Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


infections such as osteomyelitis, septic arthritis, and
soft tissue infections. Diagnosing infection with
Salmonella is dependent on culturing the organism,
usually from either stool or blood cultures. In the
case of nontyphoidal Salmonella, it is also worth
trying to culture organisms from the incriminated
food.
0017 Gastroenteritis from nontyphoidal Salmonella is
usually self-limiting, and rehydration is the most crit-
ical aspect of treatment. Antibiotic therapy is not rou-
tinely required for this aspect of Salmonella infection
and has in some instances been thought to promote
chronic carriage. When there is systemic invasion with
the bacteria and in cases of enteric fever, antibiotic
therapy is important. Third-generation cephalospor-
ins and quinolones are most frequently used, although
chloramphenicol has been the mainstay of treatment
for typhoid fever for many years and is still used in
many developing countries, but chloramphenicol does
carry a risk of complications.
0018 Antibiotic resistance has become a major problem
with many Salmonella serovars. Of particular con-
cern is the recent emergence and spread of Salmonella
DT104 that carries resistance to multiple antibiotics,
including, in some instances, to the fluoroquinolones
such as ciprofloxacin. In 2000, Salmonella strains
that were resistant to Ceftriaxone were also reported,
further raising the concern that the emergence of new
antibiotic resistance profiles were occurring.
Shiga Toxin-producing
E. coli
0019 Shiga toxin-producing E. coli (STEC) are relative
newcomers to the scene of foodborne pathogens.
The first STEC to be associated with disease in
humans was E. coli O157:H7 following two out-
breaks of hemorrhagic colitis in 1982. Since then, it
has been learned that there are in fact many different
serotypes of STEC, and at least 60 different types
have been associated with clinical disease. Recent
studies have suggested that around 1% of samples
submitted to clinical microbiology laboratories in
the USA contain STEC, of which around two-thirds
are O157:H7, the remainder being non O157. STEC
are present in the gastrointestinal tracts of many
mammalian species but appear to be especially
common in ruminants (cattle, sheep, deer, and
goats). Therefore, the main source of STEC in our
food supply is bovine products.
0020 Recently, there have been an increasing number of
reports associating STEC infection with fresh pro-
duce (lettuce, alfalfa sprouts, apple cider) and water.
This is thought to be mainly due to contamination
with fecal material from cattle pasture. Clinically,
STEC cause a variety of diseases ranging from
diarrhea, which may or may not be bloody, hemor-
rhagic colitis, and the hemolytic uremic syndrome
(HUS). HUS is a triad of renal failure, thrombocyto-
penia, and hemolytic anemia. Acutely, HUS has a
mortality rate of around 5%, and up to 50% of
HUS patients may have some degree of permanent
renal insufficiency. The main virulence factor from
STEC is the production of one or more bacterio-
phage-encoded Shiga toxins (Stx), which are of two
main types, Stx1 and Stx2. Following ingestion of the
bacteria, they colonize portions of the lower intestinal
tract and produce the toxins. Stx is then thought to
cross the intestinal epithelial cell barrier and damage
distant target sites, especially the kidney and brain, by
a direct effect on endothelial cells in the microvascu-
lature. The infectious dose of STEC may be very low,
in the region of 10–100 organisms in some instances.
Symptoms typically develop 2–4 days following in-
gestion but may occur in as little as 1 day or in as
much as 8 days. The diarrhea can be of variable type
(bloody or nonbloody) and may contain leukocytes.
The type of diarrhea is not a reliable indication of
who will go on to develop HUS. The mainstay of
treatment for STEC and its major complications is
supportive. There is a degree of controversy over
the use of antibiotics, and a number of studies have
suggested that certain antimicrobials (e.g., trimetho-
prim-sulfamethoxazole) actually increase the likeli-
hood that a patient will go on to develop serious
complications.
Yersinia
spp.
0021Of the three members of the genus Yersinia, Y. entero-
colitica and Y. pseudotuberculosis are considered to
be foodborne, whereas Y. pestis is not. Yersinia are
not very commonly found as causes of foodborne
illness compared with Salmonella or Campylobacter.
However, they are clearly transmitted in food and can
cause a significant gastrointestinal illness. The food
most frequently associated with yersiniosis is pork.
Swine are a major reservoir of these organisms, and
although they have been found in many other animals
(e.g., sheep, dogs, cats, and cattle), consumption of
undercooked pork is a common association. Milk
is another frequently reported source, and since
Y. enterocolitica can survive, and indeed multiply, in
milk at 4
C, small numbers of organisms can become
a significant health threat, even if the milk is refriger-
ated. Symptoms in Y. enterocolitica infection can
be prolonged, lasting several weeks or even longer.
Most infections are, however, self-limiting, although
complications may occur such as ulceration and in-
testinal perforation. A classic long-term complication
following yersiniosis is the development of reactive
EMERGING FOODBORNE ENTERIC PATHOGENS 2067

arthritis. As with other enteric pathogens, this is more
likely in patients who are HLA B27-positive.
Viral Foodborne Pathogens
0022 Viral agents are considered to be an increasingly
important cause of foodborne illness. A number of
different viral agents have been associated with food-
borne disease and cause a variety of illnesses varying
from a simple gastroenteritis to major systemic upset
such as hepatitis. Food and water are vehicles for
viruses, but viruses do not reproduce in food, and
nor do they produce toxins in food. Some viruses,
such as Norwalk, cause large outbreaks, and others
seem to be more frequently associated with sporadic
disease. Overall, the difficulty in diagnosing viral
illness has precluded the development of large
amounts of epidemiological data. It is beyond the
scope of this chapter to discuss all viral causes of
foodborne disease. However, Norwalk-like viruses
do deserve a specific mention.
0023 Norwalk virus is a small round-structured virus
(SRSV) and was the first virus to be clearly associated
with gastroenteritis. Norwalk is a calicivirus, and this
group of viruses causes disease worldwide and has
been associated with some large outbreaks, often in
confined environments such as cruise ships. Out-
breaks have been associated with contaminated
drinking water, swimming water, consumption of
undercooked shellfish, ice, and salads. As with the
other enteric viruses, fecal contamination of food or
water is usually found to be the ultimate source. The
incubation time following exposure is around 48 h,
and the clinical illness usually consists of vomiting
and diarrhea. The diarrhea is watery without red
cells, leukocytes or mucus. The disease is usually
self-limiting, settles in 24 h, and requires no specific
therapy. Specific diagnosis is difficult. A number of
assays are available (but not commercially), including
electron microscopy, enzyme immunoassays, and
reverse transcriptase–polymerase chain reaction.
