Bongard Frederic , Darryl Sue. Diagnosis and Treatment Critical Care
Подождите немного. Документ загружается.

ENDOCRINE PROBLEMS IN THE CRITICALLY ILL PATIENT
577
patterns: low T
3
,low T
3
and T
4
, and low thyroid-stimulating
hormone (TSH).
A. Low T
3
—This is the most common presentation. In the
early stages of nonthyroidal illness, serum T
3
(bound and
free) decreases, and reverse T
3
(rT
3
) is increased. T
4
and TSH
levels are within the normal range. The low T
3
state results in
part from decreased conversion of T
4
to T
3
because of the
inhibition of peripheral tissue 5′-monodeiodinase activity
and reduced T
3
production. Contrary to earlier belief that
the elevated rT
3
resulted from increased conversion of T
4
to
rT
3
, the elevated rT
3
results from decreased rT
3
clearance sec-
ondary to decreased 5′-monodeiodinase activity. Circulating
T
3
levels can fall below normal within 24 hours of onset of
any systemic illness, major trauma, surgery, or caloric depri-
vation, and T
3
concentrations generally become normal as
the underlying illness resolves. It has been postulated that
decreased T
3
may be a protective mechanism during acute ill-
ness because it is associated with decreased urine urea nitro-
gen excretion and decreased protein breakdown. Tissue T
3
levels
fall proportionate to serum levels.
In the early recovery phases from illness, there may be a
transient increase in serum TSH concentrations to levels seen
in patients with primary hypothyroidism. Although these
levels rarely exceed 20 μU/L, there have been several case
reports of sick euthyroid patients having TSH values
exceeding this commonly used cutoff value. This elevation
of TSH has been proposed to stimulate the thyroid gland to
increase its secretion of T
4
. Nonetheless, a TSH level greater
than 20 μU/mL is suggestive of primary hypothyroidism.
In one study of unselected ICU patients, 44% had low
free T
3
levels on admission, indicating nonthyroidal ill-
ness, 24% of these had normal TSH and 21% had low TSH
levels.
B. Low T
3
and T
4
—Free T
4
(FT
4
) is almost always normal
early in the course of nonthyroidal illness. In more severe ill-
ness of longer duration, the decreased serum T
3
levels are
accompanied by a reduction in serum T
4
. This portends a
poor prognosis: The greater the reduction in T
4
, the worse
is the outcome. This condition may occur in 25–50% of
medical patients admitted to an ICU. In patients with T
4
con-
centrations less than 3 μg/dL, the mortality rate may reach
80%. The fall in FT
4
to subnormal levels is multifactorial;
decreased TSH resulting in decreased T
4
secretion from the
thyroid, alterations in T
4
binding to plasma proteins, and
alterations in binding protein concentrations all contribute
to low T
4
concentrations. Despite the finding of very low T
4
,
these patients are not considered as having hypothyroidism,
and replacement of thyroid hormone does not improve
outcome.
C. Low TSH—Low T
3
or low T
3
and T
4
are also seen in
hypothyroidism, but a euthyroid state in these patients with
nonthyroidal illness is suggested by the clinical appearance, the
normal TSH concentration, and the normal free T
4
level by
equilibrium dialysis. Also, the TSH response may be blunted
to thyrotropin-releasing hormone (TRH) stimulation.
Not uncommonly, low TSH values (<0.1 μU/mL) rather
than normal TSH values are encountered in euthyroid hospi-
talized patients, although most patients will have only mar-
ginally depressed values of more than 0.1 μU/mL. The
availability of more sensitive third-generation TSH assays
has made it possible to distinguish between marginally
depressed TSH concentrations in euthyroid patients and the
highly suppressed levels seen with hyperthyroidism (<0.01
μU/mL). If necessary, a TRH stimulation test can be used to
confirm the result. Euthyroid patients with nonthyroidal ill-
ness and depressed TSH will show detectable responses of
TSH (>0.1 μU/mL) to TRH stimulation, whereas hyperthy-
roid patients will show the expected absence of response to
TRH stimulation.
Diagnosis
A. Laboratory Findings—When assessing thyroid dysfunc-
tion in the critically ill, perhaps the best initial screening tests
are total T
4
,T
3
resin uptake, T
3
, and sensitive TSH levels. Low
T
3
is always seen in sick euthyroid syndrome and can fall
from normal values within 24 hours of onset of acute illness.
The rT
3
level may be increased. The euthyroid state is con-
firmed if total T
4
and T
3
resin uptake are normal or if free T
4
by equilibrium dialysis is normal, along with normal TSH
values.
As described earlier, a more difficult situation arises when
both T
3
and total T
4
are reduced in more severe or prolonged
illness. Free T
4
by equilibrium dialysis is often normal but
may be misleadingly low despite the patient being clinically
euthyroid. A normal or low TSH generally confirms the find-
ing of a euthyroid state, whereas TSH greater than 20 μU/L
makes hypothyroidism a strong possibility. If there is any
doubt about the TSH response, then evaluation of pituitary
function may be helpful.
B. Disorders Associated with Altered Thyroid Function
Tests—A number of conditions can produce alterations in
thyroid function tests suggesting thyroid hormone deficiency
(Table 25–5).
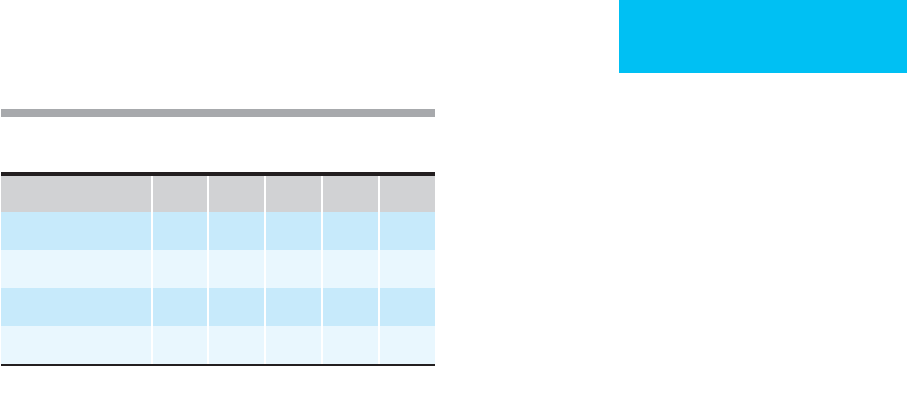
Table 25–4. Profile of thyroid hormone indices during
different phases of acute illness.
Phases of Illness T
3
T
4
FT
4
rT
3
TSH
Mild, early D N N I N
Moderate D N, D N I N
Severe D D D I N, D
Early recovery D D, N D, N I N, I
D = decreased; I = increased; N = normal.
CHAPTER 25
578
1. Malnutrition or caloric deprivation—Caloric dep-
rivation such as that seen during fasting can produce a signif-
icant fall in serum T
3
concentrations and a rise in serum rT
3
within 24 hours. Hypocaloric diets containing as few as 600
kcal/day can produce these same changes. T
4
concentrations
are usually normal, but the TSH response to TRH is blunted.
Free T
4
may rise transiently and then stabilize. It has been
suggested that caloric deprivation causes the starving body to
conserve energy by reducing the amount of metabolically
active T
3
. Administering T
3
to starving subjects induces
greater muscle catabolism. Refeeding with as little as 50 g
carbohydrate (200 kcal) normalizes serum T
3
and rT
3
.
