Bongard Frederic , Darryl Sue. Diagnosis and Treatment Critical Care
Подождите немного. Документ загружается.


PULMONARY DISEASE
547
of patients with pulmonary embolism but may not reflect
findings in patients already in the ICU. Nevertheless, deep
venous thrombosis of the proximal leg veins remains the most
frequent source of pulmonary thromboemboli.
The clinical manifestations of pulmonary thromboem-
bolism reflect two pathologic processes: obstruction of the
pulmonary circulation resulting in hemodynamic compro-
mise and gas-exchange abnormalities. The degree of circula-
tory compromise depends on the size and number of
thromboemboli and the preembolic state of the right side
of the heart and pulmonary circulation. Some authors have
used the term massive pulmonary embolism to describe the
angiographic occlusion of two or more lobar pulmonary
arteries or greater than 50% of the pulmonary circulation,
while others reserve this label for patients who have hemody-
namic instability directly related to the embolic event. The use
of this radiographic description of a “massive pulmonary
embolism” has started to fall out of favor as these large or
multiple emboli may or may not be associated with circula-
tory collapse and shock. Patients with a previously normal
pulmonary circulation and right ventricular function gener-
ally can tolerate occlusion of even a large pulmonary artery
with maintenance of sufficient cardiac output to avoid shock.
However, acute pulmonary thromboembolism in a patient
with preexisting pulmonary hypertension or heart failure
with minimal reserve may lead to acute right-sided heart fail-
ure and subsequent circulatory collapse. The same may hap-
pen to a previously normal patient in whom a very large
embolus lodges in the main pulmonary artery or who has
multiple moderately sized emboli in several major branches.
Occlusion of pulmonary arteries results in decreased
regional perfusion of the lungs. If ventilation to these areas is
maintained, then high
.
V/
.
Q areas contribute to increased dead
space ventilation. Minute ventilation requirements increase to
maintain Pa
CO
2
within normal range. Arterial hypoxemia is
much more common. Although the mechanism of hypoxemia
is not completely understood, it probably results from a com-
bination of ventilation-perfusion mismatching from atelecta-
sis, redistribution of pulmonary blood flow, and increased
blood transit time. Occasionally, acute pulmonary hyperten-
sion leads to the opening of a patent foramen ovale with intraa-
trial right-to-left shunting and severe refractory hypoxemia.
The manifestations of pulmonary thromboembolism
often appear to be greater than can be explained by the degree
of vascular occlusion by thrombi. Although this is often due
to a lack of cardiopulmonary reserve in patients with chronic
illness, it is probable that vasoactive and bronchoactive sub-
stances play a role as well as normal compensatory processes
within the lung circulation and parenchyma. Among poten-
tial candidates for participation in this response are products
released by platelets and endothelial cells. In addition, occlu-
sion of a pulmonary artery is associated with a decreased
amount and effectiveness of surfactant in the region of lung
supplied by that vessel contributing to atelectasis.
Pulmonary infarction is another potential manifestation
of pulmonary thromboembolism. However, this diagnosis
does not seem to alter outcome or management other than
causing different abnormalities on chest x-ray and somewhat
different clinical manifestations. Pulmonary infarction is
uncommon in pulmonary embolism because of the dual sys-
temic and pulmonary artery blood supply to the lung.
Patients who present with pulmonary infarction are more
frequently those with congestive heart failure, in whom both
pulmonary venous congestion and systemic perfusion may
be compromised at baseline.
Because the lungs receive the total cardiac output, a
number of other emboli can make their way into the pul-
monary arterial circulation. These include pieces of tumor,
including adenocarcinomas that have eroded into the sys-
temic veins; foreign bodies such as broken intravenous
catheters or particulate matter accidentally or deliberately
injected into veins; fat and tissue emboli from orthopedic
injury, operative procedures, or bone marrow infarction as
seen in acute chest syndrome in patients with sickle cell dis-
ease; air introduced through intravenous lines, lung rupture,
or decompression during ascent from underwater diving;
and amniotic fluid introduced into the systemic circulation
during a tumultuous obstetric delivery. The pathophysio-
logic consequences of these emboli depend somewhat on
the clinical situation, the size and number of emboli, and
concomitant medical problems. Of note, fat emboli may
result in a distinct syndrome with systemic manifestations as
a result of the breakdown of free fatty acids within the
microcirculation.
Clinical Features
The clinical features of deep venous thrombosis and pul-
monary thromboembolism are intertwined, and both can
present diagnostic difficulties. Deep venous thrombosis
causes nonspecific clinical findings and is sometimes found
in patients with pulmonary embolism in whom thrombosis
was previously unsuspected. The diagnosis of pulmonary
thromboembolism can be difficult because it too presents
with nonspecific symptoms, signs, and laboratory tests that
suggest other acute lung and heart diseases. In the critically
ill patient with preexisting cardiac or respiratory failure,
pneumonia, atelectasis, pleural effusion, or infection, diag-
nosing superimposed pulmonary thromboembolism may be
even more difficult. It is often suspected when a critically ill
patient undergoes acute deterioration from previous baseline
findings manifested by tachypnea, tachycardia, and impaired
gas exchange.
A. Deep Venous Thrombosis—
1. Symptoms and signs—Obstruction of the deep venous
system of the leg may result in edema of the lower part or the
entire leg, pain, tenderness, redness, and other nonspecific
features. Findings are notoriously unreliable and insensitive,
with as many as 50% of patients with deep venous thrombo-
sis being asymptomatic or lacking abnormal physical find-
ings. Clinically significant venous thrombi may not
completely occlude the vascular lumen, or collateral veins
and lymphatics may prevent swelling. Most blood clots do

CHAPTER 24
548
not elicit an inflammatory response unless there is additional
vascular injury, so redness and warmth are most often not
present in uncomplicated deep venous thrombosis.
Importantly, patients with proximal deep venous thrombosis
may have a somewhat greater tendency to have silent disease
compared with those who have calf vein involvement.
However, swelling above or below the knee, recent immobil-
ity, cancer, and fever were found to have diagnostic value
in proximal acute deep venous thrombosis. Only 5% of
95 patients had none of these five findings, whereas 42% had
between two and five of these features.
The differential diagnosis of deep venous thrombosis in
symptomatic patients includes cellulitis and other soft tissue
infections, popliteal cysts with below-the-knee swelling,
septic or inflammatory arthritis, lymphatic obstruction or
inflammation, external compression of deep veins, trauma,
and hematomas. The finding of unilateral leg swelling does
not rule out primary disease in pelvic iliac veins.
2. Diagnostic workup—Imaging studies for deep venous
thrombosis include contrast venography, duplex compres-
sion ultrasonography, impedance plethysmography, radio-
fibrinogen uptake scans, CT venography, and MRI. Contrast
venography uses injection of radiocontrast material into
peripheral veins of the legs to identify obstructing thrombi
in the deep venous system. Between 20% and 50% of proxi-
mal venous thrombi usually can be identified as intralumi-
nal filling defects. Costs and complications make this test less
desirable.
Duplex ultrasound uses a combination of real-time ultra-
sound imaging to demonstrate normal venous collapse with
direct compression and Doppler venous flow assessment.
Because collapse with compression is the most important
feature, a more accurate name for this technique is compres-
sion ultrasonography. Failure to collapse or demonstration of
intraluminal echoes is indirect evidence of deep venous
thrombosis. Compression ultrasonography is practical for
the popliteal vein, the common femoral vein, and often the
superficial femoral vein. It is generally regarded as having as
high as 89–98% sensitivity for proximal deep venous throm-
bosis and comparable specificity. It is less reliable in imaging
the venous plexus in the calf, and it cannot detect thrombi
limited to iliac or pelvic veins. As discussed below, compres-
sion ultrasonography has been incorporated into numerous
diagnostic algorithms for the diagnosis of pulmonary
thromboembolism.