Parasites
0024 Two emerging parasitic foodbrone pathogens that
cause predominantly watery diarrhea are Cryptospor-
idium parvum and Cyclospora cayetanensis. As can
be seen from Table 4, C. parvum causes a lot of
disease, but only 10% of it is considered to be food-
borne; in contrast, C. cayetanensis causes a lot less
disease, but 90% of it is considered to be foodborne,
much of which is based on outbreak data. Crypto-
sporidium parvum, for which proven effective ther-
apy does not exist, has gained notoriety for causing
persistent chronic diarrhea in immunocompromised
patients. C. parvum is endemic in cattle and is usually
acquired in humans from contaminated water, fresh
produce, unpasteurized milk, or person-to-person
spread. The incubation period is typically about a
week but can be as long as 28 days. C. parvum is
known to cause large outbreaks, the largest of which
was waterborne in Milwaukee, in which around
400 000 individuals became sick. C. cayetanensis in-
fection has been associated in the past with consump-
tion of imported berries most likely contaminated
with fecally contaminated water. More recently, it
has been linked with fresh basil. It can be diagnosed
by direct acid-fast microscopy of stools, but it is im-
portant to note that most microbiology laboratories
will not routinely look for C. parvum and/or C. caye-
tanensis, so they may be more common than we think.
Transmissible Spongiform
Encepalopathies
0025Mad cow disease or bovine spongiform encephalop-
athy (BSE) was first diagnosed in the UK in 1986. BSE
is a member of the family of transmissible spongiform
encephalopathies (TSEs) that are linked with neuro-
logical diseases in a variety of animals, including
humans, sheep, elk, and mink (Table 6). Historically,
TSEs were recognized in Europe as long ago as the
eighteenth century, when a neurological condition
known as scrapie was first described in sheep. How-
ever, TSEs did not become a public health or food
safety concern until the mid-1990s, when the link
between BSE and a condition in humans, known as
new variant Creutzfeldt–Jakob disease (nvCJD), was
made, and it became clear that the disease was due to
transmissible agents, that were given the name prions.
0026Although there are no clear answers as to the
origins of BSE, the generally held belief is that BSE
originated from feeding cattle rendered protein
produced from the carcasses of scrapie-infected
sheep or cattle. The rendering process involves pro-
cessing carcasses by boiling at atmospheric or higher
tbl0006Table 6 Types of transmissible spongiform encephalopathies
(TSEs) in animals and humans
TSEs in animals
Scrapie (sheep and goats)
Chronic wasting disease
Transmissible mink encephalopathy
Bovine spongiform encephalopathy
TSEs in humans
Crutzfeldt–Jakob disease
Kuru
Gerstmann–Straussler–Scheinker syndrome
Fatal familial insomnia
2068 EMERGING FOODBORNE ENTERIC PATHOGENS

pressures, which results in the generation of an aque-
ous protein solution under a layer of fat. The fat can be
removed and the protein solution further processed
into a meat and bone meal product for use as an
animal food. Changes were made in the rendering
process in the UK around 1980, and it is assumed
that this change allowed the survival of the etiologic
agent. The recycling of cattle carcasses through the
rendering process only served to concentrate the etio-
logic agent further that eventually led to the epidemic
of BSE in the UK in the late 1980s. Thus, the current
belief is that the etiologic agent can be delivered orally
and is concentrated in certain parts of an infected
animal’s body, especially central nervous tissues.
0027 In the year 2000, there were 1101 new cases of BSE
diagnosed in the UK, so infectious cattle still remain
in the UK. BSE has not been limited to the UK, and
there have been several hundred cases in other Euro-
pean countries and elsewhere in the world, although
some of these are imported rather than native cases.
Since 1994, when the first case of nvCJD was recog-
nized in the UK, there has been a steady increase in
the numbers of cases. Up to November 2000, there
had been 84 cases in the UK, and the trends in inci-
dence since 1994 indicate that the number of cases
and deaths per year are on the rise.
0028 Up to the time of this writing, there have been no
documented cases of BSE in the USA. The USDA and
FDA have taken action to prevent the importation of
BSE-contaminated material or animals into the USA.
Active surveillance for BSE is underway in cattle in
the USA with signs of neurological disease and cattle
that are nonambulatory at slaughter. Close to 12 000
brains have been examined so far, with no evidence of
BSE. Despite the lack of evidence of BSE in the USA,
there is concern that it will appear at some point. The
long incubation period and the difficulty in control-
ling all imports of potentially contaminated products
from different parts of the world make it possible that
BSE will appear in the USA, at some time in the
future. In the general context of food safety, the
risks and the likelihood of serious outcomes that are
associated with bacterial pathogens such as Campylo-
bacter, Salmonella, and E. coli are significantly
greater than the risk of developing nvCJD from BSE.
However, nvCJD is frightening, because it is incur-
able, inevitably leads to death, and there are currently
no assays available to test food for the BSE prions,
although new tests to determine the presence of ner-
vous tissue in meat products are becoming available.
See also: Bovine Spongiform Encephalopathy (BSE);
Campylobacter: Properties and Occurrence; Detection;
Campylobacteriosis; Escherichia coli: Occurrence;
Detection; Food Poisoning; Occurrence and Epidemiology
of Species other than Escherichia coli; Food Poisoning by
Species other than Escherichia coli; Food Poisoning:
Classification; Listeria: Properties and Occurrence;
Detection; Listeriosis; Parasites: Occurrence and
Detection; Illness and Treatment; Salmonella: Properties
and Occurrence; Detection; Salmonellosis; Viruses
Further Reading
Allos BM (2001) Campylobacter jejuni infections: update
on emerging issues and trends. Clinical Infectious
Diseases 32: 1201–1206.
Centers for Disease Control (2000) Surveillance for food-
borne disease outbreaks – United States, 1993–1997.
MMWR 49: 1–51.
Centers for Disease Control (2001) Diagnosis and manage-
ment of food borne illness, a primer for physicians.
MMWR 50: RR-2.
Guerrant RL, Van Gilder T, Steiner TS et al. (2001) Practice
and guidelines for the management of infectious diar-
rhea. Clinical Infectious Diseases 32: 331–350.
Hohmann EL (2001) Nontyphoidal salmonellosis. Clinical
Infectious Diseases 32: 263–269.
Kramer JM, Frost JA, Bolton FJ and Wareing DR (2000)
Campylobacter contamination of raw meat and poultry
at retail sale: identification of multiple types and com-
parison with isolates from human infection. Journal of
Food Protection 63: 1654–1659.