However, the TSH response to TRH may remain reduced,
suggesting a difference in recovery time between peripheral
5′-monodeiodination and pituitary responsiveness.
2. Chronic liver disease—Liver disease may have pro-
found effects on thyroid function. The liver is the main organ
for thyroid hormone metabolism and the major site for
extrathyroidal (peripheral) conversion of T
4
to T
3
. Serum T
3
levels are low, rT
3
is elevated, and TSH is normal in patients
with liver disease. The serum T
4
is usually normal, although
low levels are found in the most severely ill. Very low T
3
pre-
dicts a poor outcome.
3. Renal disease—Patients with chronic renal failure tend to
have multiple factors that may affect thyroid function tests.
These include poor nutrition, metabolic disturbances, medica-
tions, and hemodialysis. A reduced serum T
3
is found in many
patients, and T
3
does not increase with thyroxine replacement.
However, rT
3
is normal rather than elevated because of
increased uptake in tissues. Secondary hyperparathyroidism,
which often accompanies renal failure, also may be partly
responsible for this pattern of thyroid function tests because a
low T
3
and normal rT
3
also are seen in states of elevated parathy-
roid hormone. Free T
4
may be elevated transiently during
hemodialysis, probably representing a heparin effect. TSH levels
are usually normal. Extensive metabolic studies of patients with
renal failure and low serum T
3
concentrations indicate that they
are euthyroid.
Patients with nephrotic syndrome may lose significant
amounts of thyroxine-binding globulin (TBG) from urinary
protein losses, resulting in decreased serum T
4
levels. The T
3
resin uptake is increased in proportion to the lowered TBG
levels, and the free T
4
index is usually normal. A number of
other conditions are associated with increased and decreased
TBG levels (Table 25–6).
4. Diabetes mellitus—Diabetes and thyroid disorders
are linked at several levels. The association of autoimmune
thyroid disease with type 1 diabetes mellitus as part of the
autoimmune polyendocrinopathy syndrome is well recog-
nized. Diabetic ketoacidosis produces a similar pattern of
altered thyroid function tests seen in other severe ill-
nesses. With treatment, most patients normalize thyroid
function tests within a few days. Insulin deficiency mim-
ics a fasting state because carbohydrate is not used prop-
erly. Poorly controlled diabetes causes a marked reduction
in conversion of T
4
to T
3
;T
3
levels increase with improved
glycemic control.
5. Infection—Infection also produces changes in thyroid
hormone parameters similar to those seen in sick euthyroid
patients. These alterations are corrected by successful treat-
ment of the infection. Because malnutrition often accompa-
nies severe infection, it is thought to play a role in the
changes observed. Fever alone also may be a factor in altering
thyroid function tests. A negative correlation between tem-
perature and serum T
3
has been observed.
6. Acute myocardial infarction—A consistent pattern of
thyroid function abnormalities has been observed in acute
myocardial infarction. Serum T
3
concentrations fall after an
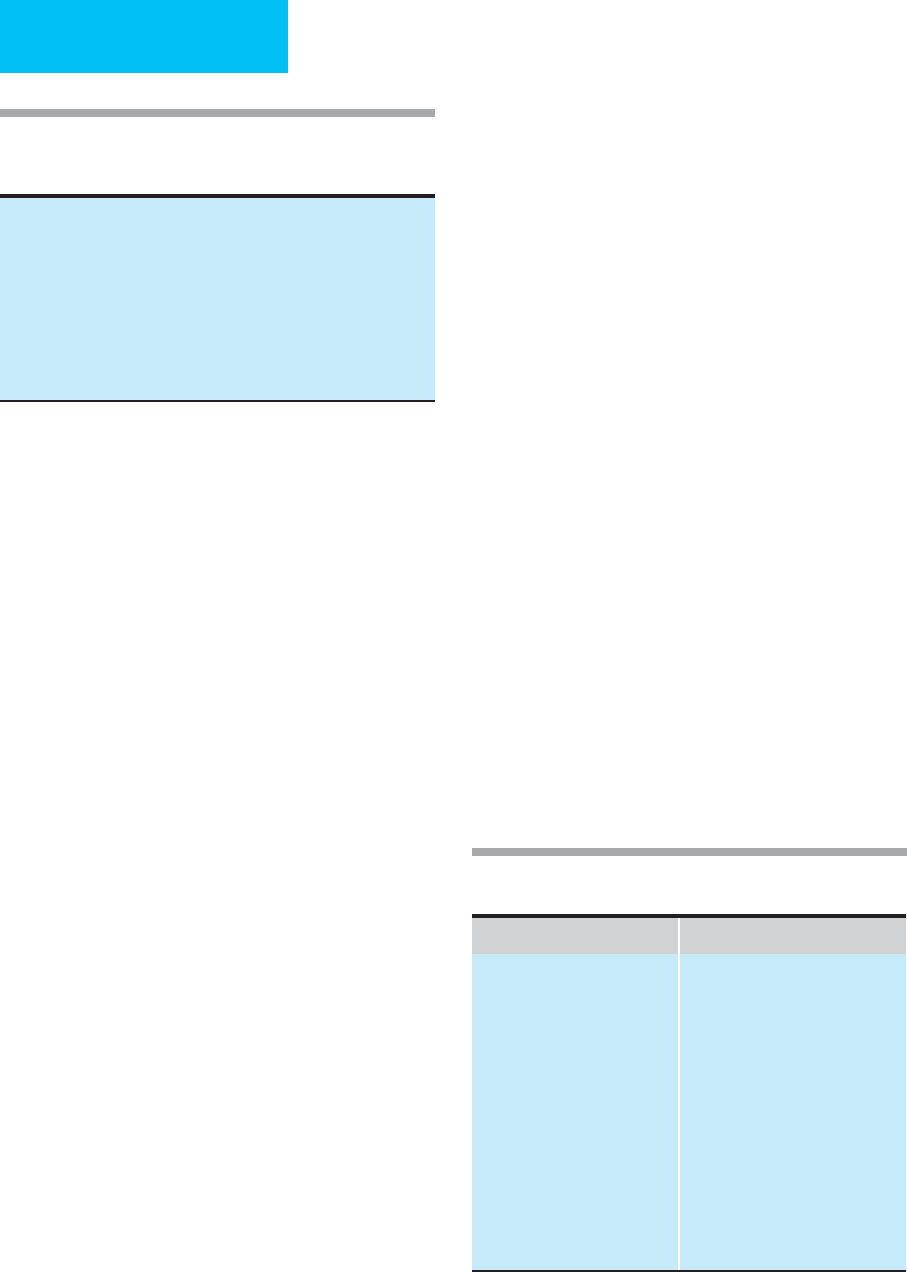
Table 25–5. Conditions that produce thyroid function
test patterns suggesting thyroid hormone deficiency
(sick euthyroid syndrome).
Malnutrition, caloric deprivation
Chronic liver disease
Renal disease
Diabetes mellitus
Infection
Acute myocardial infarction
Cancer
Surgery
Medications
AIDS
Increased TBG Decreased TBG
Physiologic conditions
Pregnancy
Newborns
Nonthyroidal illness
Acute hepatitis
Chronic liver disease
Acute intermittent porphyria
Hydatidiform mole
Lymphosarcoma
Estrogen-producing tumor
Drugs
Estrogens
Heroin and methadone
Clofibrate
Fluorouracil
Familial disorders
Nonthyroidal illness
Nephrotic syndrome
Chronic liver disease, Cirrhosis
Acromegaly
Cushing’s syndrome
Drugs
Androgens
Anabolic steroids
Glucocorticoids
Familial disorders
Table 25–6. Conditions associated with altered thyroxine
binding globulin (TBG) concentrations.

