Bongard Frederic , Darryl Sue. Diagnosis and Treatment Critical Care
Подождите немного. Документ загружается.


DIABETES MELLITUS, HYPERGLYCEMIA, & THE CRITICALLY ILL PATIENT
587
Acetoacetate crossreacts in some automated methods for
measuring creatinine and thus may artificially elevate creati-
nine concentrations; if owing to this mechanism, elevated
serum creatinine concentrations diminish rapidly as acetoac-
etate levels are cleared with therapy.
Hypertriglyceridemia occurs quite frequently in diabetic
ketoacidosis. This is of importance not only because of the
clinical manifestations of hypertriglyceridemia (eg, acute
pancreatitis) but also because high triglyceride levels inter-
fere with accurate measurement of sodium, chloride, and
bicarbonate. When hypertriglyceridemia is present, pseudo-
hyponatremia may be seen (serum is composed of a sodium-
containing water phase and a large lipid-triglyceride phase
that contributes non-sodium-containing volume). What is
less well recognized is that hypertriglyceridemia can interfere
with the colorimetric measurement of chloride and bicar-
bonate, although there is no interference with more com-
monly used ion-specific electrode assays. The effect is to
report falsely high serum chloride and bicarbonate concen-
trations that may mask the increase in anion gap expected in
ketoacidosis and lead to incorrect evaluation of the patient.
This possibility should be considered whenever the anion
gap is not elevated in an otherwise typical clinical situation
suggestive of diabetic ketoacidosis.
Differential Diagnosis
Clinical manifestations of diabetic ketoacidosis, such as dys-
pnea, nausea, vomiting, or abdominal pain, may mimic non-
diabetic acute disease. In known diabetic patients with coma
or altered mental status, ketoacidosis must be distinguished
from hypoglycemia. This is usually not difficult clinically
because the circumstances and clinical findings are usually
quite different. The patient with diabetic ketoacidosis is
volume-depleted and may be acidotic, whereas the hypo-
glycemic patient is usually cold and clammy. In either case,
bedside diagnosis of these conditions by direct measurement
of blood glucose can be made quickly, so clinical differentiation
is less relevant. In comatose patients with diabetic ketoacidosis
in whom serum osmolality is less than 340 mOsm/Kg, another
cause of coma should be considered.
A common difficulty arises when differentiating alcoholic
ketosis from diabetic ketoacidosis in a patient with diabetes
who has ingested alcohol. In such situations, whatever the
cause of ketosis, the management is that of diabetic ketoacido-
sis. Severe abdominal pain from diabetic ketoacidosis, often
accompanied by vomiting, may mimic an acute abdomen.
Acute pancreatitis is often in the differential diagnosis, and its
diagnosis is complicated by the fact that serum amylase levels
are often elevated in diabetic ketoacidosis as a result of an
increase in serum amylase from the salivary isoenzymes.
Treatment
The principles of management of diabetic ketoacidosis are to
replace losses of water and electrolytes, give sufficient insulin
(ie, stop ketogenesis, lipolysis, and gluconeogenesis), correct
blood pH, closely monitor the patient through all stages of
management, and eliminate or treat precipitating causes.
Rapid initial evaluation takes place in the emergency room in
most patients with diabetic ketoacidosis, although at times
diabetic ketoacidosis does develop in the hospital. Table 26–7
sets forth the initial steps in management when diabetic
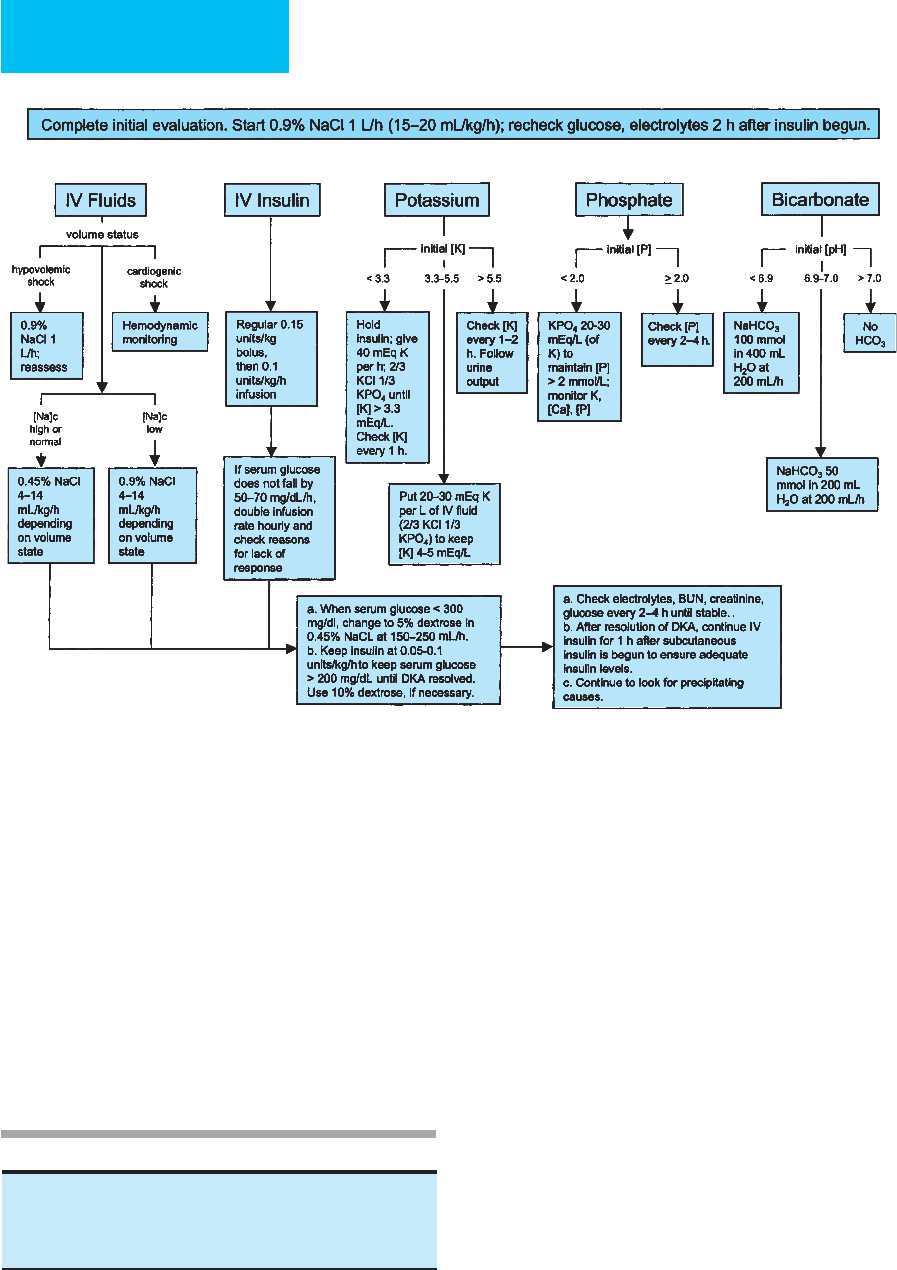
ketoacidosis is suspected. Figure 26-3 provides an overview
of management.