Mead PS, Slutsker L, Dietz V et al. (1999) Food-related
illness and death in the United States. Emerging Infec-
tious Diseases 5: 607–625.
Mosier DA and Oberst RD (2000) Cryptosporidiosis. A
global challenge. Annals of the New York Academy of
Sciences 916: 102–111.
Nataro JP and Kaper JB (1998) Diarrheagenic Escherichia
coli. Clinical Microbiology Reviews 11: 142–201.
Paton JC and Paton AW (1998) Pathogenesis and diagnosis
of Shiga toxin-producing Escherichia coli infections.
Clinical Microbiology Reviews 11: 450–479.
Prusiner SB (1998) Prions. Proceedings of the National
Academy of Sciences 95: 13363–13383.
Ranjitham M, Madan M and Chandrasekharan S (1999)
Cyclospora cayetanensis – an emerging coccidian
parasite. Journal of the Association of Physicians of
India 47: 1198–1199.
Slifko TR, Smith HV and Rose JB (2000) Emerging parasite
zoonoses associated with water and food. International
Journal of Parasitology 30: 1379–1393.
EMERGING FOODBORNE ENTERIC PATHOGENS 2069

Emission Spectroscopy See Spectroscopy: Overview; Infrared and Raman; Near-infrared;
Fluorescence; Atomic Emission and Absorption; Nuclear Magnetic Resonance; Visible Spectroscopy and
Colorimetry
EMULSIFIERS
Contents
Organic Emulsifiers
Phosphates as Meat Emulsion Stabilizers
Uses in Processed Foods
Organic Emulsifiers
T Kinyanjui and W E Artz, University of Illinois,
Urbana, IL, USA
S Mahungu, Egerton University, Njoro, Kenya
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 This chapter will narrowly focus on organic emulsi-
fiers, one category in the general class of compounds
called surface-active agents, rather than macro-
molecular stabilizers or any of the other components
involved in emulsion stabilization. Some familiar
emulsions include those occurring in foods (milk,
mayonnaise, etc.), cosmetics (creams and lotions),
pharmaceuticals (soluble vitamins and hormone
products), and agricultural products (insecticides and
herbicides).
0002 The discussion will be limited to food emulsifiers,
rather than the use of emulsifiers for nonfood
products, such as cosmetics, paints or drug-delivery
systems. Emulsifiers are substances that reduce the
surface tension at the interface of two normally im-
miscible phases, allowing them to mix and form an
emulsion. Representing one class of a broader group
of ingredients called surface active ingredients or sur-
factants, emulsifiers assert their effects at the interface
between oil, water, or air dispersed in a second
immiscible fluid. They promote the formation and
stabilization of emulsions as well as foams and sus-
pensions. There are several types of emulsions, in-
cluding oil in water (o/w) water in oil (w/o), oil in
water emulsion dispersed in oil (o/w/o) etc.
0003 Emulsions are a special kind of colloidal dispersion:
one in which a liquid is dispersed in a continuous
liquid phase of a different composition. The dispersed
phase is sometimes referred to as the ‘internal phase’
and the continuous phase as the ‘external phase.’
Structure
0004Food emulsifiers can be categorized (Table 1) on the
basis of several characteristics, including origin,
either synthetic or natural; potential for ionization,
nonionic versus ionic; hydrophilic/lipophilic balance
(HLB); and the presence of functional groups.
Lecithin and Lecithin Derivatives
0005The primary source of lecithin, the only naturally
occurring emulsifier used in any significant quantities
in the food industry, is soybeans. Soybean oil contains
anywhere from 1 to 3% phospholipids in the crude
oil. Other sources include corn, sunflower, cotton-
seed, rapeseed, and eggs. Lecithin is obtained by an
aqueous extraction of the oil extracted from soy-
beans. Phase separation occurs upon hydration of the