ENDOCRINE PROBLEMS IN THE CRITICALLY ILL PATIENT
579
acute myocardial infarction, reaching a nadir at 1–3 days with
a reciprocal increase in rT
3
. Serum TSH levels increase at day
4–5, followed by a rise in T
4
. The severity and size of the
infarct are correlated with the degree of fall in T
3
and increase
in rT
3
. The same pattern is seen with unstable angina. Low T
3
is a powerful prognostic marker of death in all forms of car-
diac disease, including congestive heart failure.
7. Cancer—Most cancer patients show a pattern of thyroid
studies similar to other nonthyroidal illness. Malnutrition
and cachexia are contributing factors. Some antitumor
chemotherapeutic agents such as fluorouracil and asparagi-
nase alter TBG levels.
8. Surgery—Elective and emergency surgeries typically
cause significant changes in thyroid hormone levels. Serum
T
3
concentrations fall during surgery and may take up to a
week to recover. This is accompanied by a reciprocal rise in
rT
3
. Serum T
4
levels usually remain stable unless there is a
prolonged recovery period. TSH may be unchanged or
reduced intraoperatively but returns to normal within a cou-
ple of days.
9. Medications—The patient in the ICU is commonly tak-
ing multiple medications, some of which may have profound
effects on thyroid function parameters. Some common drugs
and their effects are listed in Table 25–7. Patients in the ICU
commonly receive dopamine for blood pressure support or
high-dose corticosteroids for various reasons. These drugs
suppress TSH secretion, but not usually to the levels seen in
hyperthyroidism. However, in patients with primary
hypothyroidism, dopamine may suppress an elevated TSH
into the normal range, confounding diagnosis.
Other drugs likely to be encountered in the ICU that may
confuse interpretation of thyroid function tests include
octreotide, amiodarone, and β-adrenergic antagonists.
Octreotide decreases TSH secretion at high doses.
Amiodarone, an antiarrhythmic drug containing iodide, may
induce hypothyroidism or hyperthyroidism but more com-
monly results in low serum T
3
and normal or high T
4
.
Amiodarone inhibits 5′-deiodinase. Large doses of propra-
nolol, atenolol, and metoprolol decrease serum T
3
levels but
do not result in hypothyroidism.
10. AIDS—In patients with AIDS, direct infection of the thy-
roid gland by opportunistic organisms such as
cytomegalovirus, Cryptococcus, and Pneumocystis jerovici may
occur rarely in addition to infiltration by Kaposi’s sarcoma.
Patients with P. j er ov i c i thyroiditis may have hypo- or hyper-
thyroidism depending on the degree of involvement and dis-
ruption of the gland. Cytomegalovirus thyroiditis usually is
associated with sick euthyroid syndrome rather than
hypothyroidism. Some of the medications used for treating
patients with AIDS may alter thyroid function. For example,
rifampin increases T
4
clearance by hepatic microsomal
enzyme induction. Hypothyroidism has been reported after
ketoconazole treatment. One would expect to see a sick
euthyroid pattern with AIDS, but this occurs infrequently
and usually at later stages of HIV infection owing to
decreased extrathyroidal conversion to T
3
.
A unique pattern of thyroid function tests in AIDS has
been observed that includes a progressive elevation in TBG,
decreased rT
3
, and normal T
3
levels. These alterations are felt
to be part of the abnormal immunoregulation in HIV-
infected individuals and may be mediated by tumor necrosis
factor or other cytokines. The normal T
3
level is felt to be a
failure of the normal adaptive response to illness, but
whether this causes the cachexia associated with AIDS
remains to be proved. The prevalence of hypothyroidism,
both clinical and subclinical, in AIDS is higher than in the
general population and has been correlated with CD
4
cell
count.
Treatment
It is important to distinguish the sick euthyroid state from
intrinsic thyroid disease because the former does not require
thyroid hormone replacement therapy. Studies show that
treating patients with the low T
3
-T
4
syndromes with T
4
was
not beneficial and had no effect on mortality rates. In fact,
there was no increase in T
3
levels, suggesting that peripheral
conversion was not enhanced. To exclude inhibition of
peripheral conversion as a factor in nonthyroidal illness,
TSH suppression
Dopamine
Glucocorticoids
Bromocriptine
Apomorphine
Pyridoxine
Octreotide
Impaired thyroid hormone production or secretion
Thionamides (propylthiouracil, methimazole, carbimazole)
Lithium
Iodide
Amiodarone
Impaired T
4
to T
3
conversion
Propylthiouracil
Glucocorticoids
Propranolol
Ipodate sodium
Iopanoic acid
Amiodarone
Increased hepatic uptake and metabolism of T
4
Phenobarbital
Phenytoin
Carbamazepine
Rifampin
Impaired protein binding
Salicylates
Phenytoin
Table 25–7. Effect of drugs on thyroid function.
CHAPTER 25
580
some investigators have administered T
3
, but once again
these studies have not demonstrated a beneficial effect on
outcome. Supportive measures such as adequate nutrition
and specific and successful treatment of the underlying ill-
ness should result in eventual normalization of the thyroid
function alterations.
Current Controversies and Unresolved Issues
Two major issues related to sick euthyroid syndrome are the
subject of some controversy. First, the pathogenesis of the
syndrome remains unclear. The only thing that appears cer-
tain is that the mechanisms are complex, multifactorial, and
involve changes at multiple levels of the thyroid loop,
including changes in TSH and thyroid hormone secretion,
5′-deiodinase, and thyroid hormone binding to proteins
owing to a variety of inhibitors.
Second, the physiologic significance of these changes in the
thyroid function tests remains unclear. We do not know
whether these abnormalities signal a functionally hypothyroid
state or are part of the body’s adaptation to the stress of acute
illness. The answer to this question will determine whether
these patients should receive thyroid hormone replacement
therapy. Unfortunately, there are no clinically practical mark-
ers that reflect the biologic action of thyroid hormones in tis-
sues rather than their levels in the blood. Several studies in
small numbers of patients have failed to reveal any benefit of
thyroid hormone replacement therapy. On the contrary, in
patients with burns, T
3
replacement increased urinary nitro-
gen excretion. Furthermore, thyroid hormone replacement
therapy may inhibit TSH secretion and thereby delay recovery
of thyroid function as the acute underlying illness abates. Only
a large prospective study randomizing administration of thy-
roid hormone can answer this question.
Low T
3
levels are found in the vast majority of heart
donors, in potential heart transplant recipients, and in
patients who have undergone cardiopulmonary bypass.
Because cardiac dysfunction is a major problem after
transplantation, T
3
supplementation has been proposed for
both donor and recipient. There are limited data demon-
strating that T
3
supplementation in brain-dead donors
decreases both the amount and duration of inotropic
support. Recently, combined administration of growth
hormone–releasing peptide 2 (GHRP-2), TRH, and
gonadotropin-releasing hormone (GnRH) was shown to
reactivate the growth hormone, TSH, and luteinizing hor-
mone (LH) axes in men with prolonged critical illness.
Combined administration of these secretagogues evoked
beneficial metabolic effects, including reduction in urea pro-
duction and an increase in osteocalcin levels, which were not
observed with GHRP-2 infusion alone. The clinical efficacy
of such an approach should be further tested.