A. Monitoring—Close monitoring is the key to successful
management of diabetic ketoacidosis. Most management
decisions are fairly straightforward if the data are available
in timely fashion. Errors in management occur most often
when there is a lapse in monitoring so that a “catch-up” sit-
uation develops or the effects of overtreatment need to be
corrected.
Blood glucose should be monitored hourly at the bedside.
This provides information about whether the insulin dose is
adequate to cause a fall in blood glucose at the expected rate
of about 100 mg/dL per hour. Later, glucose measurement
prevents overshooting the target serum glucose level of
250–300 mg/dL, at which time infusion of dextrose should
be started. Serum electrolytes and ketones should be moni-
tored every 2 hours, and this should include measurement of
serum phosphorus as well. Arterial blood gases should be
repeated as necessary if progress in the clearing of acidosis is
slow or if there are associated pulmonary problems. If phosphate
therapy is used, serum calcium should be measured at least
once (after an initial measurement) during the first 12 hours
to detect any large decrease in serum calcium. All the data
obtained, including fluid balance measurements, should be
maintained on a flowchart for easy review and evaluation.
1. Correction of hyperglycemia—Correction of hyper-
glycemia occurs by four different mechanisms (Table 26–8).
First, the concentration of the extracellular glucose is diluted
by fluid replacement, expanding the extracellular space. The
second is by continuing—and usually increased—urinary
Rapid clinical examination.
Bedside blood glucose and urine ketone determinations.
Obtain blood for laboratory determination of glucose, ketones, electrolytes,
urea nitrogen, creatinine, phosphorus, magnesium.
Obtain arterial blood for blood gases.
Chest x-ray.
ECG (also to monitor K
+
levels).
Search for precipitating factor.
Monitoring: Hourly bedside glucose and two-hourly measurement of
anion gap, serum electrolytes, serum ketones, serum phosphorus.
Obtain arterial blood gases as needed.
Table 26–7. Initial diagnosis and management of diabetic
ketoacidosis.
CHAPTER 26
588
loss of glucose that occurs with improved renal perfusion
following expansion of the intravascular space. Third is the
effect of insulin to diminish the hepatic glucose production
rate. Considering that this is one of the important pathophys-
iologic mechanisms producing hyperglycemia in diabetic
ketoacidosis, inhibition of this process must be one of the
management goals. Indeed, it has been demonstrated that
insulin infused at the routine doses is very effective in reduc-
ing glucose production by the liver during recovery from dia-
betic ketoacidosis. Increased glucose utilization is the fourth
mechanism and is also an insulin-dependent process.
2. Correction of ketosis—Ketosis is corrected more
slowly than hyperglycemia, so while serum glucose levels
usually will reach 250–300 mg/dL within an average of 6–8
hours, it can take up to 12 hours or more for ketosis to clear.
It is particularly important to continue insulin infusion dur-
ing this period despite the fact that one major goal of ther-
apy (reduction of hyperglycemia) has been attained.
Intravenous insulin therefore should be stopped only when
ketosis has cleared.
It is not always easy to decide when a patient is no longer
in a state of ketoacidosis. Serum ketone measurements do
not provide the entire answer because they can remain posi-
tive for many hours after the acidosis is resolved, probably
because acetone is cleared much more slowly than the two
keto acids. Acetone, however, is also measured by the nitro-
prusside reaction and can give rise to persistently positive
serum ketone measurements even though it does not alter
acid-base balance. For this reason, monitoring the anion gap
is a better way of determining when ketosis has cleared.
Serum bicarbonate concentrations are useful for monitoring
progress of therapy but less useful when used alone to determine
Figure 26–3. Management of adult patients with diabetic ketoacidosis. [Na]
c
= corrected serum sodium concentra-
tion (for each 100 mg/dL glucose >100 mg/dL, add 1.6 meq/L to measured serum sodium.) (Modified from American
Diabetes Association. Hyperglycemic crises in diabetes. Diabetes Care 2004;27:S94–102.)
Dilution by expansion of extracellular volume
Urinary losses
Diminished glucose production rates
Increased glucose utilization
Table 26–8. Correction of hyperglycemia: Mechanisms.

DIABETES MELLITUS, HYPERGLYCEMIA, & THE CRITICALLY ILL PATIENT
589
that ketoacidosis has cleared because bicarbonate is slow to
normalize completely. This is so because hyperchloremic aci-
dosis accompanies saline therapy in almost all patients and
because hyperventilation persists after the patient is no
longer acidotic. Arterial blood pH measurement can be use-
ful when it is difficult to decide whether ketosis has cleared—
particularly if the anion gap has normalized and
hyperglycemia is resolved, yet the patient still appears to be
ill. The overall clinical picture is useful—in a patient whose
appetite has returned and is able to eat and retain a meal, this
generally signals resolution of ketoacidosis.
B. Fluid Replacement—Fluid losses in an adult with dia-
betic ketoacidosis average 5–7 L and may be as much as
10–15% of body weight. Fluid replacement should be initi-
ated immediately after diagnosis to prevent further deterio-
ration of hemodynamic status. Fluid therapy is begun with
intravenous 0.9% NaCl solution. A rough guide to volume
replacement is as follows: (1) 2 L can be given over the first 2
hours, (2) this can be followed by 2 L over the next 4 hours
using 0.9% or 0.45% NaCl solution, and (3) over the next 8
hours, another 2 L can be given using either 0.9% or 0.45%
NaCl. This schedule should be modified according to ongoing
assessment of volume replacement needs and serum sodium
levels. In hemodynamically compromised patients and the
elderly, close monitoring of volume status may be necessary,
and consideration should be given to use of a central venous
pressure or pulmonary artery catheter. If severe hypotension
is present, maintaining intravascular volume with albumin or
other plasma expanders also should be considered.
When the plasma glucose concentration reaches 250–300
mg/dL, 5% dextrose solution should be started with an
appropriate amount of NaCl according to the needs of the
patient at that time.
