Boggs S. Principles of Sedimentology and Stratigraphy
Подождите немного. Документ загружается.

15.4 Chronocorrelation
537
Note that chronocorrelation on the basis of physical event stratigraphy re
quires that event beds can be recognized and aced laterally in outcrop or that
they can be correlated on the basis of distinctive lithology (lithocorrelation). Be
cause they were produced as a result of an event that took place rapidly, lithocor
relation also results in chronocorrelation. Of course, the actual ages of event beds
must be established by the radiometric dating techniques discussed in the preced
ing section.
Biologic events include episodes of punctuated evolution, mass extctions,
mass mortalities (caused, for example, by major ash fall to a basin), and rapid
immigration and emigration (Kauffman, 1988; Wa llister, 1996). Some of the tech
niques and problems of ronocorrelation by biologic events are discussed in the
preceding chapter on biostratigraphy.
Event Correlation Based on T nsgressive-Regressive Events
A different approach to event correlaon is represented by local correlation based
on position within a transgressive-regressive succession or cycle (Ager, 1993b).
According to Ager, event correlation in this case is based on e correlation of cor
responding peaks of smetric sedimentary cycles that are presumed to be syn
chronous. The events represented in this type of correlation are the result of
transgressions and regressions that may represent either worldwide, simultane
ous, eustatic changes in sea level or more local changes owing to upft, subsi
dence, or fluctuation in sediment supply.
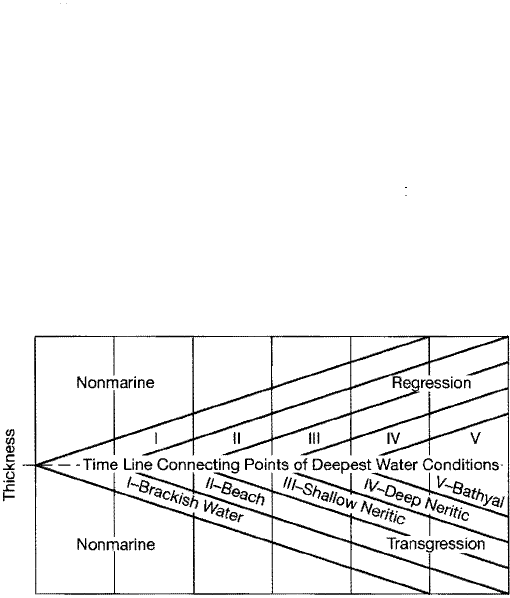
The principle of correlation based on transgressive-regressive events is illus
trated in Figure 15.6. The deposits formed during any transgressive-regressive
cycle contain one particular time plane that represents the time of maximum in
undation by the sea, that is, the time at which water depth was greatest at any par
ticular locality. Rocks lying stratigraphically below this time plane were deposited
during transgression and those above during regression. This time plane can be
identified by use of fossil data to determine depth zonation and maximum water
depth at various localities, as illustrated Figure 15.6. The position of e time
plane can be established also from liologic evidence by determining in the verti
cal stratigraphic section at each locality the position within the section where the
rocks are symmetrically distributed with respect to the most basinward facies pre
sent. A surface connecting the most basinward rocks in each of the vertical sec
tions defines the approximate position of the time plane and thus the
time-stratigraphic correlation between the sections. Figure 15.7 further illustrates
the method. Note from this illustration how time-equivalent points on the cycle
Landward
A
B
Localities
c D E
F
Seaward
Figure 15.6
Time correlation by position in a transgressive
regressive cycle. The line connecting points of
deepest-water condition is a time line. [Aer
G
lsraelski, M. C., 1949, Oscillation chart: Am . Assoc.
Petroleum Geologists Bull., v. 33, Fig. 3, p. 98.]
538
Chapter 15 1 Chronostratigraphy and Geologic me
Figure 15.7
1
E
w E
. :
.
.-.
.
·
.
·:
.
:
·
·
.
.-
.
.
·
.
:
:
.
·
.
·
.
..
·
.
.
_:
.·
·
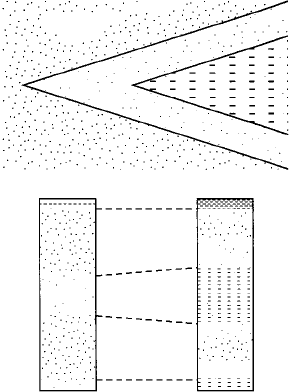
Transgressive-regressive cycle sedimentation and event correlation in the
Eocene of the Isle of Wight in southern England. [From Ager, D. V., 1993, The
nature of the stratigraphical record, 3rd ed., Fig. 7.2, p. 100. Reprinted by
permission of john Wiley & Sons Ltd.