Chinga-Alayo E et al: Thyroid hormone levels improve the predic-
tion of mortality among patients admitted to the intensive care
unit. Intensive Care Med 2005;31:1356–61. [PMID: 16012806]
Goldberg PA, Inzucchi SE: Critical issues in endocrinology. Clin
Chest Med 2003;24:583–606. [PMID: 14710692]
Iervasi G et al: Low-T
3
syndrome: A strong prognostic predictor of
death in patients with heart disease. Circulation
2003;107:708–13. [PMID: 12578873]
Peeters RP et al: Changes within the thyroid axis during critical ill-
ness. Crit Care Clin 2006;22:41–55. [PMID: 16399019]
Peeters RP et al: Serum 3,3′,5′-triiodothyronine (rT
3
) and 3,5,3′-
triiodothyronine/rT
3
are prognostic markers in critically ill
patients and are associated with postmortem tissue deiodinase
activities. J Clin Endocrinol Metab 2005;90:4559–65. [PMID:
15886232]
Plikat K et al: Frequency and outcome of patients with nonthy-
roidal illness syndrome in a medical intensive care unit.
Metabolism 2007;56:239–44. [PMID: 17224339]
Van den Berghe G, Baxter RC, Weekers F: The combined admin-
istration of GH-releasing peptide 2 (GHRP-2), TRH, and
GnRH to men with prolonged critical illness evokes superior
endocrine and metabolic effects compared to treatment with
GHRP-2 alone. Clin Endocrinol (Oxf) 2002;56:655–69. [PMID:
12030918]
581
26
Diabetes Mellitus,
Hyperglycemia, & the
Critically Ill Patient
∗
Eli Ipp, MD
Chuck Huang, MD
Patients with diabetes mellitus are seen frequently in the ICU
because of complications of poorly controlled disease,
including diabetic ketoacidosis, hyperglycemic hyperosmolar
nonketotic diabetic coma, and hypoglycemia. In addition,
diabetic patients with critical illness often will exhibit insta-
bility and poor control of blood glucose, including hyper-
and hypoglycemia. Recent studies have demonstrated that
hyperglycemia in nondiabetic patients in the ICU also can
have a significant impact on morbidity and mortality. This
chapter also will cover new approaches to management of
hyperglycemia in the ICU.
Diabetic Ketoacidosis
ESSENTIALS OF DIAGNOSIS
Acute illness in a patient with known type 1 (insulin-
dependent) diabetes mellitus, especially if the patient
is vomiting.
Evidence of precipitating illness, including infection.
Clinical symptoms and signs of volume depletion.
Clinical features of metabolic acidosis.
Laboratory features: hyperglycemia, anion gap acidosis,
ketonemia, and acidemia.
General Considerations
Diabetic ketoacidosis is the most serious metabolic compli-
cation of type 1 (insulin-dependent) and, to a smaller
extent, type 2 (non-insulin-dependent) diabetes mellitus.
There has been little change in the mortality rate associated
with diabetic ketoacidosis in recent decades despite great
improvements in our understanding of its pathophysiology
and treatment. The most effective means of reducing deaths
owing to diabetic ketoacidosis consists of teaching patients to
recognize its early signs. Close clinical and biochemical obser-
vation of every patient during treatment of diabetic ketoaci-
dosis remains the cornerstone of effective management.
A. Pathophysiology—The pathophysiology of diabetic
ketoacidosis is based primarily on an abnormal hormonal
setting: insulin deficiency combined with an excess of hor-
mones that increase the blood glucose level. This situation is
similar to the physiologic state seen during normal fasting
and is probably best considered as an abnormal and extreme
expression of the fasting state. During the fed state, insulin is
the predominant hormone, required for disposal and storage
of ingested nutrients. During fasting, the body converts to a
state in which endogenous sources of fuels need to be tapped
for ongoing support of metabolism in the brain and in mus-
cle tissue, and the hormonal milieu therefore begins to
change. Plasma insulin concentrations fall, and glucagon
concentrations rise. This change in the insulin:glucagon ratio
permits the liver to become the major source for glucose dur-
ing fasting. At the same time, decreased insulin concentra-
tions lead to lipolysis in fat depots, providing a source of free
fatty acids as a fuel for muscle and thus sparing glucose for
use by the brain. Furthermore, free fatty acids are converted
by the liver to ketones under the influence of glucagon.
Ketones constitute another alternative (nonglucose) energy
source for brain and muscle tissues. Glycerol released by
lipolysis and alanine from protein catabolism in muscle pro-
vide substrates for gluconeogenesis in the liver.
In diabetic ketoacidosis, this picture is greatly exaggerated
because of severe insulin deficiency. This is typical of patients
with type 1 diabetes, all of whom have sustained autoim-
mune beta cell destruction. However, insulin deficiency is
now also recognized as the major contributor to ketoacidosis
in type 2 diabetes, an increasingly reported entity, once
thought to be exceedingly rare. The effectiveness of circulat-
ing insulin is also reduced because patients with diabetic
∗
Eli Ipp, MD, and Tricia L. Westhoff, MD, were the authors of this
chapter in the second edition.
Copyright © 2008 by The McGraw-Hill Companies, Inc. Click here for terms of use.
CHAPTER 26
582
ketoacidosis also have considerable insulin resistance.
Historically, this resistance to insulin action was thought to
require massive doses of insulin during treatment of diabetic
ketoacidosis. Although since the 1970s “low dose” continu-
ous insulin infusion has replaced the large intermittent doses
used previously, a high level of resistance to insulin action
remains an important feature of diabetic ketoacidosis. Some
of the known causes of insulin resistance in diabetic ketoaci-
dosis are listed in Table 26–1.
In contrast to the low levels of insulin, glucagon concen-
tration is markedly elevated, with a high glucagon:insulin
ratio more striking than that seen during fasting. In addition,
diabetic ketoacidosis is characterized by large increases in the
levels of stress hormones, including the glucose counterreg-
ulatory hormones cortisol, growth hormone, and cate-
cholamines. These hormones help to define diabetic
ketoacidosis and are responsible for its two major features:
hyperglycemia and ketonemia. Glucagon has its predomi-
nant effects on the liver, enhancing long-chain fatty acid
transport into mitochondria by decreasing levels of malonyl-
CoA and increasing activity of carnitine acyltransferase I.
Cortisol enhances gluconeogenesis by increasing delivery of
gluconeogenic substrates to and transamination in the liver.
Prolonged hypersecretion of cortisol also decreases sensitiv-
ity to insulin. Catecholamines enhance lipolysis, providing
substrate for ketogenesis, and accelerate glycogenolysis and
gluconeogenesis. Finally, growth hormone also contributes
to increased lipolysis and insulin resistance.
Ketoacidosis in a pregnant diabetic is a rare example that
highlights these pathophysiologic components. This serious
complication is caused by insulin deficiency in a setting of
three factors unique to all pregnancies: accelerated starva-
tion, severe insulin resistance, and net lowered buffering
capacity owing to increased renal excretion of bicarbonate, a
consequence of increased minute ventilation and respiratory
alkalosis in pregnancy.