There is disagreement about the best approach when
serum sodium exceeds 150 meq/L at presentation. When
0.45% NaCl is given at the outset, some patients have devel-
oped hypotension during treatment. This is likely due to the
insulin effect to drive glucose intracellularly; water follows,
and the intravascular space is depleted. When hypotonic
saline is used, there is less addition of solute that will remain
in the intravascular and extracellular spaces. Therefore, a
greater proportion of the infused fluid will be ineffective in
expanding the intravascular volume.
Administering 0.9% NaCl solution provides a greater
osmotic load, and thus it is more likely to protect intravascu-
lar volume in the face of fluid shifts in diabetic ketoacidosis
patients who are strikingly volume-depleted. The concern
with infusing 0.9% NaCl (normal saline) is that hyperosmo-
lality will not be corrected. However, this approach conforms
to the physiologic principle that during states of severe vol-
ume depletion, volume maintenance takes precedence over
maintaining normal serum osmolality. Thus 0.9% NaCl is
recommended as the volume replacement fluid of first choice
even when the patient is initially hypernatremic: 0.9% NaCl
is hypo-osmolar relative to serum when serum osmolality is
greater than 308 mOsm/Kg. Once volume depletion has been
corrected, administration of 0.45% NaCl or 5% dextrose in
water to correct hypernatremia is indicated.
Fluid replacement has been given without insulin therapy
to test its effects in the absence of insulin. In a few studies
there has been considerable improvement in hyperglycemia
but with no consistent effect on serum ketones. The
improvement in serum glucose concentrations in the
absence of insulin is probably the result of increased renal
glucose losses and a dilution effect (see Table 26–8).
C. Insulin
1. Initial therapy—Insulin is started at the time of diagno-
sis at a rate of 0.1 unit/kg per hour by continuous intravenous
infusion. A bolus dose of 0.15 unit/kg may be administered
initially. Blood glucose levels should be checked at hourly
intervals using bedside (glucometer) measurements, and lab-
oratory serum glucose determinations can be obtained every
2 hours, along with serum electrolytes, to provide confirma-
tion of the glucometer measurements.
In most patients, the recommended starting dose is suffi-
cient to treat diabetic ketoacidosis because the serum-free
insulin levels achieved with this infusion rate are 7 to 10
times normal basal concentrations. However, some patients
do require even faster infusion rates. Therefore, if serum glu-
cose concentrations have not begun to fall after the first hour,
the rate of insulin infusion should be doubled—and doubled
once again if the same should happen during continuing ther-
apy. The insulin infusion should cause blood glucose to fall at
a rate of about 75 mg/dL per hour. The rate of insulin infusion
should be continued—if glucose is falling appropriately—
until serum glucose has reached 230–300 mg/dL. At that
time, a 5% dextrose infusion should be started, but the
insulin infusion must continue until ketosis clears. Because
considerable insulin resistance remains even though some of
the factors described in Table 26–1 have normalized, insulin
infusion should be maintained at the same rate and 10%
dextrose infused, if necessary, to maintain serum glucose lev-
els while awaiting clearance of ketones. Decreasing insulin
infusion rates at this time may result in slower resolution of
ketosis. Clearing of ketosis should be monitored using a
combination of the anion gap, bicarbonate, and serum
ketone measurements and, if necessary, arterial blood gas pH
measurement.
2. After resolution of ketosis—Once ketosis has
cleared—and if the patient is eating—a subcutaneous insulin
regimen can be started. The patient should be given a subcu-
taneous injection of regular insulin about 30 minutes before
stopping the insulin infusion. This allows some of the subcu-
taneously injected insulin to be absorbed before the insulin
infusion is halted. Because intravenous insulin has a half-life
of only 5–6 minutes, stopping the intravenous insulin prior
to giving subcutaneous insulin can result in temporarily
inadequate serum insulin concentrations. Low serum insulin
levels, in turn, can cause a rebound into diabetic ketoacidosis,

CHAPTER 26
590
particularly if there is significant delay in delivering the sub-
cutaneous regular insulin injection. Subcutaneous rapid-
acting (regular or analogue) insulin is also best injected
before a meal, and the insulin infusion can be halted once the
meal begins. The bolus of regular insulin thus will be deliv-
ered at a physiologically appropriate time, and the meal will
help to compensate for any error of excess in estimating the
regular insulin dosage at that time.
Split-dose insulin therapy with a combination of regular
and NPH insulin before breakfast and dinner can be started
as soon as the diabetic ketoacidosis has resolved and the
patient is able to eat. The total daily dose can be similar to pre-
hospital doses in patients with known diabetes; in newly diag-
nosed patients, regular insulin can be given before meals and
at 12 midnight using a sliding scale for 24 hours. The total
dose required then can be used to calculate split-dose therapy,
giving two-thirds of the total dose in the morning and one-
third in the evening. NPH insulin usually constitutes two-
thirds of the morning dose and three-fourths of the evening
dose, with the remainder in each case being regular insulin.
D. Potassium Replacement—Usual potassium deficits in
diabetic ketoacidosis have been estimated to be approxi-
mately 300 meq, but the deficit may be as much as 1000 meq.
This total body potassium deficit is the result of combined
renal losses owing to osmotic diuresis and potassium shifts
from intracellular to extracellular fluid that lead to further
loss through the kidneys.
If the patient is normokalemic or hypokalemic at presen-
tation, potassium replacement should be started when
insulin therapy is initiated. If the serum potassium level is
high, potassium therapy should be started only after insulin
therapy is begun and with the second liter of fluid replace-
ment. If treatment is necessary before the serum potassium
level is reported, epidemiologic data provide a basis for
determining the frequency of serum potassium abnormali-
ties (see Tables 26–5 and 26–6). Most patients initially have
either normal or high serum potassium levels; however, up to
20% of patients will have hypokalemia at the outset. In this
last group of patients, insulin infusion alone (without potas-
sium replacement) will exacerbate the hypokalemia, with
potentially severe consequences. In contrast, the fear of giv-
ing potassium to normokalemic or hyperkalemic patients is
mitigated by the effects of the concomitant insulin infusion,
intracellular rehydration, and rise in pH. Thus, if the serum
potassium concentration is unknown, the ECG does not
demonstrate hyperkalemic changes, and the patient is pro-
ducing adequate amounts of urine, potassium infusion
should be started at the same time insulin therapy is begun.
Potassium replacement is started by the addition of
potassium chloride 20–40 meq/L to the fluid replacement
solution. Potassium phosphate also may be given (alternating
with potassium chloride) if phosphate repletion is necessary.