Impedance plethysmography uses the change in electrical
impedance of the lower extremity when blood flows out of
the leg venous system after release of a pressure cuff. Failure
to change impedance is presumptive evidence of proximal
deep venous thrombosis. This test has been reported to be
highly accurate for diagnosing proximal deep venous throm-
bosis, although other forms of venous obstruction and con-
gestive heart failure can give false-positive results. In
addition, the accuracy of the test is likely to be hospital-
dependent or operator-dependent. It has been recommended
that each facility establish the accuracy of impedance
plethysmography compared with contrast venography.
Impedance plethysmography must be performed with the leg
held immobile and cannot be used if there is a plaster cast on
the suspected leg. In a comparison trial of compression ultra-
sonography and impedance plethysmography, the predictive
value of compression ultrasonography was significantly
higher in symptomatic outpatients.
Radiofibrinogen leg scans demonstrate the inclusion of
radiolabeled fibrinogen into actively forming thrombi. This
test is useful primarily for identifying calf and lower-thigh
deep venous thrombosis. Only clots that are actively forming
can be located with this method. It is poor in detecting prox-
imal deep venous thrombosis. This test is used rarely today
except in research protocols.
MRI and CT pulmonary angiography accompanied by
venography are currently being investigated for their role in
the workup of thromboembolic disease. The advantage of
these studies would be evaluation of the pulmonary vascular
system for emboli combined with evaluation of the pelvis
and lower extremity venous system for their source in a sin-
gle study.
Owing to its invasive nature, contrast venography is obvi-
ously not appropriate for screening patients at risk for deep
venous thrombosis. Both impedance plethysmography and
compression ultrasonography can be used effectively for this
purpose. In patients at high risk for deep venous thrombosis,
such as those with pelvic or hip trauma and those with criti-
cal medical illness, compression ultrasonography is probably
most sensitive and specific for proximal vein thrombosis,
even though there has been greater experience with imped-
ance plethysmography. In patients with deep venous throm-
bosis limited to the calf, serial compression ultrasonography
of patients is needed to identify the 15–20% of patients who
extend their thrombi into the proximal veins. Most clots have
been found to extend proximally within the first 7 days. Thus
studies have recommended follow-up examinations within
2–3 days and again in 7–10 days if clinical suspicion for deep
venous thrombosis remains high.
B. Pulmonary Embolism
1. Symptoms and signs—The clinical features of pul-
monary embolism have been accurately described, and
review of these findings demonstrates their nonspecificity.
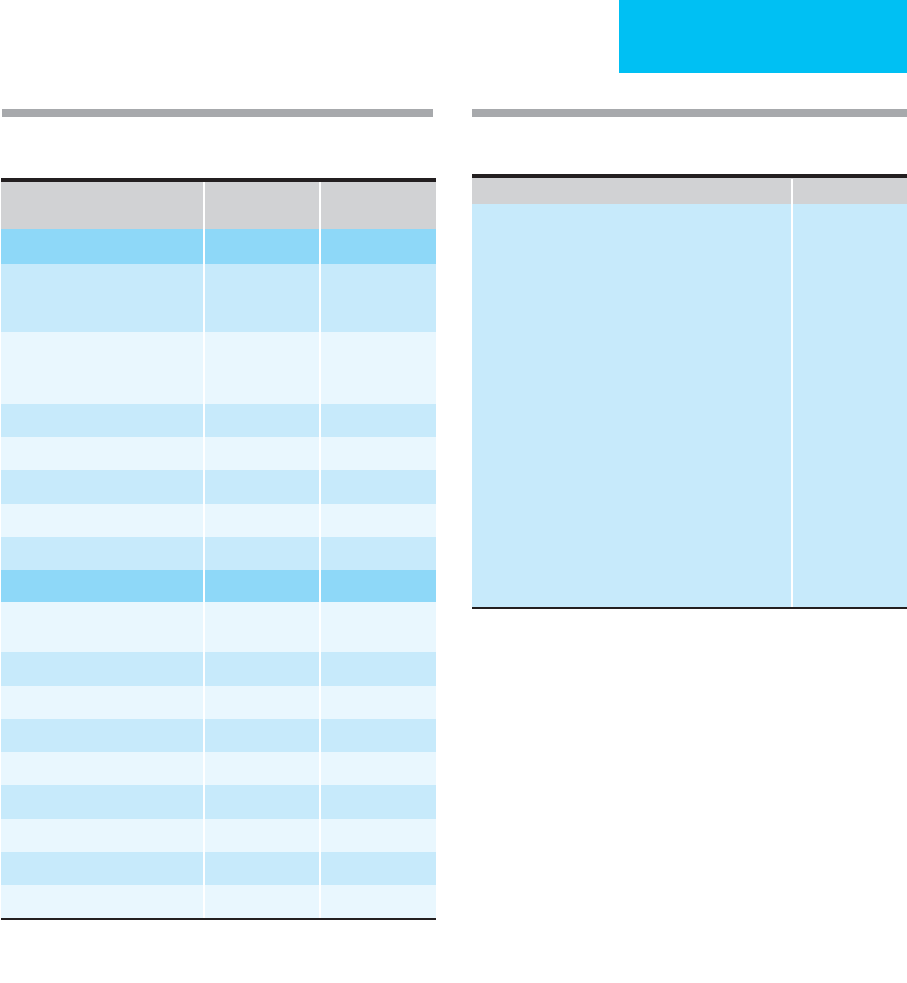
Table 24–3 summarizes data from a series of 500 patients
comparing the clinical signs and symptoms in patients with
proven pulmonary emboli by pulmonary angiography (202
patients) versus those without pulmonary embolism.
Dyspnea and chest pain were the most common complaints;
tachycardia, tachypnea, rales, and an increased intensity of
the pulmonic component of the second heart sound were the
most frequent findings on physical examination. The classic
findings of hemoptysis, chest pain, and dyspnea were
uncommon as a triad. Fewer than one-third of the total
group had symptoms or signs suggesting deep venous
thrombosis. While it is correct that these clinical findings do
PULMONARY DISEASE
549
not distinguish patients with pulmonary embolism from
those with other severe heart and lung diseases, patients sus-
pected of pulmonary embolism who have cyanosis, hypoten-
sion, shock, or syncope deserve particular consideration.
Symptoms and signs can establish meaningful probabili-
ties for pulmonary embolism. Several clinical models for esti-
mating probability have been developed and validated.
Patients suspected of pulmonary embolism were stratified
into low, moderate, and high probability, and these classifica-
tions were confirmed by subsequent testing (Table 24–4).
This clinical estimate of pulmonary embolism likelihood is
the first step in diagnosis, followed by laboratory and imaging
studies. Objectively derived clinical prediction rules should be
used for judging “pretest” probability of pulmonary
embolism. These have been derived for nonhospitalized
patients suspected of pulmonary embolism and may not be
valid in hospitalized or ICU patients.
2. Laboratory findings—Arterial blood gases most often
show mild to moderate hypoxemia, increased P(
A
–a)
O
2
, and
mildly reduced Pa
CO
2
. Almost all patients with pulmonary
embolism have a Pa
O
2
of less than 80 mm Hg, but no
absolute level of Pa
O
2
can be used to exclude the diagnosis.