phospholipids, and the two phases are separated by
tbl0001Table 1 Some organic emulsifiers
Lecithin and lecithin derivatives
Monoglycerols
Sucrose esters of fatty acids
Hydroxycarboxylic acid and fatty acid esters
Lactylate fatty acid esters
Polyglycerol fatty acid esters
Ethylene or propylene glycol fatty acid esters
Ethoxylated derivatives of monoglycerides
Sorbitan fatty acid esters
Propylene glycol monoesters
Phosphated mono and diglycerols
Sodium stearoyl-2-lactylate
Calcium stearoyl-2-lactylate
Fruit acid esters
2070 EMULSIFIERS/Organic Emulsifiers
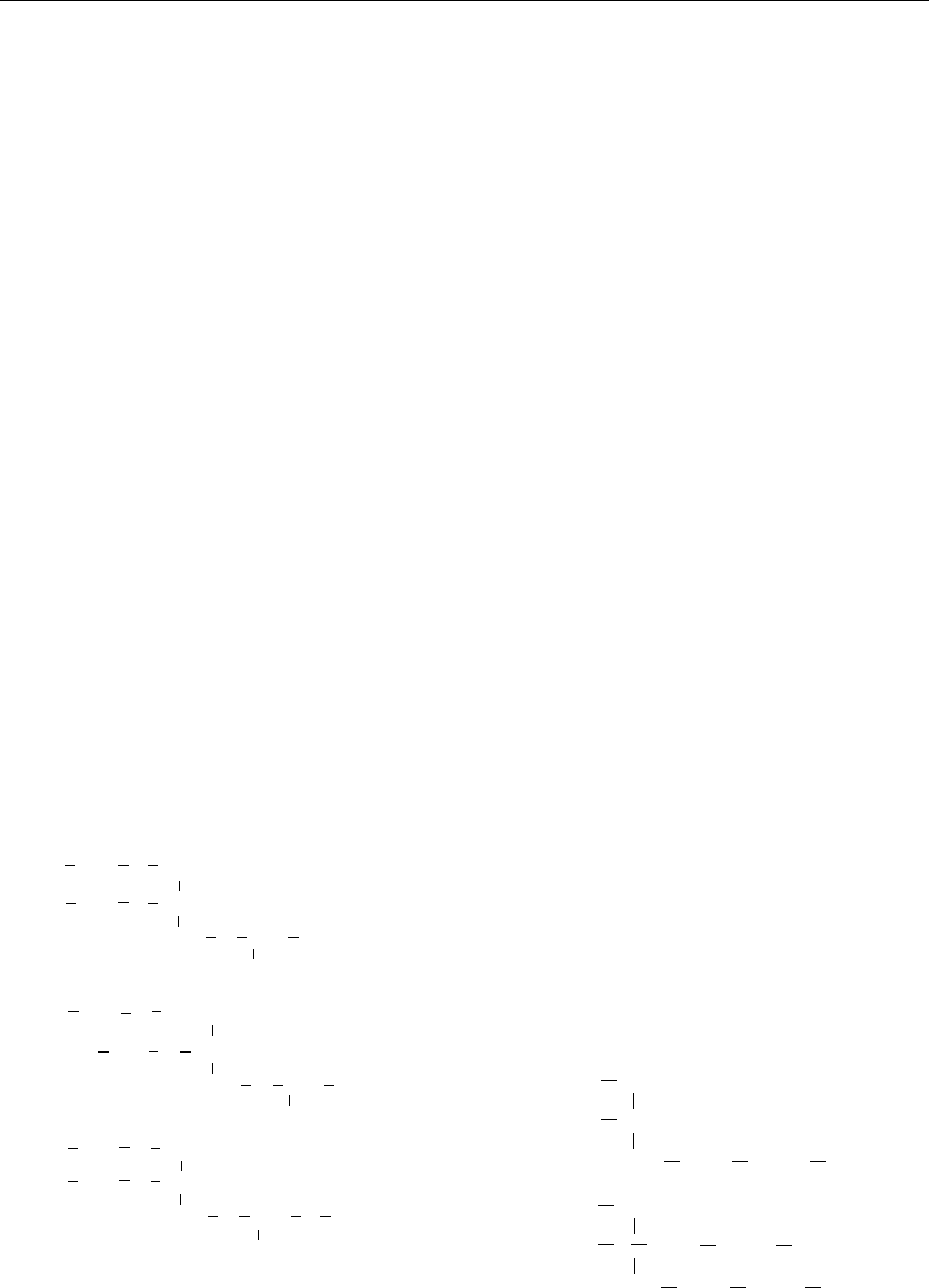
centrifugation. The crude extract, after water re-
moval, contains about 35% triglycerols and smaller
amounts of nonphospholipid materials. Extraction
with acetone is used to produce an oil-free lecithin.
The term ‘lecithin’ has been used to describe both
phosphatidylcholine and mixtures of phospholipids.
Current recommendations by the International Union
of Pure and Applied Chemistry–International Union
of Biochemistry suggest the use of 3-sn-phosphatidyl-
choline rather than lecithin to describe 1,2-diacyl-sn-
glycero-3-phosphatidylcholine. However, a commer-
cial, soybean-derived lecithin preparation contains
several different phospholipids, primarily phosphati-
dylcholine, phosphatidylethanolamine and phospha-
tidylinositol. The structures are shown in Figure 1.
(See Phospholipids: Properties and Occurrence.)
0006 Commercial lecithin preparations can be either
treated or modified chemically to provide a product
with altered functional characteristics. Treatment
with either hydrogen peroxide or benzoyl peroxide
will produce a lighter-colored product. The chemical
modification of lecithin by reaction with hydrogen
peroxide, plus lactic or acetic acid and water, will
produce a hydroxylated product. Hydroxylation
occurs at the double bonds, altering lecithin such
that its hydrophilic character is increased. The result
is a product with improved oil-in-water emulsifying
properties relative to unmodified lecithin.
0007 Triglycerols are soluble in acetone, whereas phos-
pholipids are not. Therefore, the greater the percent-
age of acetone-insoluble material, the greater the
phospholipid content in crude lecithin. Because of
this, one of the primary criteria for the evaluation of
lecithin is the percentage of acetone-insoluble mater-
ial. Lecithin is also evaluated on the basis of several
other parameters including acid value (an indication
of free fatty acids), hexane-insoluble matter (an indi-
cation of fibrous material), water, peroxide value and
metallic impurities. Individual phospholipids in soy
lecithin can be quantitated using HPLC.
Mono- and Diglycerides
0008Mono- and diglycerides are the most commonly used
food emulsifiers. They consist of esters synthesized
via catalytic transesterification of glycerol with trigly-
cerides, with the usual triglyceride source as hydro-
genated soybean oil. Mono- and diglycerides are also
synthesized directly from glycerol and fatty acids
under alkaline conditions. Molecular distillation is
used to prepare a purified product containing up to
approximately 90% monoglycerol. Monoglycerols
can be prepared from the reaction of glycidol (2,3-
epoxy-1-propanol) and carboxylic acids with a yield
in excess of 90%. Advantages of the process include
the synthesis of difficult to produce monoglycerides
and a good potential for continuous processing.
Mono- and diglycerols have also been obtained from
a butterfat fraction by chemical glycerolysis. The en-
zymatic preparation of mono- and distearin by gly-
cerolysis of ethylstearate and direct esterification of
glycerol in the presence of a lipase from Candida
antarctica has also been reported.