Subsequent potassium replacement depends on the results of
serum potassium determinations, which should be done at
2-hour intervals throughout the period of management of
diabetic ketoacidosis. In addition, electrocardiographic
follow-up with single-lead measurement is a useful way of
monitoring serum potassium for gross hyperkalemia or
hypokalemia so that emergency treatment can be initiated if
necessary. It should be kept in mind that even while replace-
ment of potassium is taking place, continuing potassium
losses occur in the kidney throughout the period of manage-
ment of diabetic ketoacidosis. For this reason, serum potas-
sium measurements also should be obtained daily once the
patient is no longer ketotic. A total body deficit of potassium
may persist despite initial correction of serum potassium,
and oral potassium replacement therapy should be given to
patients who continue to be hypokalemic.
E. Bicarbonate—Acidosis generally resolves with insulin ther-
apy and metabolism of keto acids. In most cases of diabetic
ketoacidosis, it is not necessary to treat acidemia with bicar-
bonate. Recent studies have demonstrated that bicarbonate
therapy does not alter the eventual outcome of diabetic
ketoacidosis, nor does it increase the rate at which pH is cor-
rected. In fact, some have found a counterproductive effect of
sodium bicarbonate in the treatment of diabetic ketoacidosis.
Clinical and animal studies have shown that bicarbonate
administration actually may increase ketone production.
Recent data suggest also that in children with diabetic ketoaci-
dosis, the mean duration of hospitalization for those receiving
bicarbonate was 23% longer than that of children who did not
receive bicarbonate. Lastly, a multicenter retrospective study of
the risk factors associated with cerebral edema in children with
diabetic ketoacidosis found that the relative risk of cerebral
edema was 4.2 in children treated with bicarbonate compared
with a matched control group that did not receive bicarbonate.
Bicarbonate therapy also has been associated with hypocal-
cemic tetany, decreased tissue oxygen delivery, a paradoxical
fall in pH of the cerebrospinal fluid, rebound alkalosis, greater
potassium needs, and sodium overload.
However, when pH values are extremely low, concern
about the hazards of acidemia begin to outweigh the con-
cerns with alkali therapy. Therefore, at a pH of less than 7.0,
particularly in a patient who is very ill, many clinicians will
administer bicarbonate. The effects of low pH are a negative
inotropic effect on the heart, vasodilatation, cerebral depres-
sion, insulin resistance, and depression of enzyme activity. If
treatment with bicarbonate is begun, the American Diabetes
Association (ADA) recommends that 100 mmol of sodium
bicarbonate should be added to 400 mL of sterile water and
given at a rate of 200 mL/h in severe acidosis (pH <6.9). In
patients with a pH of 6.9–7.0, 50 mmol of sodium bicarbon-
ate should be diluted in 200 mL of sterile water and infused
at a rate of 200 mL/h.
In general, taking into account the hazards of treatment,
the hazards of acidemia, and the probable benefits of bicar-
bonate administration, it is not necessary to treat routinely
with bicarbonate.
F. Phosphate Replacement—The routine administration of
phosphate in the management of diabetic ketoacidosis remains
DIABETES MELLITUS, HYPERGLYCEMIA, & THE CRITICALLY ILL PATIENT
591
controversial. Although there are theoretical reasons for replac-
ing phosphate during treatment, most studies have not demon-
strated any beneficial effect. Despite depletion of erythrocyte
2,3-diphosphoglycerate and the concern about decreased oxy-
gen delivery to the tissues resulting from a leftward shift in the
oxyhemoglobin dissociation curve, there are no deleterious
clinical consequences of a mildly low serum phosphorus con-
centration. For most patients with diabetic ketoacidosis, routine
administration of phosphate is unnecessary.
However, it is important to separate out one group of
patients in whom phosphate therapy is essential—those with
low serum phosphorus levels at presentation, which includes
about 10% of patients (see Table 26–6). Serum phosphorus
decreases in all patients, sometimes dramatically, after insti-
tution of insulin therapy. If the initial serum phosphorus
concentration is 1.5 mg/dL, however, it may fall with treat-
ment into the range of concentrations that are associated
with the hypophosphatemia syndrome (Table 26–9). This
syndrome may include decreased myocardial contractility,
respiratory muscle weakness and respiratory failure, hemol-
ysis, and rhabdomyolysis. It is important to note that none of
the studies evaluating the efficacy of phosphate therapy in
diabetic ketoacidosis included patients with severely
depressed serum levels at presentation, so the conventional
wisdom that phosphate therapy is unnecessary should not be
extended to include the group with low serum phosphate
levels at diagnosis.
Severe hypophosphatemia may not be recognized unless
frequent serum phosphorus measurements are made. These
should be obtained every 2 hours during treatment of dia-
betic ketoacidosis in all patients whose serum phosphorus
level is less than 2.5 mg/dL at presentation. Patients with
hypophosphatemia should be given supplemental phospho-
rus in the form of potassium phosphate.
Potassium phosphate can be added to the fluid replacement
solution, alternating with potassium chloride. Hypopho-
sphatemic patients generally will require 500–1000 mg
elemental phosphorus given over 12–24 hours depending on
the severity of phosphorus depletion. This is equivalent to
about 15–30 mmol of phosphate, or a total of 5–10 mL of
potassium phosphate solution (3 mmol/mL) to be added to
the fluid replacement solutions (1–4 mL potassium phos-
phate solution per liter of replacement fluid). A concern
about phosphate administration in patients with diabetic
ketoacidosis is hypocalcemic tetany, which has been
described in some patients who receive phosphate therapy. It
should be kept in mind that calcium levels may fall during
the management of diabetic ketoacidosis. Small decreases in
serum calcium therefore may not be a contraindication to
continued phosphate repletion in patients who are
phosphorus-depleted, but they do require more frequent
monitoring of serum calcium to prevent hypocalcemia.
Patients with normal or high serum phosphorus levels
at diagnosis will have a decrease in serum phosphorus
during therapy, but only to levels that do not usually
require treatment.
Complications of Diabetic Ketoacidosis
A number of complications of diabetic ketoacidosis may
occur during treatment. Hypocalcemia, hypokalemia or
hyperkalemia, hypophosphatemia, and hypoglycemia need
to be watched for during continuous monitoring of patients
with diabetic ketoacidosis (Table 26–10). Another recognized
complication is thromboembolism that occurs as a result of
Table 26–9. Hazards of hypophosphatemia and
phosphorus repletion in diabetic ketoacidosis.
Hypophosphatemia
Decreased red cell 2,3-diphosphoglycerate with decreased
O
2
delivery
Muscle weakness
Hypophosphatemia syndrome ([P] <1.0 mg/dL) with respiratory
insufficiency, decreased myocardial contractility, rhabdomyolysis,
hemolysis
Phosphorus administration
Hypocalcemia and tetany
Complication Possible Mechanism
Hypokalemia Bicarbonate therapy, inadequate replacement,
insulin
Hyperkalemia Anuria, excessive replacement
Hypophosphatemia Insulin therapy (if [P] starts out
<1.5 mg/dL)
Hypoglycemia Inadequate glucose infusion with insulin
therapy
Other
Thromboembolism
Aspiration
ARDS
Cerebral edema
Mucormycosis
Increased platelet adhesiveness, hypervis-
cosity, poor perfusion
Gastric stasis, vomiting; lack of nasogastric
suction
Use of hypotonic replacement fluids or
excess crystalloid infusion or increased
pulmonary epithelial permeability
Excessive correction of hyperglycemia
Use of hypotonic replacement fluids(?)