CROSS - BEDDED SANDS
§ MINATED BEDS
� GUCONITIC CYS
are related, resulting in a correlation in which glaucotic clays at the east end of the
succession are equated to laminated beds at the west end. Correlation is expressed,
as Ager (1993b, p. 101) puts it, in terms of degrees of "marineness." Correlaon in
this manner can be considered to be a part of sequence stratigraphy (Chapter 13).
Correlation by Stable Isotope Events
Variations in the relative abundance of certain stable, nonradioactive isotopes in
marine sediments and fossils, referred to as stable isotope geochemistry (e.g., Val
ley and Cole, 2001), can be used as a tool for chronostragraphic correlation of
marine sediments. Geochemical evidence shows that the isotopic composition of
oxygen, carbon, sulfur, and strontium in the ocean has undergone large fluctua
tions, or "excursions," in the geologic past-fluctuations that have been recorded
in marine sediments. Because the mixing time in the oceans is about 1000 years or
less, marine isotopic excursions are considered to be essentially isochronous
throughout the world. Variations in isotopic composition of sediments or fossils
allow geochemists to construct isotopic composition cues that can be used as
stratigraphic markers for correlation purposes. To be useful for correlation, fluctu
ations in isotopic composition must be recognizable on a global scale and must be
of sufficiently short duration to show up as a shift on isotopic composition curves.
Also, stratigraphers must be able to fix the relative stratigraphic position of these
fluctuations in relation to biostratigraphic, paleomagnetic, or radiometric scales.
Of the various potentially useful isotopes, oxygen isotopes seem most nearly to
meet these requirements and have proven to be parcularly useful for chronos
tratigraphic correlaon of Quaternary and late Tertiary sediments. Carbon, sulfur,
and strontium isotopes are also useful for correlating rocks of certain ages.
Oxygen Isotopes
The natural isotopes of oxygen are listed in Table 15.3. Most of the oxygen in the
oceans occurs as oxygen-16. Oxygen-18 is much rarer (about 0.2 percent of total
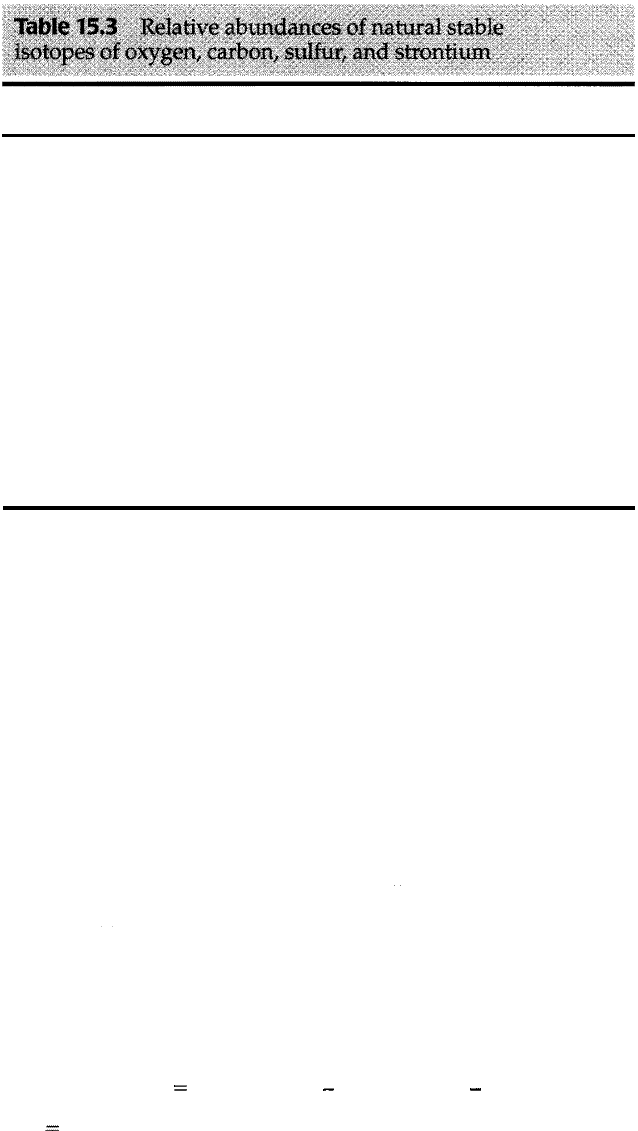
Atomic
Element
number
0
8
c
6
s 16
Sr
38
Source: CRC Handbook of Chemistry and Physics.