B. Hyperglycemia—Hyperglycemia in diabetic ketoacidosis
results from several mechanisms involving a variety of hor-
mones as well as their different target organs. Using glucose
turnover studies, it has been shown that the major physio-
logic aberration that results from the combination of mech-
anisms just described is excessive hepatic glucose
production. This, in turn, is responsible primarily for hyper-
glycemia in patients with diabetic ketoacidosis. Glucose
clearance by insulin-sensitive tissues is also reduced,
although some increase in glucose utilization is associated
with the mass-action effect of high blood glucose levels. An
important factor that determines the degree of hyper-
glycemia in diabetic ketoacidosis is the extent of renal glu-
cose losses. As long as the kidneys are well perfused, they act
as a continuing source of glucose leak from the extracellular
space and thereby prevent severe hyperglycemia.
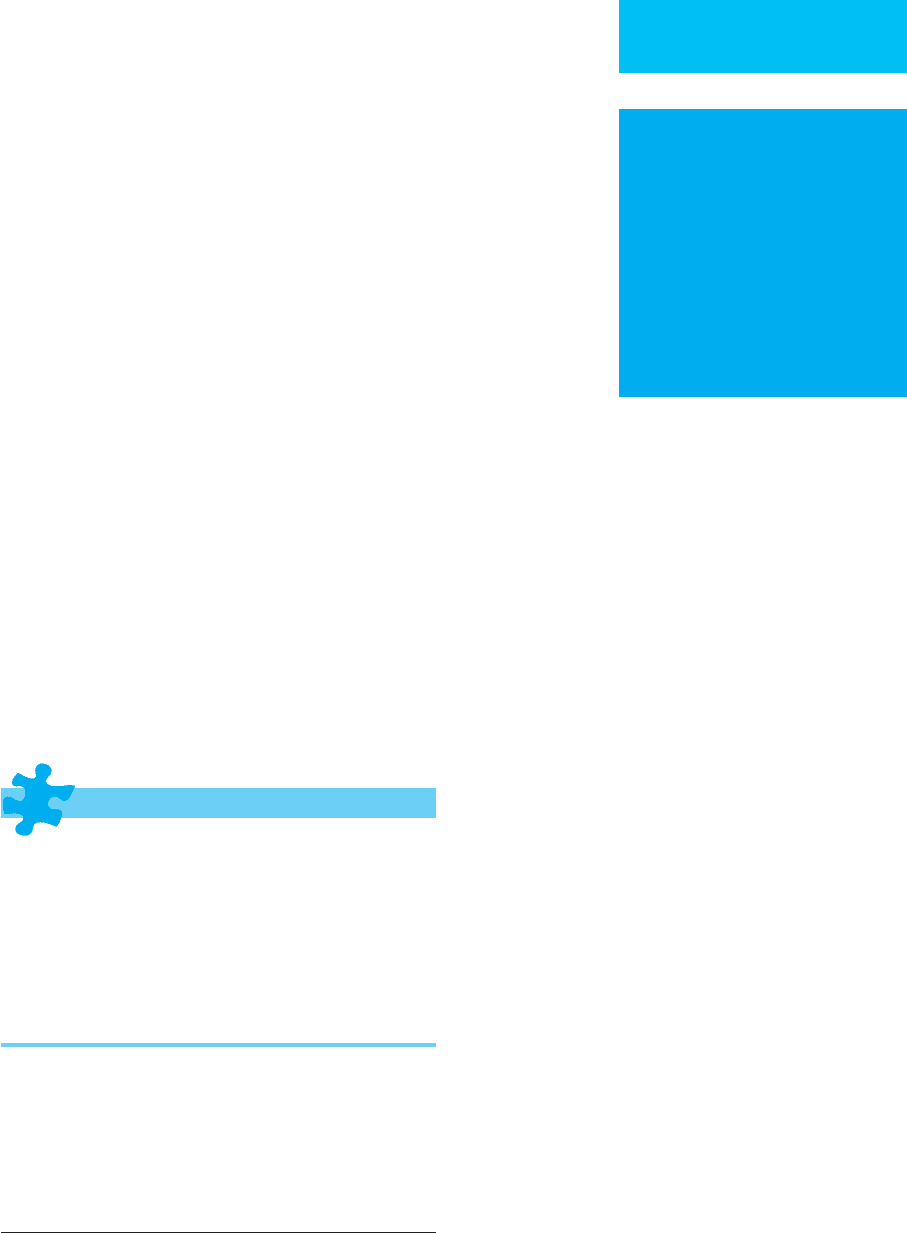
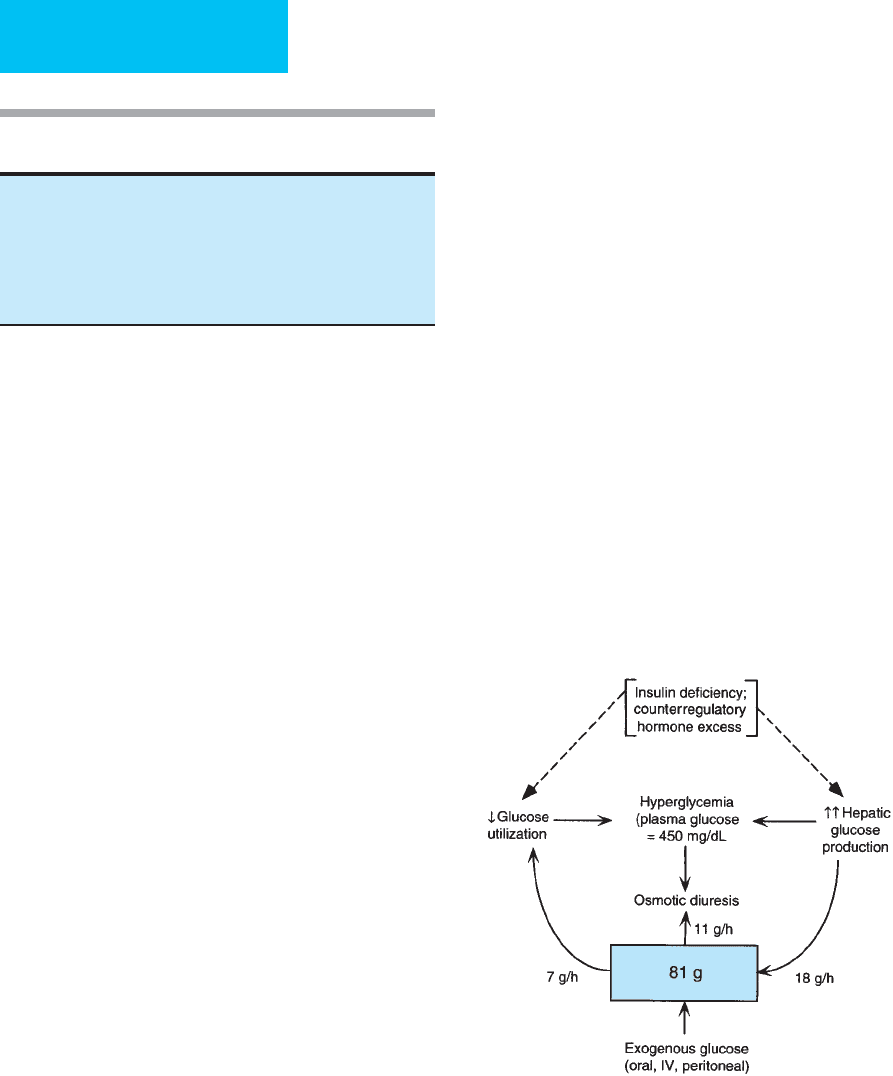
Figure 26–1 illustrates mechanisms for hyperglycemia,
with emphasis on a quantitative estimate of their contribu-
tions in diabetic ketoacidosis. The numbers presented in this
diagram are drawn from mean values reported in patients
with diabetic ketoacidosis. Insulin deficiency associated with
glucose counterregulatory hormone excess gives rise to
highly exaggerated hepatic glucose production. Although
some increase in glucose utilization owing to severe hyper-
glycemia may occur, glucose clearance remains low, and uti-
lization is insufficient to match the rise in glucose
production by the liver. In an average 70-kg patient in dia-
betic ketoacidosis with a hypothetical stable glucose concen-
tration of 450 mg/dL, hepatic glucose production is
approximately 18 g/h. Average glucose utilization is estimated
Figure 26–1. Mechanisms contributing to hyperglycemia
in a 70-kg patient with a plasma glucose level of 450
mg/dL. Values are from mean values obtained in
patients with diabetic ketoacidosis. The box represents
the extracellular glucose compartment. Lack of insulin
and increased counterregulatory hormones result in
decreased glucose utilization (7 g/h) and increased
hepatic glucose production (18 g/h). Renal glucose
losses may delay development of severe hyperglycemia.