Acidemia
Table 26–10. Complications of diabetic ketoacidosis.

CHAPTER 26
592
increased platelet adhesiveness and hyperviscosity.
Aspiration of gastric contents is associated with gastroparesis
and vomiting. Nasogastric suction should be initiated in all
patients who have altered consciousness to prevent this com-
plication. Cerebral edema and acute respiratory distress syn-
drome are other complications that will be discussed below.
One of the rare but significant conditions that should be
kept in mind in the management of diabetic ketoacidosis is
mucormycosis. This rare condition associated with ketoaci-
dosis is a treatable yet severe infection by the fungus
Rhizopus. Early diagnosis is essential so that therapy can be
instituted to prevent the severe morbidity associated with
this rapidly invasive infectious process. The hallmark of
mucormycosis is the finding of black necrotic debris in the
area of the eye, nose, or nasal cavity and histologic evidence
of vascular thrombosis or tissue infarction on biopsy. These
findings result from the propensity for invasion by
mucormycosis into the vascular system. This diagnosis is
considered to be a medical emergency that requires urgent
surgical and antifungal therapy.
Current Controversies and Unresolved Issues
A. Fluid Replacement—Fluid replacement remains a con-
troversial issue from two different points of view. The first is
volume replacement. A study comparing the effects of pro-
viding 3 L of volume replacement over the first 8-hour
period of treatment versus giving 6 L over the same period of
time showed very little difference in outcomes in the two
groups of patients. The conclusion is that smaller volumes of
fluid may be administered safely to some patients with dia-
betic ketoacidosis. It needs to be emphasized that patients
with severe volume deficits were excluded from this study,
but patients with moderate fluid deficits may do well with
slower replacement of their losses. One approach provides a
more flexible regimen for fluid replacement that recognizes
the unique requirements of each patient but consequently
requires careful monitoring. Fluid is replaced at a rate of
15–20 mL/kg per hour for 1 hour and then at 4–14 mL/kg
per hour thereafter according to need, as determined by care-
ful monitoring of fluid balance.
The type of fluid replacement also has been an issue of
controversy. Some investigators feel that colloid therapy is
better than crystalloid infusion in the management of dia-
betic ketoacidosis. Their concern is that insufficient fluid is
maintained in the intravascular space during therapy and
that much of the fluid, when given as crystalloid, finds its way
into the interstitial space, where edema is the result. It has
been suggested that the syndromes of cerebral edema and
pulmonary edema may be a consequence in part of the
sequestration of fluid outside the intravascular space.
However, no controlled studies of colloid infusion have been
performed. Colloid solutions should be considered in the
management of hypotension in patients who present with
diabetic ketoacidosis, but at this time, crystalloid infusion
remains the management of choice.
B. Cerebral Edema—This serious complication is fortu-
nately quite rare. Cerebral edema manifests as a deteriorating
level of consciousness at a time when other parameters of dia-
betic ketoacidosis are improving in response to treatment.
Clinical evidence for this complication generally appears
early in the course of treatment; almost two-thirds of
patients who develop neurologic deterioration begin to do
so within 12 hours of initiation of therapy. Cerebral edema
occurs mainly in children and young adults and is associ-
ated with a greater than 90% mortality. However, despite its
rarity, this complication has influenced one of the basic
aspects in our management of diabetic ketoacidosis because
of its catastrophic consequences. As mentioned earlier, it is
accepted practice to start glucose infusion to prevent serum
glucose concentrations from falling below 250–300 mg/dL.
This recommendation derives from studies of experimentally
induced cerebral edema in diabetic dogs that demonstrated
an association of cerebral edema with greater decrements in
plasma glucose concentrations during treatment.
In an observational study of children who developed cere-
bral edema with ketoacidosis, only treatment with bicarbon-
ate was associated with cerebral edema. Neither the initial
serum glucose concentration nor the rate of change in the
serum glucose concentration during therapy was associated
with the development of cerebral edema. Symptomatic cere-
bral edema has developed in a few children with diabetic
ketoacidosis before the initiation of therapy, suggesting that it
may not necessarily be caused by therapy. Children with dia-
betic ketoacidosis who have low partial pressures of arterial
carbon dioxide (relative risk 2.7 per decrease of 7.8 mm Hg)
and high serum urea nitrogen concentrations (relative risk 1.8
per increase of 9 mg/dL) at presentation were at increased risk
for cerebral edema. This supports the hypothesis that cerebral
edema in children with diabetic ketoacidosis is related to
decreased perfusion of the brain. Both hypocapnia, which
causes cerebral vasoconstriction, and extreme dehydration
would be expected to decrease brain perfusion. Furthermore,
because the developing brains of children are more sensitive
to hypoxia, this may explain the greater incidence of cerebral
edema in children than in adults.
Subclinical cerebral edema appears to be quite common
in patients with diabetic ketoacidosis because compression of
the subarachnoid and ventricular spaces during treatment
has been observed by CT scan without any obvious clinical
manifestations. The high frequency of subclinical edema has
raised questions about the rate and composition of fluid
replacement discussed earlier in this section. Mannitol is
used in therapy of symptomatic cerebral edema.
C. Acute Respiratory Distress Syndrome (ARDS)—
Another unusual but serious complication of ketoacidosis is
ARDS. Patients present with progressive dyspnea and hypox-
emia; the earliest sign appears to be an increased P(
A
–a)
O
2
,
and pulmonary edema is found on chest x-ray. ARDS in dia-
betic ketoacidosis tends to occur most often in patients
under 50 years of age, and the mortality rate associated with
DIABETES MELLITUS, HYPERGLYCEMIA, & THE CRITICALLY ILL PATIENT
593
this syndrome is extremely high. The pathogenesis of ARDS
in diabetic ketoacidosis is unknown, but it also has been sug-
gested that the syndrome may be associated with crystalloid
infusion and perhaps with excess volume replacement. It is
unlikely that either of these factors is the sole cause of ARDS
because it is too unusual a complication of diabetic ketoaci-
dosis to be simply fluid-dependent given the wide range of
replacement regimens used. However, it is possible that there
is an association with excessive fluid replacement in patients
who have some underlying pulmonary disease process asso-
ciated with increased pulmonary epithelial permeability.