Diagnostic accuracy may be improved somewhat by using
the P(
A
–a)
O
2
difference rather than the Pa
O
2
,but again,a
clear distinction between those with and those without pul-
monary embolism cannot always be made. In the Prospective
Investigation of Pulmonary Embolism Diagnosis (PIOPED)
study, 7% of patients with angiographically documented
pulmonary emboli had completely normal arterial blood gas
measurements. Conversely, another study found that
patients suspected of having a venous thromboembolic event
who had a normal P(
A
–a)
O
2
had only a 1.8% chance of hav-
ing a pulmonary embolism. Severe hypoxemia refractory to
oxygen administration may indicate the opening of a patent
foramen ovale. Hypercapnia is unusual and suggests the
presence of underlying lung disease.
PE Present
(
n
= 202)
PE Absent
(
n
= 298)
Symptoms
Dyspnea
Sudden onset
Gradual onset
78%
6%
29%
20%
Chest pain
Pleuritic
Nonpleuritic
44%
16%
30%
10%
Orthopnea 1% 9%
Fainting 26% 13%
Hemoptysis 9% 5%
Cough 11% 15%
Palpitations 18% 15%
Signs
Tachycardia
HR >100 beats/min
24% 23%
Cyanosis 16% 15%
Hypotension (SBP <90 mm Hg) 3% 2%
Neck vein distention 12% 9%
Unilateral leg swelling 17% 9%
Fever >38°C 7% 21%
Crackles 18% 26%
Wheezes 4% 13%
Pleural friction rub 4% 4%
Data from Miniati M et al: Accuracy of clinical assessment in the
diagnosis of pulmonary embolism. Am J Respir Crit Care Med
1999;159:864–71.
Table 24–3. Symptoms and signs in 500 patients with
suspected pulmonary embolism (PE).
Table 24-4. Estimating clinical probability of pulmonary
embolism (PE).
Points
Risk factors
Age >65 years 1
Previous DVT or PE 3
Surgery under general anesthesia or fracture 2
of the lower limb within 1 month
Active malignant condition (solid or hematologic 2
malignant condition, currently active or
considered cured <1 year)
Symptoms
Unilateral lower limb pain 3
Hemoptysis 2
Clinical signs
Heart rate 75–94 beats/min 3
Heart rate ≥95 beats/min 5
Pain on lower limb deep venous palpation and 4
unilateral edema
Clinical probability of pulmonary embolism
Low (8%) Total = 0–3
Intermediate (28%) Total = 4–10
High (74%) Total ≥11
Modified from Le Gal G et al: Prediction of pulmonary embolism in
the emergency department: The revised Geneva score. Ann Intern
Med 2006;144:165–71.

CHAPTER 24
550
Sinus tachycardia is a frequent and nonspecific finding in
acute pulmonary embolism. Supraventricular tachycardia
and atrial fibrillation are sometimes present. Features
suggesting acute right-sided heart strain on the ECG occur
relatively infrequently; these include acute right-axis devia-
tion, P pulmonale, right bundle branch block, and
inverted T waves and ST-segment changes in right-sided
leads. Electrocardiographic patterns such as an S wave in lead
I, a Q wave in lead III, and an inverted T wave in lead III
(“S1Q3T3”) and S waves in leads I, II, and III (“S1S2S3”)
were considered highly predictive of pulmonary embolism.
These observations were found in fewer than 12% of patients
with pulmonary emboli. In the differential diagnosis of pul-
monary embolism, the ECG is particularly useful to assess
the presence of myocardial ischemia and infarction.
D-dimer, a fibrin degradation product, is found in the
plasma of patients with deep venous thrombosis and pul-
monary embolism. D-dimer is the result of plasmin action
(thrombolysis) on fibrin monomers that have undergone
cross-linking by factor XIII to form fibrin polymers. Various
methodologies are available to measure D-dimer levels. ELISA
D-dimer assays have a higher sensitivity and negative predictive
value (91–100%) when compared with latex agglutination
techniques. However, the older ELISAs were more labor-
intensive, required skilled personnel, and took hours to com-
plete, making them less useful clinically in an emergent
situation, whereas the semiquantitative latex agglutination
studies can be performed at the bedside. A D-dimer level of less
than 500 μg/L by ELISA is considered the cutoff for excluding
venous thromboemboli. Newer latex whole blood agglutina-
tion techniques have demonstrated consistently high negative
predictive values in patients with low pretest probability of dis-
ease. Current recommendations are to combine this laboratory
finding with the pretest clinical probability as well as some
other noninvasive evaluation to guide decision making for
diagnosis and management. The one exception would be in the
face of a low clinical pretest probability, when the finding of a
low D-dimer may be enough to exclude venous thromboem-
bolic disease. Lastly, D-dimer is of limited use in a number of
clinical scenarios, which are associated with elevated D-dimer
levels as part of the disease state including surgery or trauma in
the past 3 months, underlying malignancy, sepsis with or with-
out disseminated intravascular coagulation (DIC), inflamma-
tory states, pregnancy, or abnormal liver function.
Elevation in cardiac troponins in the setting of an acute
pulmonary embolism has been described. In patients with
either a moderate or large pulmonary embolism, troponin T
(TnT) levels greater than 0.1 ng/mL were seen in 32% of
patients in one trial. None of the patients with small emboli
had an elevation of this cardiac marker. In another study,
tropoinin I (TnI) levels greater than 0.4 ng/mL were seen in
21% of patients with pulmonary embolism, with levels
exceeding 2.3 ng/mL in 4% of patients. It is thought that the
strain on the right ventricle from the increased pulmonary
arterial resistance in the face of an acute embolism leads to
the right ventricular myocardial ischemia in these patients
and is associated with a poorer prognosis.
3. Imaging studies—Radiographic studies include nonspe-
cific tests such as chest x-rays, examinations of the pul-
monary circulation such as perfusion lung scans, CT
pulmonary angiography (spiral or helical CT scans), MRI,
and pulmonary angiograms, as well as studies directed at
finding deep venous thrombosis.
a. Chest x-ray—The chest x-ray is most useful in identi-
fying coexisting problems such as pneumonia, lung mass,
lymphadenopathy, pulmonary edema, atelectasis, or pleural
effusion. The most common findings in pulmonary
embolism without coexisting disease are nonspecific, includ-
ing no visible abnormality, enlarged cardiac silhouette, ele-
vated hemidiaphragm, atelectasis, or small pleural effusion.
A normal chest x-ray in a patient with shortness of breath
and hypoxemia should prompt a further evaluation for pul-
monary embolism. Findings suggestive of pulmonary vascu-
lar occlusion, such as an apparent cutoff of a segmental or
lobar pulmonary artery, regional hyperlucency of the lung
parenchyma or oligemia (Westermark’s sign), or a wedge-
shaped density consistent with pulmonary infarction
(Hampton’s hump if located in the periphery), may suggest
pulmonary embolism but are insensitive and lack specificity.
b. Radionuclide ventilation-perfusion scan—The venti-
lation-perfusion lung scan was previously the test used most
frequently to diagnose pulmonary embolism. Newer imaging
studies have replaced this nuclear medicine study in many
centers. In addition, scan results must be considered carefully
because these tests do not have perfect diagnostic accuracy,
and over 70% of patients undergoing this radiographic eval-
uation have indeterminate results. To perform the perfusion
scan, radionuclide-labeled macroaggregated albumin is
injected into a peripheral vein, after which the labeled parti-
cles become trapped in the pulmonary capillary bed.