0009Several tests are used for characterizing commer-
cial sources of mono- and diglycerides, including total
monoglycerides, hydroxyl value, iodine value, and
the saponification value. With the monoesters, the
fatty acid can be attached at either the alpha or beta
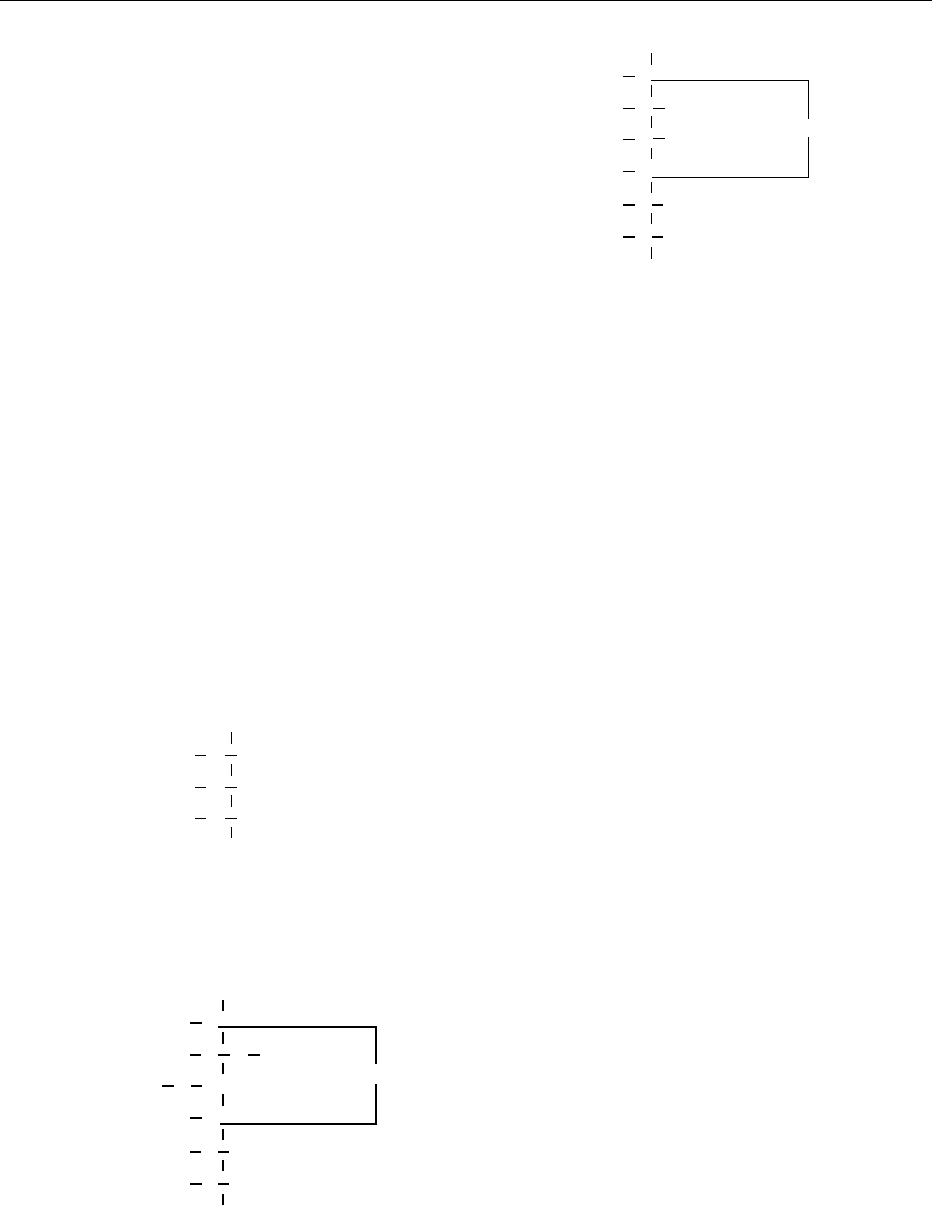
positions, as with the diglycerides (see Figure 2).
Hydroxycarboxylic and Fatty Acid Esters
0010To produce an emulsifier with an increased hydroph-
ilic character relative to monoglycerides, small organic
acids are esterified to monoglycerols (Figure 3). Some
R
1
C(O) CH
2
O
R
2
C(O) CH
O
R
1
C(O) O CH
2
CH
2
O P(O) OCH
2
CH
2
N
+
(CH
3
)
3
CH
2
O P(O) OCH
2
CH
2
NH
+
R
2
C(O) O CH
O
−
R
1
C(O) O CH
2
R
2
C(O) O CH
CH
2
O P(O) O C
6
H
11
O
5
O
−
O
−
(a)
(b)
(c)
3
fig0001 Figure 1 Primary phospholipids reported in commerical leci-
thin, where R
1
and R
2
are fatty acids: (a) phosphatidylcholine;
(b) phosphatidylethanolamine; (c) phosphatidylinositol.
HO CH
2
HO CH
CH
2
OC(O)
(CH
2
)
16
CH
3
(a)
HO CH
2
CH
2
OC(O)
(CH
2
)
16
CH
3
(b)
HO C
(CH
2
)
16
CH
3
OC(O)
fig0002Figure 2 (a) Mono- and (b) diglycerides.
EMULSIFIERS/Organic Emulsifiers 2071

of the acids used are acetic, citric, fumaric, lactic,
succinic, and tartaric. Succinylated monoglycerols
are synthesized from succinic anhydride and distilled.
They are used by the baking industry as dough condi-
tioners and crumb softeners. Acetic acid esters of
mono- and diglycerides are synthesized from fatty
acids plus acetic anhydride or by transesterification.
The product is lipid-soluble and water-insoluble.
Functions in food include control of fat crystalliza-
tion and improvement of aeration properties of high-
fat foods. They are often added to shortenings or cake
mixes. (See Fats: Classification.)
0011 To synthesize other acid esters, citric acid esters of
mono- and diglycerides, glycerol is esterified with a
mixture of citric acid and fatty acids. It can also be
prepared by the direct esterification of citric acid with
glyceryl monooleate. The product is hot water- and
lipid-soluble. Functions in food include emulsifica-
tion, antispattering agent in margarine, improvement
of bakery product characteristics, a fat replacement in
high-fat foods and a synergist and solubilizer for
antioxidants.
0012 Diacetyl tartaric acid esters of monoglycerides
(DATEM) are synthesized from diacetyl tartaric acid
anhydride and monoglycerides. The emulsification
properties of DATEM depend primarily upon the
type of fatty acid and the percentage of esterified
tartaric acid. Emulsifier quality is based upon results
from the analyses for tartaric and acetic acid, acid
value, total fatty acids, saponification value, and
metallic residues.
0013 Lactic acid esters of mono and diglycerides consist
of a mixture of lactic and fatty acid esters of glycerin.
The emulsifier is dispersible in hot water. Important
qualitative parameters include the percentage of
monoglycerides, total lactic acid, acid value, free
glycerin, and the amount of water.
Lactylate Fatty Acid Esters
0014 Polymeric lactic acid esters of monoglycerides are also
available, commonly known as sodium or calcium
stearoyl-2-lactylates (see Figure 4). Typically, there
are two lactic acid groups per emulsifier molecule.
To produce the emulsifier, a mixture of the fatty
acid, polylactic acid, and calcium or sodium carbonate
is heated at about 200
C for about 1 h with agitation
in an inert atmosphere. The calcium salt is less dispers-
ible in water than sodium stearoyl-2-lactylate.
Polyglycerol Fatty Acid Esters
0015Polyglycerol esters (Figure 5) of fatty acid are also
used in food products, primarily in baked goods.
They consist of mixed partial esters synthesized from
the reaction of polymerized glycerol with edible fats.
Polyglycerols will vary in degree of glycerol polymer-
ization with an average specified. The source of fatty
acids as well as the degree of polymerization can vary,
providing a wide range of emulsifiers, from hydro-
philic to very lipophilic.