Glaser NS et al: Mechanism of cerebral edema in children with dia-
betic ketoacidosis. J Pediatr 2004;145:164–71. [PMID: 15289761]
Kamalakannan D et al: Diabetic ketoacidosis in pregnancy.
Postgrad Med J 2003;79:454–7. [PMID: 12954957]
Kitabchi AE et al: Hyperglycemic crises in adult patients with
diabetes: A consensus statement from the American Diabetes
Association. Diabetes Care 2006;29:2739–48. [PMID:
17130218]
Linfoot P, Bergstrom C, Ipp E: Pathophysiology of ketoacidosis in
type 2 diabetes mellitus. Diabet Med 2005;22:1414–9. [PMID:
16176205]
Hyperglycemic Hyperosmolar Nonketotic
Coma
This disorder is probably not a distinct entity but rather a
variant of the diabetic ketoacidosis syndrome because a large
overlap exists in the clinical presentation and pathogenesis of
diabetic ketoacidosis and nonketotic coma. Management of
the two conditions is essentially identical. However, there are
clearly major differences in clinical presentation between
typical patients with nonketotic coma and diabetic ketoaci-
dosis, who probably represent opposite extremes of this
metabolic syndrome.
The patient with nonketotic coma typically is an elderly
person who presents with diabetes for the first time, is
severely dehydrated, is more often in coma, and has severe
associated diseases and a poor outcome. In contrast, the
common first presentation of diabetic ketoacidosis is in a
teenager who is otherwise healthy, not often in coma, and has
an excellent chance of recovery. Biochemically, the typical
patient with hyperglycemic hyperosmolar nonketotic coma
has more severe hyperglycemia (eg, serum glucose often >
1000 mg/dL) and hyperosmolarity, more pronounced elec-
trolyte abnormalities, and no ketosis. Between these two
extremes are a large number of patients in whom the clinical
syndromes overlap, so some degree of ketosis is often present
in a patient who otherwise would be classified as having typ-
ical nonketotic coma; on the other hand, hyperosmolarity
and severe hyperglycemia occur in what otherwise appears to
be typical diabetic ketoacidosis.
The pathogenesis of hyperglycemic hyperosmolar nonke-
totic coma is not well understood. The severity of the hyper-
osmolarity can be ascribed to a greater degree of volume
depletion. Indeed, severe volume depletion is the most
important common feature and can best be explained by the
degree of hyperglycemia. Figure 26–1 provides an explana-
tion for this severe hyperglycemia. Since volume depletion is
a characteristic feature, renal blood flow and the glomerular
filtration rate are reduced, thus diminishing the urinary
escape of glucose. However, the hormonal setting ensures a
continuing high rate of hepatic glucose production (see
above for further discussion of Figure 26–1), and this has the
capacity to rapidly increase blood glucose levels. If this
sequence of events is not halted, severe hyperglycemia and
hyperosmolarity ensue.
The other major feature of nonketotic coma—the rela-
tively low level of serum keto acids—also may play an impor-
tant role in the development of severe hyperglycemia.
Ketoacidosis makes the patients feel more ill and thus pre-
vents the syndrome from going undiagnosed for prolonged
periods. The lack of ketosis in hyperglycemic hyperosmolar
nonketotic coma may delay presentation, resulting in ongo-
ing osmotic diuresis that results in more severe volume
depletion. The mechanism for the lower levels of ketosis is
unexplained. The hormonal picture at presentation is not
helpful because concentrations of circulating counterregula-
tory hormones do not appear to be different from those seen
in diabetic ketoacidosis, nor do insulin levels seem to differ.
Nevertheless, it is thought that a restraining effect on lipoly-
sis by small increases of circulating insulin—and, as a result,
less availability of substrate for ketogenesis—may explain
low ketone concentrations in this syndrome. It also appears
that hyperosmolarity may itself play a role in diminishing
ketosis; this is based on in vitro studies in which hyperosmo-
larity was shown to suppress lipolysis.
Management is not different from that of diabetic
ketoacidosis. Plasma glucose concentrations should be
allowed to decrease at a rate of about 100 mg/dL per hour,
and fluid replacement should begin with normal saline as
discussed earlier. Early aggressive volume replacement is
important to prevent hypotension that may result from
fluid shifts that accompany insulin-mediated glucose
transport into the intracellular compartment. Many of
these patients are elderly, and monitoring fluid balance
with central venous catheters therefore is advised. Because
of the massive osmotic diuresis that precedes presentation
in these patients, electrolyte losses may be severe. It is par-
ticularly important to monitor serum potassium concen-
tration carefully during early management and in
subsequent days so as to provide potassium supplements if
they become necessary. The mortality rate is high, with
deaths often due to concurrent illnesses or thrombotic and
infectious complications.
Kitabchi AE et al: Hyperglycemic crises in adult patients with
diabetes: A consensus statement from the American Diabetes
Association. Diabetes Care 2006;29:2739–48. [PMID:
17130218]

CHAPTER 26
594
MANAGEMENT OF THE ACUTELY ILL PATIENT
WITH HYPERGLYCEMIA OR DIABETES MELLITUS
Management of patients in the ICU setting has undergone a
strategic change in a matter of a few years since the publica-
tion of a landmark work known as the Leuven study in 2001.
This study demonstrated unequivocally that management of
hyperglycemia is of fundamental importance in critically ill
patients and has changed the way in which hyperglycemia
and diabetes are managed in the ICU.
For many years it was thought that moderate control of
blood glucose in inpatients and the critically ill was appro-
priate care. This was a result of the prevailing interpretation
of the hyperglycemia of injury as a benign adaptive process
that contributes to survival. Mild or moderate hyperglycemia
is common in critically ill patients, even in those not previ-
ously known to have diabetes. Hyperglycemia is due to a
combination of physiologic changes including insulin resist-
ance and increased hepatic glucose production despite
increased secretion of insulin and was seen as necessary to
provide glucose as fuel during severe injury. The Leuven
study challenged these concepts after demonstrating that
tight glucose control (<110 mg/dL) improved mortality and
morbidity in critically ill patients and thereby changed not
only the practice of medicine in the ICU but also how we
think about glucose regulation in acute illness.
Hyperglycemia
Pathophysiology
The most frequent pathophysiologic abnormality of glucose
metabolism induced by acute illness is an increase in insulin
resistance. The molecular mechanisms are not well under-
stood, but a number of associated abnormalities contribute to
the development of insulin resistance. Increased secretion of
glucose counterregulatory hormones occurs in the acutely ill
patient. Increased concentrations of cortisol, catecholamines,
growth hormone, and glucagon lead to increased hepatic glu-
cose production by gluconeogenesis and glycogenolysis, as well
as insulin resistance at the level of muscle and adipose tissue.