Uniform distribution of the radionuclide throughout the
lung fields implies the absence of significant localized pul-
monary arterial obstruction, whereas a pulmonary
embolism occluding a pulmonary artery will result in a per-
fusion defect. Unfortunately, perfusion defects commonly
result from other causes, including focal vasoconstriction
accompanying atelectasis, pneumonia, or bronchospasm.
To improve diagnostic value, uniformity of ventilation is
assessed using a ventilation scan, performed by inhalation of
either radioactive Xenon or an aerosol containing a radiola-
beled solute. The perfusion and ventilation scans are then
compared. A sufficiently large perfusion defect without a cor-
responding ventilation defect in the same area (ie, mis-
matched defect) generally is considered supportive of the
diagnosis of pulmonary embolism. On the other hand, a
matched ventilation-perfusion defect generally is considered
indeterminate and not helpful in making the diagnosis of
pulmonary embolism or other kinds of heart or lung disease
such as pneumonia or bronchospasm.
By convention, ventilation-perfusion lung scans are inter-
preted as normal (no perfusion defects), low or high proba-
bility for pulmonary embolism, or intermediate probability
(sometimes called indeterminate) for pulmonary embolism.
Prospective studies have led to accepted diagnostic strategies
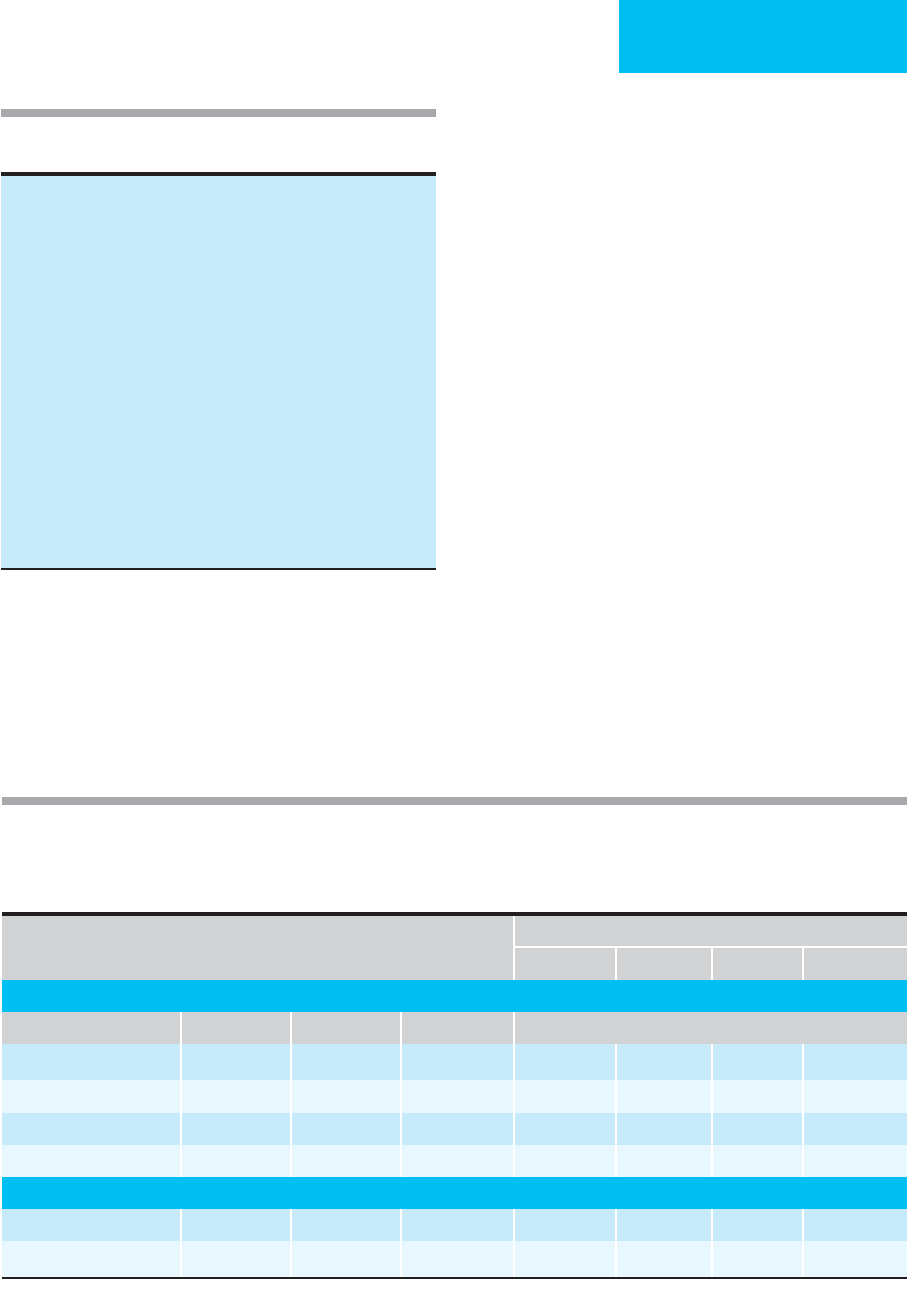
PULMONARY DISEASE
551
for pulmonary embolism. The PIOPED multicenter study
compared lung scan results with pulmonary angiography in
patients with suspected pulmonary embolism. Table 24–5 is
a modified summary of lung scan categories and criteria
used in 931 patients included in this study.
Of patients with suspected pulmonary embolism, lung
scans indicated a high probability of pulmonary embolism in
13% and were normal or nearly normal in 14%. Intermediate
or low probability was the conclusion in 73%. The sensitiv-
ity, specificity, and likelihood ratios of lung scan interpreta-
tions for angiographically diagnosed pulmonary emboli are
shown in Table 24–6. The likelihood of pulmonary embolism
parallels the interpretation of the lung scans, especially when
used in conjunction with the pretest clinical likelihood of
pulmonary embolism. A reading of any probability (low,
intermediate, or high) of pulmonary embolism on lung scan
resulted in 98% sensitivity but low specificity. Unfortunately,
only 41% of cases of pulmonary embolism had high-
probability lung scans, whereas 42% had intermediate-
probability scans and 17% had low-probability scans. It is
emphasized that patients with low-probability lung scans are
found to have pulmonary emboli about 15–30% of the time.
These and other data show that ventilation-perfusion lung
scans are of greatest utility when they show high probability for
pulmonary embolism (87% likelihood of pulmonary embolism
in all patients suspected of pulmonary embolism; 96% if clinical
suspicion is high) or when they are normal (nil to 4% likelihood
of pulmonary embolism during long-term follow-up even if clin-
ical suspicion was high). Thus high-probability lung scans effec-
tively predict pulmonary embolism, whereas a normal scan (no
perfusion defects) effectively excludes pulmonary embolism.
Treatment or withholding therapy can be guided by this rule with
considerable confidence. Unfortunately, the majority of patients
suspected of having pulmonary embolism fall into intermediate
or low probability, and substantial numbers within each
Table 24–5. PIOPED lung scan interpretation criteria
(modified).
High probability
≥ 2 large (>75% of the segment), OR
≥ 2 moderate (25–75% of the segment) plus one large, OR
≥ 4 moderate mismatched perfusion lung scan defects
Low probability
Nonsegmental perfusion defects only, OR
One moderate mismatched segmental perfusion defect with normal
chest x-ray, OR
Any perfusion defect with a larger chest x-ray abnormality, OR
Limited number of large or moderate perfusion defects with
matching ventilation defects (with normal or mildly abnormal
chest x-ray)
Intermediate probability
Not falling into normal, low-, or high-probability categories
Borderline high or borderline low
Difficult to categorize as high or low
Normal
No perfusion defects, OR
Perfusion outlines exactly the shape of lungs seen on chest x-ray
(chest x-ray or ventilation lung scan may be abnormal)
Table 24-6. Sensitivity, specificity, likelihood ratios, and posttest probabilities of ventilation-perfusion radionuclide
lung scans and CT pulmonary angiograms for pulmonary embolism.
