amino
acids/glucose/
phosphate
cotransporter
Loop of Henle (thick
ascending limb)
20–25% Presence Sodium-2 chloride–
potassium ion
cotransporter
Arginine vasopressin Loop diuretics
Prostaglandin E
2
Cytochrome P450
metabolites of
arachidonic acid
Distal convoluted tubule 10% Absence Sodium–chloride
ion cotransporter
Aldosterone Thiazide diuretics
Angiotensin II
Sympathetic nerves
Collecting duct 5% Absence Sodium channel Aldosterone
b
Potassium-sparing
diuretics
b
Arginine vasopressin
b
Atrial natriuretic
peptide
b
Bradykinin
b
a
2
-Adrenergic
agonists
b
Atrial natriuretic
peptide
c
Prostaglandin E
2
c
a
Factors affecting the sodium–hydrogen ion exchanger.
b
Factors affecting the sodium channel at the cortical collecting duct.
c
Factors affecting the sodium channel at the medullary collecting duct.
2044 ELECTROLYTES/Water–Electrolyte Balance
for people with a low sodium intake, and the tubulo-
glomerular feedback seems to be more important in
excreting excessive sodium for people with a very
high sodium intake.
Potassium Balance
Potassium Content in the Body
0030 The total body potassium content is dependent on
sex, age, and, most importantly, muscle mass (which
contains 60–75% of total body potassium), and is
approximately 40–45, and 50–55 mmol per kilogram
of body weight for normal adult women and men,
respectively. These values decrease with age and are
20% less in the elderly because of a decrease in
muscle mass.
0031 About 90% of the body’s potassium is in the ICF.
Only 0.4% of the body’s potassium is distributed in
the vascular space of the ECF (Figure 6).
0032Potassium is the major cation in the ICF, and the
intracellular potassium concentration is approxi-
mately 150 mmol l
1
. In the ECF, however, the
plasma potassium level normally ranges from only
3.5 to 5.0 mmol l
1
. The intracellular-to-extracellular
potassium concentration ratio is the most important
determinant of the resting membrane potential of
neuromuscular tissue. Disturbances in this ratio will
predispose patients to several clinical disorders, such
as cardiac arrhythmia and neuromuscular problems.
Factors that Maintain the Potassium Distribution
Between ICF and ECF
0033Potassium leaks passively from the cell to the ECF
space through the ion-selective potassium channels
in the cell membrane. A high intracellular potassium
concentration is maintained by Na
þ
–K
þ
–ATPase
pumps that actively transport potassium molecules
back into the cells. Factors that alter the activity
of Na
þ
–K
þ
–ATPase pumps and ion-selective potas-
sium channels affect the transcellular potassium
Sodium
exchanger
Sodium
co-transporter
Specific sodium
channel
Paracellular
pathway
Lumen
K
+
or H
+
Na
+
Na
+
Na
+
Na
+
Na
+
Other
solutes
Renal
tubular
cells
Na
+
−K
+
−
ATPase
pump
Na
+
−K
+
−
ATPase
pump
Na
+
−K
+
−
ATPase
pump
Peritubular
capillary
Basolateral
membrane
3Na
+
3Na
+
3Na
+
2K
+
2K
+
2K
+
Na
+
fig0005 Figure 5 Renal transport of sodium. K
þ
, potassium ion; H
þ
, hydrogen ion; Na
þ
, sodium ion; Na
þ
–K
þ
–ATPase pump, sodium–
potassium–adenosine triphosphatase pump. The sodium exchanger moves Na
þ
into the cell in exchange for another cation (e.g., K
þ
and H
þ
), sodium cotransporter couples sodium entry with the entry of other solutes (e.g., amino acids, glucose, phosphate, K
þ
and
chloride ion), and the Na
þ
–K
þ
–ATPase pump pumps out three Na
þ
and transports in two K
þ
.
ELECTROLYTES/Water–Electrolyte Balance 2045
distribution, resulting in hypo- or hyperkalemia with-
out any change in the total body potassium content.
These factors are listed in Table 4.
Potassium Intake and Excretion
0034 Potassium is present in all protein-containing foods,
particularly meats. Each gram of dietary protein con-
tains approximately 1 mmol of potassium. Hence,
potassium intake is directly related to protein intake.
The average Western diet provides 60–100 mmol
of potassium each day. In the steady state, about
80–95% of the daily potassium intake is excreted in
urine, 5–20% is excreted in the stools (mainly by the
colon), and less than 5% is eliminated in sweat.
However, in renal failure (with a glomerular filtration
rate of less than 20 ml min
1
) or diarrhea, potassium
loss from the gastrointestinal tract can be significant.
Moreover, potassium loss in sweat may be important
if perspiration is excessive.
Kidneys and Potassium Balance
0035About 720 mmol of potassium is filtered through the
glomeruli each day. Both potassium reabsorption
and secretion occurs in renal tubules. The majority
of the filtered potassium (66–75%) is reabsorbed
passively at the proximal convoluted tubules and
actively (via the sodium-2 chloride–potassium ion
cotransporter) in the ascending limbs of the loops
of Henle so that only 5–10% of the filtered potas-
sium remains in the early distal convoluted tubules.
Fine regulation of potassium excretion occurs at the
distal nephron. The collecting duct is the major site
of potassium secretion. Under the effect of Na
þ
–K
þ
–
ATPase pumps at the basolateral membrane of the
tubular cell (resulting in a high intracellular potas-
sium concentration) and the effect of sodium absorp-
tion via the sodium channels at the luminal
membrane (resulting in a negative electrical gradient
in the lumen), an electrochemical gradient is set up to
promote potassium secretion via the potassium se-
lective channels on the luminal surface of the princi-
pal cells of the collecting duct. Factors that modify
tbl0004 Table 4 Factors that regulate the transcellular potassium
distribution
Movement of potassium
Acid^base status
Acidosis by mineral acid (e.g.,
hydrochloric acid)
ICF !ECF
Acidosis by organic acid (e.g., lactic
acid, b-hydroxybutyric acid,
methylmalonic acid)
No effect
Alkalosis ECF !ICF
Insulin ECF !ICF
Glucagon ICF !ECF
b
2
-Adrenergic agonist ECF !ICF
a-Adrenergic agonist ICF !ECF
Aldosterone ? ECF !ICF
Hyperosmolality (e.g., mannitol,
hyperglycemia)
ICF !ECF
Exercise ICF !ECF
Transcellular water, 1%
Dense connective tissue, 0.4%
Interstitium, 1%
Plasma, 0.4%
Bone matrix, 7.6%
Extracellular
compartment
10.4%
Intracellular
compartment
89.6%
fig0006 Figure 6 Distribution of body potassium, expressed as a percentage of total body potassium content.
2046 ELECTROLYTES/Water–Electrolyte Balance