Although insulin secretion increases in response to rising glu-
cose concentrations, it is insufficient to prevent hyperglycemia,
which is usually mild in nondiabetic patients. Catecholamines
may play a role in restraining an adequate insulin secretory
response by their action to suppress beta cell function.
The ability to continue to secrete increasing (although
inadequate) amounts of insulin in response to elevated serum
glucose is probably what prevents the development of ketoaci-
dosis in most patients with type 2 diabetes when acute illness
supervenes, although insufficient to prevent hyperglycemia.
The presence of even low levels of insulin may be sufficient to
inhibit lipolysis in adipose tissue and thereby suppress ketoge-
nesis in the liver. Indeed, it is unusual for patients with type 2
diabetes to develop diabetic ketoacidosis even though they
have the same pattern of glucose counter-regulatory hormone
release. If diabetic ketoacidosis occurs in type 2 diabetes, it is
due to more severe insulin deficiency than in equally ill type
2 patients without ketosis.
Clinical Benefits of Intensive Insulin Therapy
in the Critically Ill
A brief outline of the Leuven study will be provided to illustrate
the benefits of the use of insulin-mediated tight glycemic con-
trol in the ICU. Critically ill patients, almost all post–cardiac
surgery and most not previously known to have diabetes, were
studied with the intention of testing the role of maintaining
strict normoglycemia (<110 mg/dL) in the ICU. Those patients
randomized to the control group also were treated with insulin
if the blood glucose level exceeded 200 mg/dL, so average blood
glucose concentrations were 150–160 mg/dL, previously
thought to be adequate glycemic control in this setting.
Mortality was reduced dramatically in the intensive insulin ther-
apy group, especially among patients with prolonged critical ill-
ness in the ICU. In those requiring intensive care for more than
5 days, mortality was reduced from 20% to about 10%. Time in
the ICU was decreased significantly. Morbidity also was reduced
significantly, including prevention of severe complications such
as acute renal failure, nosocomial infections, liver dysfunction,
critical illness neuropathy, muscle weakness, and anemia. Other
recent reports confirm the findings in this landmark study,
including medical ICU patients, although less strikingly in this
population. Carefully monitored intravenous insulin titration
regimens are recommended to induce normoglycemia without
the risk of significant hypoglycemia.
This approach is also applied to patients with diabetes,
although in known diabetic patients hyperglycemia may be
more severe to begin with. Patients who have inadequate
insulin secretory reserve but who are not frankly diabetic are
at great risk for severe metabolic decompensation owing to
intercurrent disease. These patients are unable to respond to
increasing insulin needs, but unlike in previously diagnosed
diabetes, they may not necessarily recognize the symptom
complex associated with hyperglycemia. This can be exacer-
bated if large quantities of glucose are infused or ingested.
For example, in patients who ingest a large amount of
sucrose from sugar-containing soft drinks because of
extreme thirst induced by diuresis or diarrhea, ensuing
osmotic diuresis can make this a self-perpetuating disorder.
Similarly, peritoneal dialysis using solutions with high glu-
cose concentrations, used in an attempt to remove excess
extracellular fluid, may be associated with hyperglycemia and
even hyperosmolar diabetic coma.
An example of how control of diabetes influences the
management of another disorder is when diabetes insipidus
and diabetes mellitus coexist. In diabetes insipidus, large
volumes of free water are lost in the urine because of an
inability to concentrate urine. During treatment, equally
large quantities of electrolyte-poor solutions—usually 5%
dextrose—are infused to match urine output. If the urine
output is large, 6–10 L of 5% dextrose may be given within a
DIABETES MELLITUS, HYPERGLYCEMIA, & THE CRITICALLY ILL PATIENT
595
24-hour period, representing a load of 300–500 g of glucose
into the extracellular pool (compared with about 20 g in the
extracellular pool in a patient with normal serum glucose
concentration; see Figure 26–1). Nondiabetic subjects handle
this glucose load by increasing the secretion of insulin.
However, in patients who have underlying or overt diabetes
and a poor insulin secretory response, serum glucose
increases, and glycosuria and an osmotic diuresis develop
unresponsive to the effect of vasopressin. Continuing urine
volume losses result in the administration of even larger vol-
umes of dextrose, perpetuating a vicious circle. Proper man-
agement consists of preventing glycosuria by tightly
controlling blood glucose concentration. This can be achieved
using an intravenous insulin infusion in the ICU while simul-
taneously treating the diabetes insipidus with vasopressin.
Hypoglycemia
Some diabetic patients appear to have decreased insulin
requirements or a decreasing need for oral hypoglycemic
agents when acute illness supervenes. A decrease in insulin
requirements may result from a number of different
mechanisms.
Pathophysiology
A. Decreased Insulin Resistance—Weight loss may occur
prior to or during the acute illness. It is well known that
weight loss reduces insulin resistance in diabetes mellitus,
and small decreases in weight often are sufficient to produce
a substantially decreased requirement for insulin or other
hypoglycemic agents. Another cause of decreased insulin
resistance is a deficiency of one of the glucose counterregu-
latory hormones. Adrenal or pituitary insufficiency may
occur as part of an intercurrent disease process. Decreased
growth hormone or cortisol can diminish the need for hypo-
glycemic agents by enhancing peripheral insulin sensitivity
and decreasing gluconeogenesis.
B. Decreased Clearance of Insulin or Oral Hypoglycemic
Agents—Because the liver and the kidneys are the primary
organs involved in metabolism of insulin and the sulfony-
lurea drugs, development of renal or hepatic failure may
delay drug clearance and result in hypoglycemia. In the case
of the sulfonylureas, this may be associated with severe, pro-
longed hypoglycemia and coma. Concomitant use of other
drugs also may influence the metabolism of sulfonylurea
agents (Table 26–11). A relatively common cause of unex-
pected hypoglycemia occurs during acute episodes of con-
gestive heart failure in insulin-treated patients. As a result of
liver congestion and possibly decreased insulin clearance
with reduced hepatic blood flow, insulin requirements are
transiently diminished. If the insulin dose is not reduced,
hypoglycemia may occur.
C. Drug Interactions—Drug interactions are of particular
importance with the first generation of oral hypoglycemic
agents that are tightly protein-bound and may be displaced
by other protein-bound drugs. Table 26–11 summarizes pos-
sible interactions between drugs used in management of dia-
betes and therapeutic agents that may affect glucose control.
Because multiple drugs are used often in acutely ill patients,
particularly the elderly, the possibility of drug interactions
giving rise to hypoglycemia always needs to be considered.