Elevated counterregulatory hormones
Acidemia
Hypertonicity
Phosphate depletion
Elevated plasma free fatty acids
Hyperaminoacidemia
Glucose toxicity
Table 26–1. Mechanisms of insulin resistance in diabetic
ketoacidosis.

DIABETES MELLITUS, HYPERGLYCEMIA, & THE CRITICALLY ILL PATIENT
583
to be about 7 g/h. The square in the diagram represents the
extracellular space, in which there is a total of 81 g glucose
at this time. Considering that the input into the system
(hepatic glucose production) exceeds the output (glucose
utilization), stable glucose concentrations can persist only if
there is another source of glucose loss. Thus renal losses of
glucose are an important component of the protection from
severe hyperglycemia afforded patients with developing dia-
betic ketoacidosis. The average amount of glucose lost
through the kidneys in this example is estimated to be
approximately 11 g/h.
If, without any increase in hepatic glucose production, the
leak of glucose in the urine were diminished by only one-
fifth—for example, as a result of diminished perfusion of the
kidney owing to volume depletion—considerable net accu-
mulation of glucose would occur rapidly in the extracellular
space. In this example, a reduction of about 2 g/h of urinary
glucose losses would result in a further accumulation of about
50 g glucose added to the 81 g in the extracellular space over
24 hours. If the rate of glucose utilization were unchanged—
and with contraction of the extracellular space owing to fluid
losses—this could result in a doubling of serum glucose con-
centrations within a 24-hour period. The features of this dia-
gram accentuate the important role of the kidneys and the
liver in the generation of hyperglycemia in diabetic ketoaci-
dosis. Although not demonstrated in this diagram, these fac-
tors will be discussed later as an important mechanism for
reduction of hyperglycemia once treatment begins.
The degree of volume depletion has an important effect
on the development of hyperglycemia. In a study of insulin
withdrawal in type 1 diabetes, volume depletion (>3% of
weight) increased plasma glucose concentrations compared
with control subjects. Glucose production and disposal were
increased during the volume-depletion study compared with
the control study. Although the study did not evaluate renal
perfusion, a likely explanation of the increased glycemia is a
reduction of glucose excreted owing to reduced glomerular
filtration during volume depletion. Much of the variability of
glycemia in ketoacidosis may be the result of lack of fluid or
energy intake prior to or during metabolic decompensation.
Given the common occurrence of volume depletion in
patients who present with diabetic ketoacidosis, it is proba-
ble that the variability in severity of this factor—as well as the
severity of insulin deficiency and underlying illness—
explains much of the glycemic variability observed in dia-
betic ketoacidosis.
C. Ketosis and Metabolic Acidosis—Ketosis is the second
major manifestation of diabetic ketoacidosis and results
from the accumulation of keto acids generated by the liver.
Ketosis is predominantly a disorder of increased synthesis
of ketones, although inability of peripheral tissues to use
the excess ketones probably plays a small role. The keto
acid measured in the blood during diabetic ketoacidosis is
predominantly β-hydroxybutyrate rather than acetoacetate.
This reflects an altered redox state in the liver.
The increase in keto acids results in an increase in the
serum anion gap that develops because of buffering by bicar-
bonate of hydrogen ion. If the acidosis in diabetic ketoacido-
sis is due only to ketosis, the fall in serum bicarbonate is
equal to the increase in anion gap. It is evident, however, that
acidosis in diabetic ketoacidosis may have additional mecha-
nisms. Many patients have a reduction in serum bicarbonate
concentration that is greater than the increase in anion gap,
indicating, in addition, the presence of a non-anion gap
hyperchloremic acidosis. Previously, hyperchloremic acidosis
was recognized as a common manifestation of the later treat-
ment stages in diabetic ketoacidosis. It is now appreciated
that it also may be present at initial presentation, where it
appears to occur in patients who are less severely volume-
depleted. Recognition of hyperchloremic acidosis is impor-
tant because hyperchloremic acidosis takes longer to resolve
during treatment than ketoacidosis. This is so because keto
acids are metabolized to generate bicarbonate in equimolar
amounts, whereas hyperchloremic acidosis depends for its
correction on regeneration of bicarbonate by the kidneys.
Another cause of acidosis in diabetic ketoacidosis is lactic
acidosis. Lactic acidosis also contributes to the increase in the
anion gap, with a corresponding further decrease in serum
bicarbonate.
D. Fluid and Electrolyte Imbalance—Extensive losses of
fluids and electrolytes comprise the third important feature
of diabetic ketoacidosis and are a consequence of the forego-
ing abnormalities. Fluid and electrolytes are lost in the
osmotic diuresis caused by glycosuria that occurs as a result
of marked hyperglycemia in diabetic ketoacidosis. Fluid
losses are generally about 5–8 L in a 70-kg person, and deple-
tion of sodium, potassium, and chloride may be 300–500
mmol or more at presentation (Table 26–2). Magnesium and
phosphate are also lost, but in smaller quantities. While water
losses are usually easily appreciated clinically, serum elec-
trolyte concentrations do not generally reflect the large losses
that occur in these patients. This is especially true for potas-
sium because, despite large urinary losses, normal or even
high serum levels are seen at presentation as a result of a shift
of potassium from the intracellular to the extracellular
fluid—a consequence of acidosis and the loss of water from
Water 5–8 L
Sodium 400–700 mmol
Chloride 300–500 mmol
Potassium 300–1000 mmol
Calcium 100 mmol
Magnesium 50 mmol
Phosphate 50 mmol
Bicarbonate 350–400 mmol
Table 26–2. Approximate fluid and electrolyte deficits in
patients with diabetic ketoacidosis.

CHAPTER 26
584
the extracellular space. The water shift is in response to the
hyperosmolar extracellular space brought on by hyper-
glycemia; intracellular potassium accompanies the water
shift. This fluid shift also plays a role in determining serum
sodium concentration. Because osmotic diuresis is associated
with greater water loss than sodium or chloride, hyperna-
tremia might be expected to occur, but this is not seen com-
monly because of the fluid shift from the intracellular space.
This mechanism explains the mild hyponatremia often
found at diagnosis. In contrast, normal serum chloride con-
centrations are the rule, for the reason that chloride losses are
less than sodium losses. This is so because sodium is also lost
as the cation accompanying ketones excreted in the urine.