Polyethylene or Propylene Glycol Fatty Acid Esters
0016Fatty acids can be esterified directly to polyethylene
glycol ethers or by enzymatic preparation, which
allows better control of the reaction. Propylene glycol
fatty acid (C12, C14, C16, C18, and C18:1) mono-
esters (Figure 6) have been produced by lipase-
catalyzed reactions. Propylene glycol monoesters
of docosahexaenoic acid and eicosapentaenoic acid
that are potentially health-beneficial w/o emulsifiers
useful in the food industry have been synthesized
by lipase-catalyzed esterification. The HLB of the
emulsifier is altered by adjusting the degree of ethox-
ylation. Fatty acid polyglycol esters are good o/w
emulsifiers.
Ethoxylated Derivatives of Monoglycerides
0017Ethoxylated mono- and diglycerides are produced
from the reaction of several moles of ethylene oxide
and mono- or diglycerols under pressure. Ethoxyla-
tion of monoglycerols results in a product that is
much more hydrophilic relative to monoglycerols.
OR
2
R
1
[O
CH
2
CCH
2
]
n
OR
3
H
fig0005Figure 5 Polyglycerol esters of fatty acids, where R
1
,R
2
, and R
3
are each a fatty acid and/or a hydrogen, and where the average
value of n is greater than 1.
RC
O O
OHO
[C
C]
n
Na
CH
3
fig0004Figure 4 Sodium stearoyl-2-lactylate, where n normally
averages 2, and R is a fatty acid moiety.
H
H
H
C
OR
1
H
C
OR
2
H
C
OR
3
fig0003 Figure 3 Organic acid ester of monoglycerol, where at least
one R is a short-chain organic acid, for example, acetic acid.
2072 EMULSIFIERS/Organic Emulsifiers
0018 The end product of the synthesis is actually a mix-
ture with a distribution range and peak; therefore,
many often vary among manufacturers.
Sorbitan Fatty Acid Esters
0019 Polyoxyethylene sorbitan esters are synthesized by
the addition, via polymerization, of ethylene oxide
to sorbitan fatty acid esters. These nonionic hydro-
philic emulsifiers are very effective antistaling agents
and, thus, are used in a wide variety of bakery prod-
ucts. These emulsifiers are much more widely known
as the polysorbates, e.g., polysorbate 20, 60, and 80
(Figure 7). Polysorbate 20, 60, and 80 utilize lauric,
stearate, and oleate, respectively, for the fatty acid
portion of the molecule. Polysorbate 60 is a mono-
stearate, whereas polysorbate 65 is a tristearate.
Miscellaneous Derivatives
0020 Fatty acids can be esterified directly to compounds
other than glycerol, for example, sugar alcohols like
sorbitol, mannitol, and maltitol, and sugars like
sucrose, glucose, fructose, lactose, and maltose.
0021 Sorbitol or sorbitan esters are formed from 1,4-
anhydro-sorbitol and fatty acids (see Figure 8). Typ-
ically, the emulsifier consists of a mixture of stearic
and palmitic acid esters of sorbitol and its mono- and
dianhydrides. Ethoxylated derivatives can also be
prepared by the addition of several moles of ethylene
oxide to the sorbitan monoglycerol ester and,
depending on the number of moles of ethylene oxide
added, have a wide range in HLB.
0022Lactitol (the hydrogenation product of lactose)
palmitate is synthesized by direct esterification at a
temperature of approximately 160
C. Workers have
reported the enzymatic synthesis of acetylated glu-
cose fatty acid esters. Two immobilized lipases from
Candida antarctica (SP 382) and Candida cylindra-
ceae catalyzed the synthesis of novel acetylated glu-
cose fatty acid esters with glucose pentaacetate and
Trisun 80 (80% oleic) vegetable oil or methyl oleate
as substrates in organic solvents. The incorporation
of oleic acid on to the glucose ranged from 30 to
100%. It was possible to catalyze the synthesis of
glucose fatty acid esters with free glucose as the
sugar substrate. Other researchers have reported the
synthesis of a novel nonionic surfactant, dialkyl glu-
cosylglutamate from d-gluconolactone, glutamic acid
and alkyl alcohols. Sucrose fatty acid esters (Figure 9)
can be synthesized using a variety of solvents or by
direct esterification. The first description of a prac-
tical commercial process for the preparation of su-
crose esters of fatty acids was reported in 1956.
Enzymatic synthesis of carbohydrate esters of fatty
acids has also been reported for the esters of sucrose,
glucose, fructose, and sorbitol with oleic and stearic
acid and fructofuranose. The reports by researchers
indicate the enzymatic preparation of three different
1,6-diacyl fructofuranoses. At low temperatures
(5
C), the synthesis produces quantitative yields of
the diesters by simple addition of the original sugar to
a solution of the fatty acid in a solvent (acetone),
which is accepted by the European Commission
(EC) for use in the manufacture of additives. By
varying the degree of esterification, the HLB and,
hence, the functionality can be controlled. Sucrose
H
H
H
C
H
C
H
C
OH
OH
OR
H
HO
C
H
C
H
C
O
fig0008Figure 8 Sorbitan stearate, where R represents a fatty acid
moiety, for example, stearic acid, oleic acid, lauric acid, or
palmitic acid.
H
H
H
C
H
H
C
OR
1
H
C
OR
2
fig0006 Figure 6 Propylene glycol esters of fatty acids, where R
1
and
R
2
represent a fatty acid and/or a hydrogen, and where at least
one R represents a fatty acid.
H
H
H
C
H
C
H
C
O
(C
2
H
4
O)
w
OH
O
C
H
C
(C
2
H
4
O)
y
R
2
H
C
(C
2
H
4
O)
z
R
3
R
1
(OC
2
H
4
)
x
O
fig0007 Figure 7 Polysorbates, where w þ x þ y þz ¼ 20 (approx.) and
Rs represent a single fatty acid and hydrogens for polysorbate
20, 40, 60, and 80. For polysorbate 65, each R represents a stearic
acid moiety. The fatty acids are lauric, palmitic, stearic, and oleic
acid for polysorbate 20, 40, 60, and 80, respectively.
EMULSIFIERS/Organic Emulsifiers 2073

monoesters have an HLB value greater than 16 while
the triesters have an HLB value less than 1. Monoe-
sters are particularly useful for the stabilization of
o/w emulsions, whereas diesters are best for w/o
emulsions. With esterification equal to or greater
than 5 moles of fatty acid per mole of sucrose, the
emulsification properties of sucrose fatty acid esters
are lost. But, at that degree of esterification, the su-
crose fatty acid polyester can be used as a low-calorie
fat replacement since it is neither digestible nor
absorbable.
0023 The consistency of both o/w and w/o emulsions can
be affected with the addition of ethylene or propylene
glycol monostearate. The most common ethylene and
propylene glycol esters used as emulsifiers are the
monostearate and monopalmitate.