Clinical Features
Hypoglycemia in hospitalized patients occurs most com-
monly in patients with diabetes, and this is usually due to a
decrease in caloric intake related to illness or hospital routine
with continued administration of hypoglycemic drugs. Some
of the causes of hypoglycemia observed in hospitalized
patients with diabetes are presented in Table 26–12.
Concomitant diseases play an important role in increasing
the risk of hypoglycemia in patients with diabetes, for exam-
ple, renal insufficiency, malnutrition, and sepsis—three dis-
orders known to be associated with hypoglycemia even in
nondiabetic hospitalized patients—as well as alcohol inges-
tion, liver disease, shock, pregnancy, and malignancy. A high
mortality rate has been reported in nondiabetic critically ill
patients who develop hypoglycemia, and in some, this may
represent a terminal phenomenon associated with malnutri-
tion or kidney and liver disease.
Pentamidine, used for treatment for Pneumocystis carinii
infection, is associated with hypoglycemia probably owing to
destruction of pancreatic beta cells with acute insulin release.
Treatment
Several factors determine appropriate management for
patients with diabetes mellitus and acute illness to avoid the
consequences of hyperglycemia and hypoglycemia.
Interaction Drug
Displacement of sulfonylureas
from plasma proteins
Clofibrate, phenylbutazone,
salicylates, sulfonamides
Reduced hepatic sulfonylurea
metabolism
Dicumarol, chloramphenicol,
monoamine oxidase inhibitors,
phenylbutazone
Decreased urinary excretion of
sulfonylureas or metabolites
Allopurinol, probenecid, phenylbu-
tazone, salicylates, sulfonamides
Intrinsic hypoglycemic activity Insulin, alcohol, beta-adrenergic
agonists, salicylates, guanethidine,
monoamine oxidase inhibitors
Table 26–11. Pharmacokinetic and pharmacodynamic
interactions that augment the hypoglycemic actions of
sulfonylurea drugs.

CHAPTER 26
596
A. Goal for Serum Glucose—Based on the data of the
Leuven study, target glucose concentrations in acutely ill
patients have been changed to attempt to achieve normo-
glycemia, defined as concentrations of less than 110 mg/dL.
In medical patients, a target of 130–140 mg/dL may be more
appropriate. This is best achieved using continuous intra-
venous insulin infusion with a titration algorithm. Once
patients are out of the critical care setting, other regimens
may be appropriate.
B. Fasting Patients—Patients who for any reason are not
being fed do not need bolus injections of rapid-acting insulin
to maintain glucose homeostasis postprandially; a continu-
ous intravenous insulin infusion is more appropriate.
Intravenous insulin is given routinely if a patient is receiving
total parenteral nutrition because of the large load of calories
given to patients during this therapy. However, even in the
absence of an exogenous source of glucose, such as total par-
enteral nutrition, it is important to recognize that hyper-
glycemia in patients with diabetes is the result of a
continuous delivery of large amounts of glucose from an
endogenous source, that is, the liver. It is logical, therefore,
that serum glucose concentration be controlled by continu-
ous levels of insulin, and this can be done with intravenous
insulin infusion. The response should be evaluated by moni-
toring blood glucose level at the bedside at 2–3-hour intervals.
An alternative to the use of intravenous infusion of insulin for
patients who are no longer critical is the use of long-acting sub-
cutaneous insulin (eg, glargine insulin or insulin-detemir).
These insulins analogues provide the equivalent of a basal
infusion rate, but because they do not provide the possibility
of hour-to-hour titration, they are unsuitable for anyone but
very stable patients, in whom day-to-day titration of insulin
is often sufficient.
C. Patients with Intermittent Food Intake—Patients who
are acutely ill, are vomiting, or have decreased food intake
can be managed somewhat differently. Under these circum-
stances, longer-acting insulins may be inappropriate because
it is difficult to predict insulin needs for more than a few
hours in advance. Thus, even if NPH insulin were adminis-
tered in an appropriate dose, inability to eat a meal hours
later could put the patient at risk for hypoglycemia. Rapid-
acting insulin given before each meal and at midnight is
preferable.
Rapid-acting insulin can be given to patients four times a
day using a variable insulin dosage (2–10 units) based on the
bedside blood glucose determination (sliding scale). Glucose
is measured preprandially, immediately before each insulin
dose is to be administered, and at midnight. This provides
insulin coverage for the expected glucose excursion with each
meal, and the dose can be reduced if the patient is not going
to eat the next meal. As soon as the patient begins to stabilize
and is able to eat consistently, an attempt should be made to
convert the patient to morning and evening split-dose
insulin injections using a combination of NPH and regular
insulin or multiple-dose insulin using a combination of pre-
meal rapid-acting analogues and a basal insulin (eg, glargine
or detemir).
D. Patients Eating Regularly—Patients who are eating
meals or receiving feeding in bolus fashion in reasonably
consistent manner can be treated with a split-dose regimen
of NPH and rapid-acting insulin. Alternatively, basal insulin
(eg, glargine or detimir) with premeal rapid-acting ana-
logues can be used. If necessary, a sliding scale of supplemen-
tary preprandial rapid-acting insulin doses can be added to
provide good glycemic control. There is no need for pro-
longed use of sliding scales in this situation because with the
sliding-scale approach the doses of insulin are variable from
day to day, and more stable patients will do better if managed
with a more constant regimen.
Once patients have stabilized, in addition to preprandial
glucose measurements, which are the basis for the sliding
scale, blood glucose measurements also should be performed
2 hours after meals to evaluate whether the dose of rapid-
acting insulin given prior to those meals is appropriate for
adequate glucose control. These 2-hour postprandial glucose
measurements are most useful for determining the required
dose of rapid-acting insulin. Rapid-acting insulin dosages
can be increased or decreased depending on the change in
glucose concentration from that obtained immediately prior
to the meal (keeping in mind that there is a normal rise in
blood glucose levels of about 40–50 mg/dL after a meal).
Table 26–12. Proximate causes of hypoglycemia in 42
diabetic patients receiving insulin (60 episodes) or oral
hypoglycemia drugs (four episodes).
Cause % Episodes
Decreased intake of calories
Nausea, vomiting, anorexia, lethargy
NPO because of diagnostic test or surgical
procedures
Enteral tube feedings held for measurement
of residual
Meals not delivered
Adjustment of insulin dosage
Treatment of diabetic ketoacidosis or nonketotic
coma
Attempt at tighter metabolic control
Sliding scale insulin doses too high for patient with
renal insufficiency
Failure to reduce insulin dose after one hypoglycemia
episode
Less insulin needed as infection resolved
Incorrect dose of insulin given
No cause identified
45
23
14
5
3
39
17
9
6
5
2
8
8