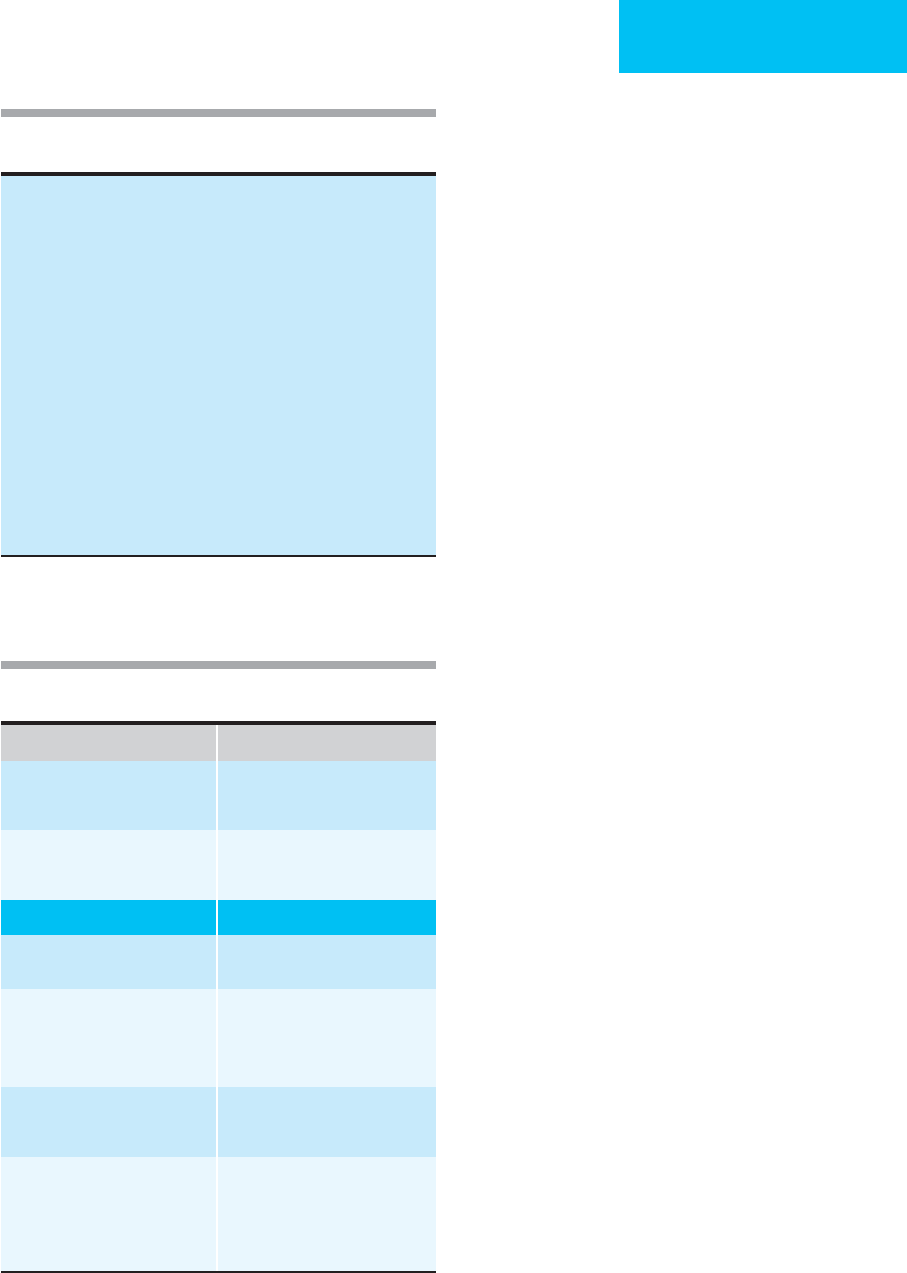
E. Altered Mental Status—The altered state of conscious-
ness observed in diabetic ketoacidosis has not been
explained. The closest correlation with impaired conscious-
ness is the serum osmolality. There appears to be an almost
linear relationship between the degree of mental obtunda-
tion and the level of serum osmolality, and most patients
with mental impairment have been found to have serum
osmolality greater than 350 mOsm/Kg (Figure 26–2). If a
patient has altered consciousness in association with a serum
osmolality of less than 340 mOsm/Kg, another cause for the
neurologic problems should be considered.
F. Precipitating Disease—Although diabetic ketoacidosis
can occur in the absence of any coexisting disease, in all
patients with diabetic ketoacidosis (or even milder metabolic
decompensation in diabetes mellitus), a precipitating factor
should be looked for. The most commonly identified pre-
cipitating factors are withdrawal of insulin, infection, and
undiagnosed diabetes during the initial presentation of the
disease. Intercurrent illnesses increase the requirements for
insulin by increasing insulin resistance, a consequence of the
hormonal mechanisms outlined earlier. In the absence of an
appropriate increase in the dose of insulin, patients become
acutely insulin-deficient; the effects of stress increase the
counterregulatory hormones; and the stage is set for the
development of diabetic ketoacidosis.
Clinical Features
A. Symptoms and Signs—Most patients present with a his-
tory of polyuria and polydipsia, weakness, and weight loss.
Duration is often as short as 24 hours, but the history usually
extends over several days, and in newly diagnosed diabetes,
symptoms often go on for weeks. Patients are usually
anorexic and may have vomiting and abdominal pain.
Abdominal pain associated with ketosis is most common
in children, although it may occur in adults as well, and
occasionally has features of an acute abdomen. Fatigue and
muscle cramps are also presenting features of diabetic
ketoacidosis.
Signs of volume depletion are characteristic. Decreased
skin turgor, dry mucous membranes, sunken eyeballs, tachy-
cardia, orthostatic hypotension, and even supine hypoten-
sion may be present. If severe acidosis is present, deep and
slow Kussmaul respirations may be noted as well as the char-
acteristic odor of ketones on the breath. Additional findings
include alteration in CNS function ranging from drowsiness
to coma. Only about 10% of patients who present with dia-
betic ketoacidosis are actually in coma, and about 20% have
clear mentation. The rest have various degrees of altered con-
sciousness. Abdominal tenderness may be noted.
Hypothermia may be present, but fever is not associated with
diabetic ketoacidosis alone. The features of an associated pre-
cipitating illness often dominate the clinical picture.
Precipitating factors for diabetic ketoacidosis are listed in
Table 26–3. The most commonly recognized causes fall
into three groups: (1) undiagnosed type 1 diabetes mellitus,
(2) reduction of insulin dose in association with intercurrent
illness (particularly patients who have anorexia and vomiting
and reduce the insulin dose for fear of hypoglycemia) or non-
compliance (in recent times, interruption of nonconven-
tional, nondepot insulin delivery—for example, insulin
infusion pumps—also may be responsible for an unintended
reduction in insulin dose), and (3) infection, where two
important and potentially confounding features of diabetic
ketoacidosis should be recognized: Fever is not caused by dia-
betic ketoacidosis alone and therefore suggests the presence of
infection, and a white blood cell count of 15,000–40,000/μL
may be seen in the absence of any infection. In some patients,
precipitating factors cannot be identified.
Unusual presentations of patients with diabetic ketoaci-
dosis must be recognized in order to make the correct
diagnosis and provide appropriate therapy (Table 26–4).