For a patient with suspected pulmonary embolism, estimate pretest probability (clinical information or clinical information plus results of prior tests).
Look up posttest probability at intersection of pretest probability (column) and test result (row).
Pretest Probability
∗
8% 28% 50% 74%
Ventilation-Perfusion Radionuclide Scan
Scan Result Sensitivity Specificity Likelihood Ratio Posttest Probability
High probability 41% 97% 17.1 60% 87% 94% 98%
Indeterminate 41% 62% 1.1 9% 30% 52% 76%
Low probability 16% 60% 0.4 3% 13% 29% 53%
Near-normal or normal 2% 81% 0–0.1 0–1% 0–4% 0–9% 0–22%
CT Pulmonary Angiogram
Positive 78–97% 53–100% 3.5–32 23–74% 58–93% 78–97% 91–99%
Negative 0.05–0.48 0–4% 2–16% 5–32% 12–58%
∗
Using the revised Geneva Score (see Table 24–4).
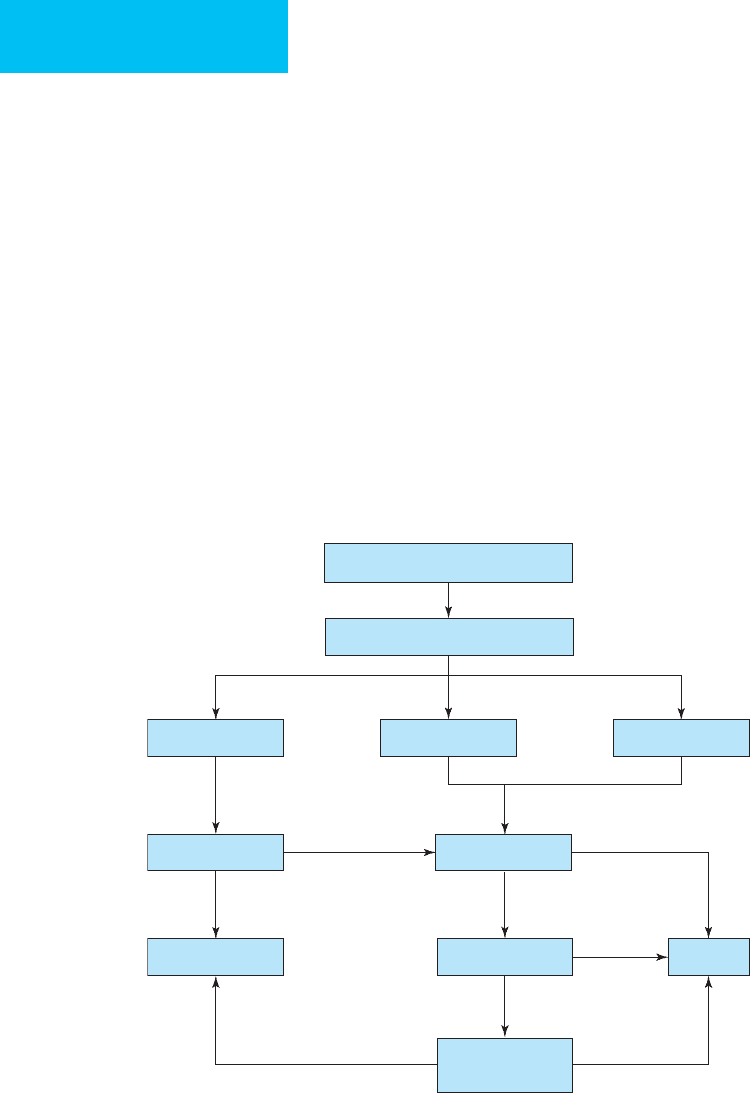
CHAPTER 24
552
category will or will not actually have the disease. It should be
emphasized that these studies have characterized mostly patients
who were not critically ill, in whom the appropriate studies could
be performed and compared. The predictive value of lung scans
may not be comparable in patients in the ICU.
c. Combination imaging—Multiple approaches for
improving diagnostic accuracy when lung ventilation-
perfusion scanning is nondiagnostic have been described
(Figure 24–1). The traditionally used strategy is to perform
pulmonary angiography for all suspected patients in whom
intermediate probability lung scans are found (pulmonary
embolism present by angiography in 16–66% depending on
clinical suspicion) or in whom a low-probability lung scan is
associated with high or uncertain clinical suspicion (pul-
monary embolism found in 16–40%). A normal pulmonary
angiogram is quite accurate in excluding pulmonary
embolism, with fewer than 1% of patients with negative pul-
monary angiograms subsequently proving to have pulmonary
embolism without treatment during long-term follow-up or
at autopsy. This approach would be ideal if it were not for
problems encountered with obtaining pulmonary angiogra-
phy, including limited availability, uncertain reliability in all
hospitals, and risks of vascular catheterization and radi-
ographic contrast agents. Small amounts of contrast material,
selective pulmonary angiography, nonionic contrast material,
and careful preinjection measurement of pulmonary artery
pressure reduce the frequency of complications from pul-
monary angiography. Pulmonary hypertension is a relative
contraindication because of reported morbidity and mortal-
ity from injecting additional volume into a system already
under high pressure. Other complications, including death,
respiratory distress leading to intubation, renal failure, and
hematoma requiring transfusion, are reported to occur in
up to 4% of critically ill patients undergoing pulmonary
angiography. Pulmonary angiography used to be per-
formed in all patients being considered for thrombolytic
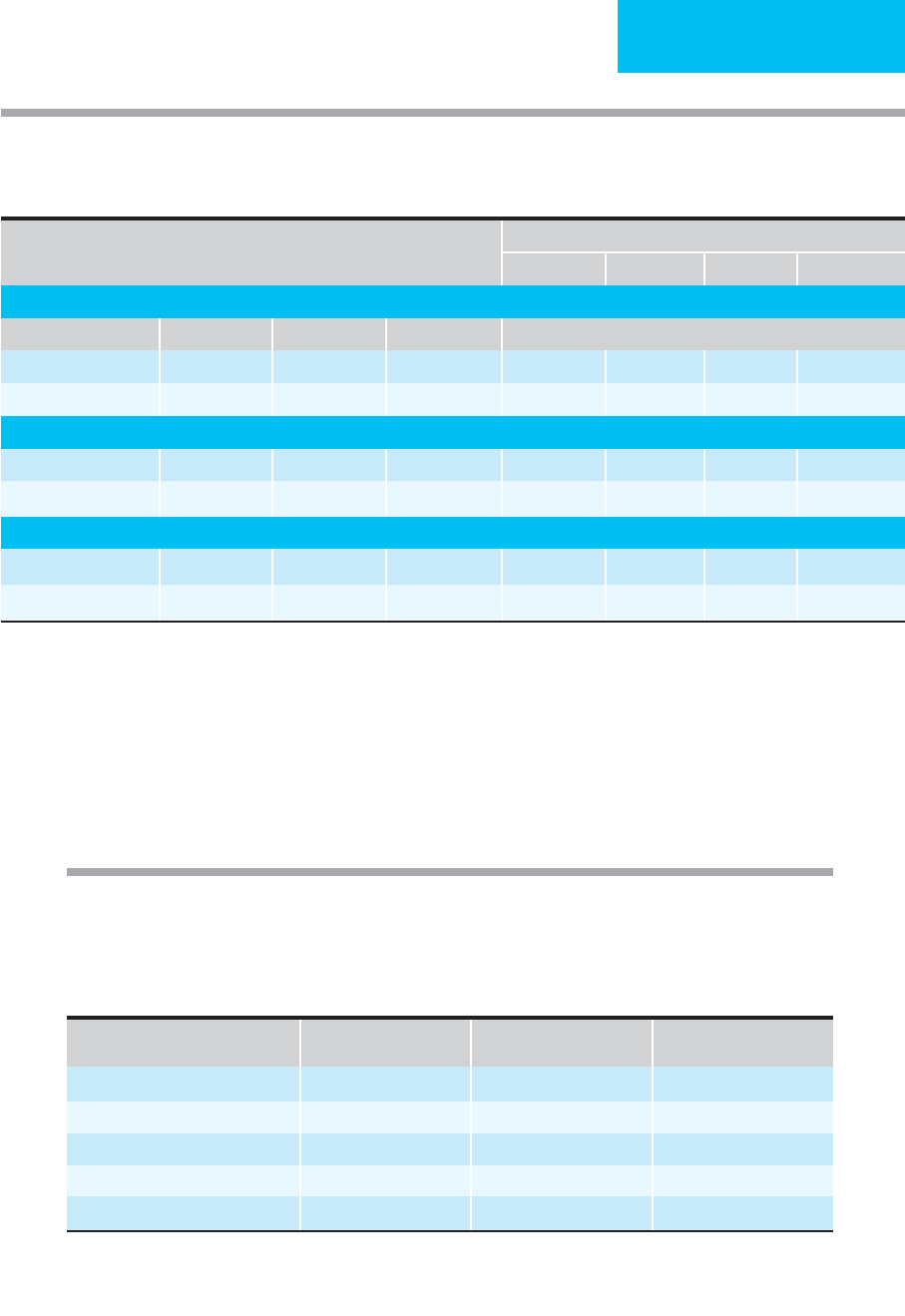
Figure 24–1. One suggested approach to a patient with suspected pulmonary embolism. After estimation of clinical
probability (see Table 24–4), most patients should have CT pulmonary angiogram performed unless a contraindication exists.