Emulsion Stability
0024 Small droplets and the presence of an interfacial film
on the droplets in emulsions make them stable; that
is, the suspended droplets do not settle out or float
rapidly, and the droplets do not coalesce quickly.
0025 Emulsifiers are generally classified as: anionic
emulsifiers, cationic emulsifiers, amphoteric emulsi-
fiers and nonionic emulsifiers. Proteins (caseins) are
known to stabilize emulsions, owing to their ampho-
teric nature. The oil phase viscosity also contributes
to the stability of the emulsion stability, but this
depends on the adsorption and interfacial behavior
of the emulsifiers.
0026 One basis for a theory of emulsion stability con-
cerns the balance between the attractive and repulsive
forces of the particle. In other words, attractive (Van
der Waals forces or London dispersion forces) would
tend toward destabilizing an emulsion, whereas the
repulsive forces (electrostatic repulsion between elec-
trical double layers of like sign) would stabilize the
emulsion by keeping the droplets separated.
0027 There are several phenomena that can cause emul-
sion destabilization. Each is affected by the presence
of emulsifiers. To understand best the mechanism of
emulsion formation and ultimately the forces stabil-
izing emulsions, the mechanisms of destabilization
should be understood.
Mechanisms of Destabilization
0028Emulsion destabilization can be due to one or all of
five possible mechanisms; flocculation, coalescence,
sedimentation or creaming, Ostwald ripening, and
phase inversion.
0029-Flocculation The adherence of droplets to form
aggregates or clusters and the buildup of these aggre-
gates are referred to as ‘flocculation.’ It occurs when
the attractive forces between the droplets exceeds that
of the repulsive forces, without a breakdown in the
structural integrity of the interfacial film surrounding
the droplets.
0030-Coalescence When aggregates or flocculates of the
dispersed phase combine to form a single, larger drop,
the phenomenon is referred to as ‘coalescence.’ Co-
alescence is really a reflection of the nature of the
interfacial film on the surface of the droplet. A strong,
stable film on the surface of the droplet, owing to
the addition of the correct concentration of the ap-
propriate emulsifier, will minimize this type of desta-
bilization.
0031-Ostwald ripening If the two phases forming the
emulsion are not totally immiscible, and there are
differences in droplet size within the emulsion, larger
droplets will form at the expense of smaller droplets
owing to a process known as ‘Ostwald ripening.’
Ostwald ripening is always a factor since variations
in initial droplet size always occur in macroemul-
sions, and both phases are never completely immis-
cible. The driving force for Ostwald ripening is the
difference in chemical potential between droplets of
difference sizes. Equilibrium will only exist when all
droplets are the same size, which really means a single
‘drop’ or the presence of two continuous and separate
phases.
0032-Phase inversion The viscosity of an emulsion will
increase gradually as more and more of a given phase
is added until a critical volume is reached. If more
of that same phase is added, exceeding the critical
volume, the emulsion will invert, i.e., the discontinu-
ous phase will become the continuous phase.
Mechanisms of Stabilization by Emulsifiers
0033There are several factors, some of which are depend-
ent on the emulsifiers and stabilizers added, involved
in emulsion stabilization. The first is the reduction of
interfacial tension by the emulsifiers. Next, is repul-
sion between droplets due to similar electrical charges
on the surface of the droplets. A third is the formation
of mesophases or liquid-crystalline phases, which will
R
1
OCH
2
C
5
H
7
O
4
C
4
H
4
O
3
CH
2
OR
2
O
CH
2
OR
3
fig0009 Figure 9 Sucrose fatty acid esters, where at least one of either
R
1
,R
2
,orR
3
represents a fatty acid, and the remainder may
represent a fatty acid or a hydrogen; the degree of substitution
is 1–3.
2074 EMULSIFIERS/Organic Emulsifiers

provide the most stable configuration for a specified
set of conditions. A fourth is the addition of macro-
molecules or particulate material, which can substan-
tially increase emulsion viscosity and stability. An
increase in viscosity of the continuous phase adds to
the kinetic stability. However, without a concurrent
energy barrier, viscosity will have a small effect on
stabilization. Viscosity enhancers increase the stabil-
ity of the energy barrier. Stable oil-in-water emulsions
of argan oil with two different types of mixtures of
nonionic emulsifiers have been produced. Three dif-
ferent types of oil (Israeli argan oil, Moroccan argan
oil, and soybean oil) have been emulsified with mix-
tures of Span 80 and Tween 80. The optimum HLB
value for argan oil is 11.0. The argan o/w emulsions
are stable for more than 5 months at 25
C. Synergis-
tic effects have been found in enhancing the stability
of emulsions prepared with sucrose monostearate.
The origin of the oil and the internal content of
natural emulsifiers, such as monoglycerides and phos-
pholipids, have a profound influence on its interfacial
properties and on the stability of the argan o/w
emulsions.
0034 Emulsion stability is also dependent upon the con-
ditions under which the emulsion is formed. This
includes not only the constituents of the emulsion
but also the emulsifier concentration, emulsion tem-
perature, and physical state (crystalline vs. fluid) of
the emulsifier. The order of addition of the constitu-
ents is also an important factor. Addition of lecithin
to the lipid phase prior to the addition of the aqueous
phase can substantially alter the droplet size, liquid
crystal formation, and emulsion stability. Another
contributing factor is the nature of the internal and
continuous phases. Both affect emulsion stability.
Two types of emulsions, those prepared with unsatur-
ated emulsifiers and unsaturated oil and those pre-
pared with saturated emulsifiers and saturated oil, are
more stable than those prepared with emulsifiers and
oil of intermediate or mixed saturation.
Functions of Emulsifiers
Interfacial Tension
0035 The reduction of interfacial tension through the add-
ition of emulsifiers is a key factor in emulsion forma-
tion. It allows emulsion formation with considerably
less energy input than would be required without
the presence of an emulsifier. Once the interfacial
film consisting of emulsifier is formed, it acts as an
effective barrier to droplet coalescence. It has been
found that, in the presence of emulsifiers, the droplet
interface may acquire viscoelastic properties, which
are important in the prevention of coalescence. A
strong interaction between the hydrophilic portion
of the emulsifier and the aqueous phase leads to
a large reduction in the surface tension of the water;
this also affects the type of emulsion formed. A
weak interaction between water and the hydro-
philic portion of the emulsifier molecule will favor a
w/o emulsion, whereas a strong interaction will favor
a o/w emulsion.