Diabetic ketoacidosis should be considered in any patient
(51)
(48)
(17)
(6)
370
360
350
340
330
320
310
300
Mean osmolality (mOsm/Kg)
Ranges in osmolality
( ) = Number of patients
Alert Drowsy Stupor Coma
Mental status
Figure 26–2. Relationship between serum osmolality
and level of consciousness in patients with diabetic
ketoacidosis. (Reproduced, with permission, from Kitabchi
AE, Wall B: Diabetic ketoacidosis. Med Clin North Am
1995;79:10–37.)
DIABETES MELLITUS, HYPERGLYCEMIA, & THE CRITICALLY ILL PATIENT
585
with type 1 diabetes who develops an acute illness. The need
to rule out diabetic ketoacidosis in a patient with type 1 dia-
betes who is becoming ill applies equally in the outpatient or
inpatient setting. When patients are at home, ketosis can be
tested easily by checking urine ketones. The possibility of
developing diabetic ketoacidosis provides a rationale for
teaching patients to test their urine for glucose and ketones
even though urine glucose measurement is no longer used to
monitor glycemic control. In inpatients, urine or serum
ketones will help to exclude diabetic ketoacidosis as the cause
of a change in a patient’s condition. Effective bedside meth-
ods for measurement of serum β-hydroxybutyrate now have
been developed, and this should contribute to more rapid
diagnosis of ketosis in hospitalized patients.
B. Laboratory Findings (See Table 26–5)—
1. Hyperglycemia—Serum glucose usually ranges from
500–800 mg/dL in patients with diabetic ketoacidosis.
Although it is often the blood glucose measurement that
alerts the staff to the possibility of diabetic ketoacidosis in an
undiagnosed patient, it is important to keep in mind that
severe hyperglycemia does not always occur. The diagnosis
should be suspected even if serum glucose levels are not
greatly elevated. This is particularly important in patients
who are fasting and may have imbibed alcohol recently
(which inhibits gluconeogenesis) or in pregnant women,
whose serum glucose levels can be normal or only slightly
elevated during severe diabetic ketoacidosis.
2. Metabolic acidosis—Total keto acids average 10–20
mmol/L; lactate is often elevated. As shown in Table 26–5,
however, less severe acidosis may be seen, based on the bicar-
bonate and pH measurements, at initial presentation. This
may be a result in part of possible earlier access to medical
care, but it also may be due to a shift in the strictness of the
criteria used to classify acute metabolic decompensation in
diabetes.
Absence of acidemia or only mild acidemia in diabetic
ketoacidosis may occur if the patient has had vomiting severe
enough to result in a mixed metabolic alkalosis and acidosis.
Absence of ketones in a patient with acidosis, hyperglycemia,
and volume depletion should make one suspect a predomi-
nance of the β-hydroxybutyrate component of the serum
ketones. With an altered redox state—in particular, in the
presence lactic acidosis—acetoacetate is converted to β-
hydroxybutyrate. The nitroprusside reagent that is used to
measure ketones semiquantitatively in most laboratories
recognizes only acetoacetate. A predominantly β-
hydroxybutyrate acidosis therefore may be missed because it
will not be picked up by this method. Use of bedside
methodology for β-hydroxybutyrate measurement should
help to eliminate this factor as a source of confusion in the
diagnosis of diabetic ketoacidosis.
3. Serum electrolytes—Table 26–6 summarizes electrolyte
abnormalities seen at the time of presentation in diabetic
1. New-onset type 1
2. Reduction of insulin dosage
Anorexia and vomiting
Nonadherence
Failure of nondepot insulin delivery
3. Infection
4. Acute disease
Trauma
Pancreatitis
Myocardial infarction
Cerebrovascular accident
5. Treatment
Corticosteroids
Pentamidine
Peritoneal dialysis
6. Endocrine disorders
Hyperthyroidism
Pheochromocytoma
Somatostatinoma
Table 26–3. Precipitating factors in patients with
diabetic ketoacidosis.
Typical Exceptions
Acute illness in a patient with
(suspected) type 1 diabetes
Diagnosis of diabetic ketoacidosis
may be delayed in older patients
with new-onset type 2 diabetes.
Clinical symptoms and signs of
volume depletion
Absence of volume depletion in
patient with diabetic ketoacidosis
and oliguric renal failure.
Laboratory features
Hyperglycemia Less severe hyperglycemia in
pregnancy or after alcohol intake.
Increased anion gap Hypertriglyceridemia may mask
increase in anion gap by interfer-
ing with serum Cl
–
and HCO
3
–
measurements.
Metabolic acidemia Vomiting may cause concurrent
metabolic alkalosis, decreasing
severity of metabolic acidosis.
Ketonemia Beta-hydroxybutyrate is not
detected by nitroprusside reaction.
If this ketone predominates,
ketonemia may be underesti-
mated or absent.
Table 26–4. Typical and atypical features of diabetic
ketoacidosis.
CHAPTER 26
586
ketoacidosis. Mild hyponatremia is the most common abnor-
mal finding and is due to a shift of water from the intracellular
compartment into the hypertonic extracellular fluid. In addi-
tion, a total sodium deficit occurs as a result of the osmotic
diuresis and the obligate cations accompanying ketones
excreted in the urine. Hypochloremia is less common because
chloride is not lost with urinary ketones and because of the
high incidence of hyperchloremic acidosis mentioned earlier.
Despite total body losses of other electrolytes, serum chloride
concentrations usually are normal or low normal at presenta-
tion. Most patients have normokalemia or hyperkalemia at
presentation, but it is important to recognize that up to 20%
will be hypokalemic at that time. Ten percent of patients have
hypophosphatemia at diagnosis, and less than 10% of patients
present with hypomagnesemia. Hypocalcemia occurs in almost
30% of patients. Serum osmolality is rarely measured directly
but instead is calculated from the electrolytes. Hyperosmolality
of varying degrees is a typical feature.
4. Interference with other laboratory tests—
Abnormalities observed in diabetic ketoacidosis may contribute
to artifactual interference with other laboratory tests.
Foster and McGarry
∗
Kitabchi
†
Ipp and Linfoot
‡
Glucose (mg/dL) 476 616 542
Sodium (meq/L) 132 134 132
Potassium (meq/L) 4.8 4.5 4.9
Bicarbonate (meq/L) <10 9.4 16
Anion gap (meq/L) — — 20
Blood urea nitrogen (mg/dL) 25 32 25
Acetoacetate (mmol/L) 4.8 — —
Beta-hydroxybutyrate (mmol/L) 13.7 9.1 9.1
Lactate (mmol/L) 4.6 — 2.0
Blood pH — 7.12 7.26
∗
Data from Foster DW, McGarry JD: The metabolic derangements and treatment of diabetic ketoacidosis. N Engl
J Med 1983;309:159–69.
†
Data from Kitabchi AE et al: Diabetes Care 2001;24:131–53.
‡
Unpublished results.
Table 26–5. Mean laboratory values in patients with diabetic ketoacidosis on admission
to hospital.
Low Normal High Comment
Sodium 67 26 7
Body stores depleted; serum [Na
+
] depends on
[glucose] and relative water loss.
Chloride 33 45 22
Potassium 18 43 39 Body stores depleted.
Magnesium 7 25 68
Phosphate 11 18 71 Decreases with insulin treatment.
Calcium 28 68 4
Table 26–6. Serum electrolyte concentrations in patients with diabetic ketoacidosis at presentation
(% of patients).