If the cumulative probability of clinical estimation plus CT angiogram results is sufficient to begin or withhold treatment,
diagnostic studies are completed. If not, further studies are required (eg, compression ultrasonography or pulmonary
angiography) until a decision can be reached. Any decision to begin or withhold treatment must take into account the risk
of treatment compared with the potential benefits of treatment.
Suspected pulmonary embolism
Estimate clinical probability
Low (8%) Moderate (28%) High (78%)
D-dimer (ELISA)
No treatment
DVT studies Treat
CT angiogram
1
High
Positive
Positive
Positive
Low Negative
Negative
Negative
Pulmonary
angiogram
1
If CT is contraindicated due to iodine allergy or creatinine clearance is prohibitive, consider
starting with V/Q scan or compression ultrasonography

PULMONARY DISEASE
553
therapy, but this recommendation is being questioned
because of the subsequent risk of bleeding (as high as 20%).
The major disadvantage of this approach is that a large num-
ber of patients would require pulmonary angiography
because of abnormal but nondiagnostic lung scans. In some
studies, patients making up this group comprise 40–60% of
the total enrolled.
A second approach recognizes the close relationship
between pulmonary thromboembolism and deep venous
thrombosis. Proximal vein deep venous thrombosis is found
on initial testing in about 50% of patients with pulmonary
embolism. In patients with low- or intermediate-probability
ventilation-perfusion lung scans but with high or uncertain
clinical probability of pulmonary embolism, compression
ultrasonography or impedance plethysmography may be
performed. A combination of high or uncertain clinical sus-
picion, abnormal (but not high-probability) lung scan, and
positive noninvasive test for deep venous thrombosis
strongly supports the diagnosis of pulmonary embolism.
Treatment should be initiated on the basis of this result. The
absence of evidence of deep venous thrombosis, however,
should not rule out pulmonary embolism because the false-
negative rate of a single noninvasive test for deep venous
thrombosis ranges from as low as 3% to as high as 30%.
Therefore, these patients must undergo pulmonary angiog-
raphy or serial duplex ultrasonography if the initial ultra-
sound is negative (see below). The combination of lung scan
and noninvasive deep venous thrombosis studies decreases
the number of pulmonary angiograms needed in these
patients from about 72% to 33%. Completely noninvasive
strategies for selected patients also have been proposed. In
patients suspected of pulmonary embolism who have abnor-
mal but nondiagnostic lung scans (ie, low or intermediate
probability) and adequate cardiopulmonary reserve (eg, lack
of respiratory failure, hypotension, severe underlying lung
disease, and severe tachycardia), serial noninvasive tests for
proximal lower extremity deep venous thrombosis are per-
formed (eg, impedance plethysmography or compression
ultrasonography). If evidence of deep venous thrombosis is
found initially or subsequently, treatment is started.
However, if no evidence of deep venous thrombosis is found,
anticoagulation is withheld while noninvasive deep venous
thrombosis studies are repeated at least twice over the next
10–14 days. In a study of 627 untreated patients with sus-
pected pulmonary embolism with nondiagnostic lung scans
and negative serial impedance plethysmographic studies over
2 weeks, pulmonary thromboembolism occurred in only
1.9% over the next 3 months. Treatment therefore can be
withheld in this group of patients with acceptable results. In
fact, this approach clarifies the natural history of pulmonary
thromboembolism in that extension of the pulmonary
embolus itself rarely occurs without treatment, and treat-
ment is directed solely at prevention of extension of the
venous thrombus. This noninvasive approach can be used to
avoid pulmonary angiography in some patients with nondi-
agnostic lung scans. For critically ill patients, however, this
strategy may not be feasible because of concomitant heart
and lung disease and lack of cardiopulmonary reserve. In
addition, patients being considered for therapy other than
anticoagulation, such as thrombolytic therapy, cannot be
evaluated appropriately using this method.
Another completely noninvasive strategy included the com-
bined use of CT angiography, a ventilation-perfusion scan,
plasma D-dimer measurements, and in some patients compres-
sion ultrasonography of the lower extremities. In 247 patients
evaluated using this strategy, the 3-month risk of developing an
embolic event without therapy if none of these studies revealed
venous thromboembolism was only 1.7%. In general, the diag-
nostic protocols that combine these noninvasive studies either
have obtained all studies during the initial assessment and made
treatment decisions based on all study results altogether or have
obtained a single study at a time and continue to obtain addi-
tional diagnostic data if the results are not conclusive until a
definitive diagnosis is obtained or excluded.
d. CT pulmonary angiography—Imaging studies that
attempt to assess the pulmonary vasculature bed directly have
been evaluated for their role in the diagnostic workup of pul-
monary thromboembolism. The development of helical
(spiral) and electron-beam CT pulmonary angiography has
nearly replaced the ventilation-perfusion scan in many cen-
ters with its reported sensitivities in most studies of greater
than 80% and specificities of greater than 90% for diagnosing
pulmonary emboli in the main, lobar, and segmental pul-
monary arteries. The lower sensitivity of this imaging study
results in part from the poor performance in the diagnosis of
subsegmental emboli. The clinical impact of emboli in these
subsegmental arteries is unclear, and they may not pose the
same morbidity and mortality risks as emboli in larger seg-
ments. However, in patients with a limited cardiopulmonary
reserve, emboli in subsegmental arteries potentially could be
devastating. Newer imaging techniques that are available
today, including thin collimation (1–3-mm instead of 5-mm
slices) and faster acquisition timing have improved the evalu-
ation of these subsegmental vessels. An additional advantage
of these imaging studies is their ability to provide additional
information with regard to other disease processes within the
thorax that may be responsible for the patient’s clinical pres-
entation, such as pneumonic infiltrates, pleural disease, medi-
astinal lymphadenopathy, or parenchymal masses. CT
imaging requires the administration of intravenous contrast
material and a degree of patient cooperation with the ability to
lie still and breath-hold for approximately 25 seconds in some
protocols to obtain good-quality pictures of the vasculature.