Electrical Charge
0036Ionic emulsifiers provide an additional mechanism
for emulsion stabilization relative to nonionic emulsi-
fiers, through ion–ion and ion–solvent interactions.
In addition, the introduction of charged groups on the
surface of the emulsion droplets increases the repul-
sive forces. Ionic emulsifiers will form an electrically
charged double layer in the aqueous solution
surrounding each oil droplet.
Liquid Crystal Stabilization
0037Macroemulsions, although thermodynamically un-
stable, can attain rather long-term stability, strongly
suggesting an intermediate stability level. This was
attributed to the formation of a liquid crystalline
state by the emulsifier. It has been shown that the
presence of a liquid crystalline state reduces the rate
of coalescence, even if droplet flocculation occurs.
(See Crystallization: Basic Principles.)
Stabilization by Macromolecules and Finely Divided
Solids
0038Emulsion stability can be increased by the addition of
macromolecules like gums and protein. Colloids like
xanthan gum, carboxy methyl cellulose and guar gum
significantly increase emulsion stability. With both a
constant emulsifier and colloid concentration, emul-
sion stability is enhanced by increased emulsification
temperature, increasing the degree of shear, and
increase pH, in the range of 3–6. Colloids act by
either increasing the viscosity or partitioning into
the o/w interface and providing a physical barrier to
coalescence.
0039To evaluate emulsion stability and thereby charac-
terize the potential of an emulsifier, the rate at which
the combined destabilization phenomena occur must
be determined. These rates can be determined from
the changes in the size and distribution of the oil
droplets with time. There are several methods avail-
able for this determination. Nuclear magnetic reson-
ance is often preferred as a better indication of
stability to HLB values. Other methods, used to evalu-
ate the effects of processing on emulsion stability,
include centrifugation, turbidity light micoscopy
scanning electron microscopy, etc.
EMULSIFIERS/Organic Emulsifiers 2075

Emulsifier Selection
0040 Emulsifier selection is based upon the final product
characteristics, emulsion preparation methodology,
the amount of emulsifier added, the chemical and
physical characteristics of each phase, and the pres-
ence of other functional components in the emulsion.
Food emulsifiers have a wide range of functions. The
most obvious is to assist stabilization and formation
of emulsions by the reduction of surface tension at the
o/w interface.
0041 Perhaps the most important factor in preparing an
emulsion is the selection of the appropriate emulsifier.
Several methodologies have been developed to assist
in such an endeavor. These include the HLB system
of Griffin, the H/L numbers, the water number, the
phase inversion temperature, and the emulsion inver-
sion point. Even with the best of methods, selection
can be very difficult, except perhaps for the few foods
that are relatively straightforward emulsions, like
mayonnaise and margarine. Often, one of the best
sources of information is the emulsifier manufacturer.
Several parameters should be considered during
emulsifier selection. These parameters include: (1) ap-
proval of the emulsifier by the appropriate govern-
ment agency, (2) desired functional properties, (3)
end-product application, (4) processing parameters,
(5) synergistic effect of other ingredients, (6) home
preparation, and finally (7) cost.
0042 Obviously, before an emulsifier can be used in a
food product, it must be approved by the appropriate
regulatory agency. Assuming this criterion is met, the
most important considerations would be both the re-
quired functional properties of the selected emulsifier
and the application. Delineating the required func-
tional properties such as emulsification, starch com-
plexation and crystallization control and the specific
end-product application are the two major factors in
emulsifier selection. An exact determination of these
two parameters should focus attention on a limited
number of emulsifiers. The processing methodology
and equipment available in the processing facility
could further limit the range of emulsifiers that are of
potential use. It is at this stage that ingredient sup-
plier(s) could begin to provide helpful assistance.
0043 By far, the most widely used rule for the selection of
food emulsifiers is the HLB number. The HLB index is
based upon the relative percentage of hydrophilic to
lipophilic groups within the emulsifier molecule. The
assigned values range from 1 to 20. Lower HLB values
indicate a more lipophilic emulsifier, whereas higher
values indicate a more hydrophilic emulsifier. Emulsi-
fiers with HLB numbers in the 3–6 range are best for w/
o emulsions, whereas emulsifiers with HLB numbers
in the range of 8–18 are best for o/w emulsions.
Depending upon the application and the types of
oils to be emulsified, there is an optimum HLB.
0044There is an equation that can be used to determine
the HLB number for several types of nonionic emulsi-
fiers, particularly the ethoxylated alcohols and the
polyhydric fatty acid esters. To determine the HLB
for the fatty acid ester type, the following equation
can be used:
HLB ¼ 20ð1 S=AÞ,
where A is the acid number, and S is the saponification
number of the ester.
0045For the polysorbate type of emulsifier, the HLB
value can be determined from the equation:
HLB ¼ðE þ PÞ=5,
where E is the weight percentage of oxyethylene, and
P is the weight percentage of polyhydric alcohol.
0046However, there are several factors that reduce the
utility of the HLB selection system. One factor is that
the HLB system does not work well for ionic emulsi-
fiers, a problem further complicated by the fact that
the charge varies with pH. Another factor is that com-
mercial preparations usually contain two or more
emulsifiers. These emulsifiers can have a significant
synergistic effect that makes it very difficult to apply
the HLB system. Another limitation is due to the fact
that the HLB system is based upon the molecular
structure of the emulsifier and does not take into ac-
count the combined oil/aqueous phase/emulsifier
system. In addition, once the appropriate emulsifier
has been selected, the correct concentration can not be
determined from the HLB value. The concentration
required is really a function of the droplet size. The
smaller the droplet, the greater the surface area and,
therefore, the greater the amount of emulsifier that is
required for monolayer coverage of each droplet. In
spite of these limitations, the HLB is still the most
widely used index of emulsifier functionality.
See also: Colloids and Emulsions; Fatty Acids:
Properties; Phospholipids: Properties and Occurrence
Further Reading
Artz WE (1990) Emulsifiers. In: Branen AL, Davidson MP
and Salminen S (eds) Food Additives, pp. 347–393. New
York: Marcel Dekker.
Artz WE and Myers MR (1994) Supercritical fluid extrac-
tion and chromatography of emulsifiers. Journal of the
American Oil Chemists Society 72: 219.
Brian V (1999) Dispersion stabilization and destabilization
by polymers. In: Dickinson E and Patino RJ (eds) Food
Emulsions and Foams – Interfaces, Interactions and
Stability, p. 20. Cambridge: Royal Society of Chemistry.
2076 EMULSIFIERS/Organic Emulsifiers