In a small but important proportion of studies, the results are
not acceptable because of movement artifacts or inadequate
concentration of contrast material in the pulmonary arteries.
The exact role of these CT imaging techniques with the
diagnosis of pulmonary thromboembolic disease is continu-
ing to evolve, and these techniques are included in a number
of combined-strategy models. In fact, some investigators
have adopted CT imaging as the key starting diagnostic test
for all patients considered likely to have a pulmonary

CHAPTER 24
554
embolism. A meta-analysis evaluated the 3-month clinical
outcome in patients with suspected pulmonary embolism
managed solely based on the results of CT pulmonary
angiography. The rate of subsequent venous thromboem-
bolism after a negative CT result in untreated patients was
1.4%, which is similar to the rate seen in previous studies
with negative pulmonary angiograms. Because concern still
exists among many physicians about the overall sensitivity of
CT imaging but with acceptable specificity, the helical CT
can be used to diagnose pulmonary thromboembolism if a
thrombus is seen but may not be able to exclude significant
pulmonary artery thrombi if clots are not visualized in
patients with a high pretest probability of having this disease
state. Nevertheless, a diagnostic strategy based on clinical
probability, D-dimer, and CT pulmonary angiography
proved highly effective. In those in whom pulmonary
embolism was “excluded,” only 1.3% of untreated patients
had recurrent venous thromboembolism.
CT pulmonary angiography combined with CT venogra-
phy of the pelvis and lower extremities to evaluate the venous
system for the source of the embolus during the same study
(ie, CTA-CTV) was investigated in the PIOPED II trial. While
the addition of venography increased the sensitivity of this
combined CT imaging study, the authors concluded that
both CT angiography alone or combined with CT venogra-
phy had high predictive values when concordant with the
pretest probability of disease. However, when these imaging
studies were discordant with the pretest probability, addi-
tional studies were needed before excluding venous throm-
boembolic disease from the differential diagnosis for the
patient’s clinical signs and symptoms. Therefore, the addi-
tion of CT venography to diagnostic algorithms has not been
strongly recommended based on the little it adds to predic-
tive value of CT angiography and clinical probability. MRI of
both the pulmonary vasculature and the venous system of
the pelvis and lower extremity also may have a future role in
the workup of this disease. The advantage MRI studies of the
vasculature would be the avoidance of iodine-based contrast
material and its associated risks, including anaphylactic reac-
tions and renal impairment.
4. Diagnostic approach to pulmonary thromboem-
bolism—Many algorithms optimizing diagnostic strategies
have been proposed. A logical approach is to assess clinical
pretest probability based on symptoms and signs and then
apply tests that increase or decrease the probability of disease
(posttest probability). In this way, the likelihood of pul-
monary embolism can be estimated and the risks of treat-
ment compared. Table 24–5 assigns low, moderate, and high
clinical estimates for disease based on clinical findings for
patients evaluated in an emergency department setting.
Validation of these data confirmed that these groups had 8%,
28%, and 74% likelihood of pulmonary embolism.
The clinician must decide whether these probabilities are
a sufficient basis for decisions about whether or not to treat
a patient. If a more certain diagnosis is warranted—and this
is almost always the case with clinical information alone—
another diagnostic test is applied. The first diagnostic study
in many algorithms in the past was the ventilation-perfusion
radionuclide scan, but this has now been replaced in many
centers by CT pulmonary angiography, especially in patients
with preexisting obstructive or parenchymal lung disease
likely to result in uninterpretable scans. Table 24–6 compares
posttest probability using typical sensitivity and specificity
values for various imaging results for selected clinical pretest
probabilities. If posttest probability is sufficiently high to jus-
tify starting treatment or sufficiently low to justify withhold-
ing treatment, further diagnostic tests are not indicated. If
more diagnostic certainty is desired, then noninvasive stud-
ies—compression ultrasonography or pulmonary angiogra-
phy—can be performed. For decision-making purposes, the
pulmonary angiogram is assumed to have 100% specificity;
that is, a negative study excludes pulmonary embolism. The
probabilities of pulmonary embolism when assessed by com-
pression ultrasonography, D-dimer, and other diagnostic
tests are shown in Table 24–7. These tests can be applied
sequentially to support the diagnosis or to exclude the diag-
nosis; although to be certain, these estimates are derived
from a number of studies of different populations. While
they are not strictly independent estimates, the combined
probabilities are still likely to have value. Any decision to
begin or withhold treatment must take into account the risk
of treatment compared with its potential benefit especially in
the ICU patient population.
Treatment
A. Anticoagulation—Anticoagulation is the major therapy
for deep venous thrombosis and pulmonary embolism.
Heparin, either unfractionated heparin (UFH) or low-
molecular-weight heparin (LMWH), is most often given for
4–5 days, overlapping with oral anticoagulant therapy begin-
ning on day 1. Oral anticoagulant agents, mainly in the form
of vitamin K antagonists such as warfarin, are given for a
minimum of 3 months. There are a number of other treat-
ment schedules depending on the underlying cause of the
event, the history of previous thromboembolic events, and
the patient’s underlying medical history. Heparin therapy
should be started immediately before any definitive tests are
performed if there is a strong clinical suspicion and no con-
traindications exist. Heparin also should be started once a
diagnosis of deep venous thrombosis or pulmonary
embolism is confirmed, if not started prior to the diagnosis.
Anticoagulants do not directly affect existing thrombi, but if
given in sufficient amounts, they can prevent further clot
propagation until the patient’s inherent fibrinolytic system
can begin to break down the thrombus or embolism.
In the absence of contraindications, initial treatment
should be with heparin in the form of continuous intra-
venous infusion of UFH or subcutaneous LMWH. To begin
therapy with continuous UFH, an infusion of heparin is
given at a dose that achieves and maintains a stable activated
partial thromboplastin time (aPTT) of 1.5–2.5 times control
when measured at 6-hour intervals. The aPTT then can be
PULMONARY DISEASE
555
measured at approximately daily intervals once this goal
range has been achieved. Failure to achieve an aPTT at least
in the lower level of this range within the first 24 hours of
therapy has been associated with recurrent venous throm-
boembolism in patients with deep venous thrombosis.
Standard heparin dose-adjustment protocols or nomo-
grams for deep venous thrombosis and pulmonary
thromboembolism are highly desirable and have been
shown to increase therapeutic efficacy and reduce bleeding
complications. One weight-based nomogram is shown in
Table 24–8. A bolus of UFH at 80 units/kg of body weight is
given, followed by 18 units/kg per hour. For a 60-kg adult, this
corresponds to 4800 units as a bolus followed by 1080 units/h
or about 26,000 units/day. Concern has been expressed that
Table 24-7. Sensitivity, specificity, likelihood ratios, and posttest probabilities of pulmonary embolism from selected
studies of compression ultrasonography and D-dimer.
Instructions:
For a patient with suspected pulmonary embolism, estimate pretest probability and find posttest probability at intersection of pretest probability
(columns) and test results (rows).
aPTT (s)
Rate Change
(units/kg/h)
Additional Action Next aPTT
<35 (<1.2 × normal) +4 Rebolus with 80 units/kg 6 hours
35–45 (1.2–1.5 × normal) +2 Rebolus with 40 units/kg 6 hours
46–70 (1.5–2.3 × normal) 0 None 6 hours
71–90 (2.3–3.0 × normal) –2 None 6 hours
>80 (>3 × normal) –3 Stop infusion for 1 hour 6 hours
∗
During the first 24 hours, repeat aPTT every 6 hours. Thereafter, obtain aPTT every morning unless it is outside the
therapeutic range.
Modified from Hyers TM: Venous thromboembolism. Am J Respir Crit Care Med 1999;159:1–14.
Table 24–8. Body weight-based dosing of intravenous unfractionated heparin.
Initial dosing
Loading: 80 units/kg
Maintenance infusion: 18 units/kg/h using 25,000 units in 250 mL D
5
W (100 units/mL)
Obtain aPTT before and 6 hours after starting heparin
Subsequent dose adjustments based on aPTT measured at 6-hour intervals
∗
Pretest Probability
8% 28% 50% 78%
Compression Ultrasonography (for Patients with Symptoms of DVT)
Sensitivity Specificity Test Result Likelihood Ratio Posttest Probability
95% 95% Positive 19.00 62% 88% 95% 98%
Negative 0.05 0.40% 2% 5% 12%
Compression Ultrasonography (for Patients without Symptoms of DVT)
62% 97% Positive 21.00 65% 89% 95% 98%
Negative 0.39 3% 13% 28% 53%
D-Dimer
85–100% 45–68% Positive 1.6–2.7 12–19% 38–51% 62–73% 82–88%
Negative 0.09–0.22 0–1.80% 3–8% 8–18% 20–39%

CHAPTER 24
556
the maintenance dose of heparin is frequently underesti-
mated, resulting in recurrent deep venous thrombosis or pul-
monary thromboemboli. The mean heparin requirement in
several studies was approximately 1300 units/h (about 31,000
units/24 h) compared with older studies suggesting that
1000 units/h usually was adequate. The dose is adjusted
upward or downward as needed based on the aPTT.
Although this nomogram should be strictly used only when
the same reagent is employed as in the development of the
nomogram, it should prove useful as a guide in all hospitals.
LMWH differs from standard UFH in its pharmacoki-
netics, bioavailability, and anticoagulant activity and has
found a role in the treatment of both deep venous throm-
bosis and pulmonary embolism. These heparin fractions
have greater bioavailability when given subcutaneously,
longer duration of action, allowing for once- or twice-daily
dosing, and predictable anti-factor Xa activity. The antico-
agulation effects of LMWH can be correlated with body
weight for dosing purposes, and this diminishes the need
for following laboratory parameters to ensure adequate
anticoagulation. In direct comparisons, LMWH has been
associated with fewer major bleeding complications, less
thrombocytopenia, and a lower incidence of osteoporosis
than UFH. All LMWH formulations crossreact with UFHs
and cannot be used as an alternative form of anticoagulation
in patients with heparin-induced thrombocytopenia syn-
drome. Dosage adjustments may be necessary in patients
with morbid obesity and renal insufficiency. Each LMWH
formulation has its own distinct pharmacokinetic profile,
so data on one form cannot be readily extrapolated to
another form in the same class. In the treatment of deep
venous thrombosis and pulmonary thromboembolism, the
2004 American College of Chest Physicians (ACCP) con-
sensus recommendations are as follows: LMWH treatment
administered subcutaneously in doses adjusted to body
weight or dose-titrated intravenous UFH for at least the
first 5 days of therapy; LMWH is preferred over UFH in
patients with “nonmassive” pulmonary emboli, whereas
UFH is preferred in patients with severe renal impairment.
In addition to heparin, warfarin can be started on day 1
unless there are contraindications to its use. Warfarin, an oral
anticoagulant that inhibits synthesis of vitamin K–dependent
coagulation factors, becomes an effective anticoagulant only
after disappearance of previously synthesized circulating
coagulation factors. Thus several days are needed for war-
farin to have an antithrombotic effect, whereas its anticoag-
ulant effect occurs sooner. In the past, concern has been
raised about a potential hypercoagulable state induced by
warfarin because synthesis of proteins C and S, naturally
occurring anticoagulants, is also inhibited by this agent. This
problem is encountered very rarely.
Almost all patients can be started with a single oral dose
of warfarin at 5 mg/day. The goal is to achieve an interna-
tional normalized ratio (INR) of 2.5 (range 2.0–3.0). This
corresponds roughly to a prolongation of the prothrom-
bin time to 1.3–1.5 times normal (using the usual tissue
thromboplastin assay employed in laboratories in North
America). Nomograms for warfarin dosing suggest that the
INR measured at least 15 hours after the first dose is helpful
in deciding on subsequent doses. If the first INR is greater
than 1.5, a very low maintenance dose (1 mg) is probably suf-
ficient; an INR of between 1.2 and 1.3 calls for a dose of 2–3
mg/day. All other patients should receive a second oral dose
of 5 mg and should be monitored by continued daily INR
measurements. Heparin can be discontinued after 4–5 days if
the INR is greater than 2.0 for two consecutive days.
The dose of warfarin should be adjusted according to the
prothrombin time. Initially, the prothrombin time should
be measured daily until a stable INR is achieved. Thereafter,
twice-weekly measurements followed by weekly measure-
ments should be adequate. A number of drugs interact with
warfarin, both increasing and decreasing its effectiveness.
Antibiotics in particular may decrease bacterial flora of the
gut that are responsible for a significant amount of vitamin
K synthesis. In the ICU, other drugs that may prolong the
prothrombin time by potentiating the action of warfarin
include aspirin, nonsteroidal anti-inflammatory drugs
(NSAIDs), omeprazole, and amiodarone, as well as antimi-
crobial agents such as most cephalosporins, erythromycin,
fluconazole, and metronidazole. On the other hand, barbi-
turates, rifampin, and carbamazepine may reduce the effect
of warfarin on the prothrombin time by increasing the rate
of its metabolism in the liver. Warfarin and other oral vita-
min K antagonists are contraindicated during pregnancy
because of the potential for abnormal fetal development.
The optimal duration of anticoagulation therapy for deep
venous thrombosis and pulmonary embolism has not been
determined for all clinical situations and depends on under-
lying predisposing risk factors. Studies have shown that
heparin followed by 3 months of warfarin results in an
acceptably low (<5%) frequency of recurrent deep venous
thrombosis in patients who have a transient and identifiable
risk factor. This applies to patients with a first event whose
predisposing risk factor, such as immobilization from a bro-
ken bone, surgery, or trauma, has resolved. For patients with
a first episode of idiopathic venous thromboembolism (no
identifiable risk factor), at least 6 months of anticoagulation
therapy is recommended. On the other hand, patients in
whom risk factors are long term and poorly reversible, such
as those with chronic congestive heart failure or hypercoagu-
lable states, should receive a longer period of anticoagulation
therapy on the order of 12 months or even indefinitely. In
patients with a hypercoagulable state associated with malig-
nancy, the effectiveness of anticoagulation is highly variable.
Patients unable to receive warfarin should be given either
subcutaneous UFH at a dosage sufficient to prolong the
aPTT more than 1.5 times control (adjusted-dose subcuta-
neous heparin) or daily LMWH.
The major complication of UFH therapy is bleeding,
occurring in approximately 5% of patients (ranges from 1% in
those with low risk for bleeding to 10% in those with high risk).
LMWH has imposed a lower risk of major bleeding events