Isotope
16
17
18
12
13
32
33
34
36
84
86
87
88
15.4 Chronocorrelation
539
Relative Isotopic
abundances (%)
99.789
0.037
0.204
98.89
1.11
95.0
0.76
4.22
0.014
0.56
9.86
7.02
82 .56
oxygen), but it is present in measurable amounts. The ratio of
180
/
160
in the
ocean at any given time in the past is built into contemporaneous marine carbon
ate minerals and the calcium carbonate shells of marine organisms as a permanent
record of the isotopic composition of the ocean at those times. Fluctuations in oxy
gen isotope ratios in the ocean with time thus show up in the geologic record as
fluctuations in e isotopic ratios of these marine carbonates and fossils. Classifi
cation of deep-sea sedents on the basis of oxygen isotope ratios in the shells of
calcareous marine orgisms, particularly foraminifers, has given rise to a new
stratigraphy for Quaternary sediments. This stratigraphic method is commonly
referred to as oxygen isotope stratigraphy. It was first used by Emiliani (1955),
who studied the isotopic composition of foraminifers in deep-sea cores and used
oxygen isotope ratios to subdivide the core sediments. Oxygen isotope stratigra
phy has now developed into a major tool for correlating Quaternary and late Te r
tiary marine successions, as explained below.
The 180/160 ratio in biogenic marine carbonates reects both the tempera
ture and the 180
/
160 ratio of the water in which these carbonates formed. The re
lationships
of ocean paleotemperature (T) to oxygen isotopic composition has
been shown by Shackleton (1967) to be
T(0C)
16.9 - 4.38(de dw) + 0.10(de dw
)2
(15.1)
where de the equilibri oxygen isotope composition of calcite and dw = oxygen
isotope
composition of the water from which the calcite was precipitated. The de
and dw notations refer not to the actual oxygen isotopic abundances in calcite and
water but to the per mil (parts per thousand) deviation of the 180/160 ratio in cal
cite and water from that of an arbitrary standard. A coonly used standard for
oxygen isotopes in the past was the University of Chicago PDB standard, where
PDB refers to a particular fossil belemnite from the Pee Dee Formation of South
Carolina. More commonly now, the isotope composition of ocean water (Standard

540
Chapter 15 I Chronostratigraphy and Geologic me
Mean Ocean Water, or SMOW) is used as a stan
d
ard (e.g., Coplen, Kendall, and
Hopple, 1983). The per mil deviation from the standard, referred to as a
1s
o, is ex
pressed by the relationship
[(
1S
0/
16
0)
sample - e
s
0/
16
0) standard]
a
1s
o =
x 1000 (15.2)
e
s
0/
16
0)
standard
Oxygen isotope stratigraphy is based on the fact that a
1s
o values in biogenic ma
rine carbonates reflect both e temperature and the isotopic composition of the
water from which e calcite precipitates. These factors are both, in tum, a func
tion of the climate. When water evaporates at the surface of e ocean, e lighter
16
0 isotopes
are preferentially removed in the water vapor, leaving the heavier
IS
o
the ocean. This isotopic fractionation process thus causes water vapor to be
depleted of
IS
O with respect to e seawater from which it evaporates. When
water vapor condenses to form rain or snow, the water containing heav� oxygen
will tend to precipitate first, leaving the remaining vapor depleted in
s
o com
pared to the initial vapor. Thus, the precipitation that falls near the coast and runs
back quickly to the ocean will contain heavier oxygen than that which falls in e
interior of continents or in polar regions, where it returns more slowly to the
ocean. The
1s
o;
16
0 ratio of precipitates also correlates with the air temperature.
The colder e air, the liter the rain or snow (Odin, Renard, and Grazzini, 1982).
For example, the overall average oxygen isotope composition of seawater is
.28 %o (per mil); however, the precipitation that falls in e crests of the Green
land Ice Sheet is about -35%o and in relavely accessible parts of the Antarctic
Ice Sheet it is as negative as -58 %o .
The
1s
0-depleted moisture that falls in polar regions is locked up as ice on
land and is thus prevented from quickly retuing to e ocean. Because of this re
tention of light-oxgen water in the ice caps, the ocean becomes progressively en
riched in
lS
o as
s
O-depleted ice caps build up during a glacial stage. Marine
carbonates that precipitate in the ocean during a glacial stage, particularly bio
genic carbonates such as foraminifers, will be enriched in
1s
0 relative to those that
precipitate during times when the climate is warmer and ice caps are absent, or
are
much smaller, on land. Changes in e a
1s
o content of biogenic marine calcite
us reflect changes in the volumes of ice on land and concomitant changes in sea
leveL That is, sea level drops as ice masses build up on land during glacial e�isodes
and rises when continental ice masses melt durg interglacial stages. The a
8
0 val
ues of seawater track these changes, becoming higher (more posive values) dur
ing glacial stages when heavy oxygen is concenated in the ocean and lower (more
negative) during terglacial stages as melng of polar ice caps retus light-oxy
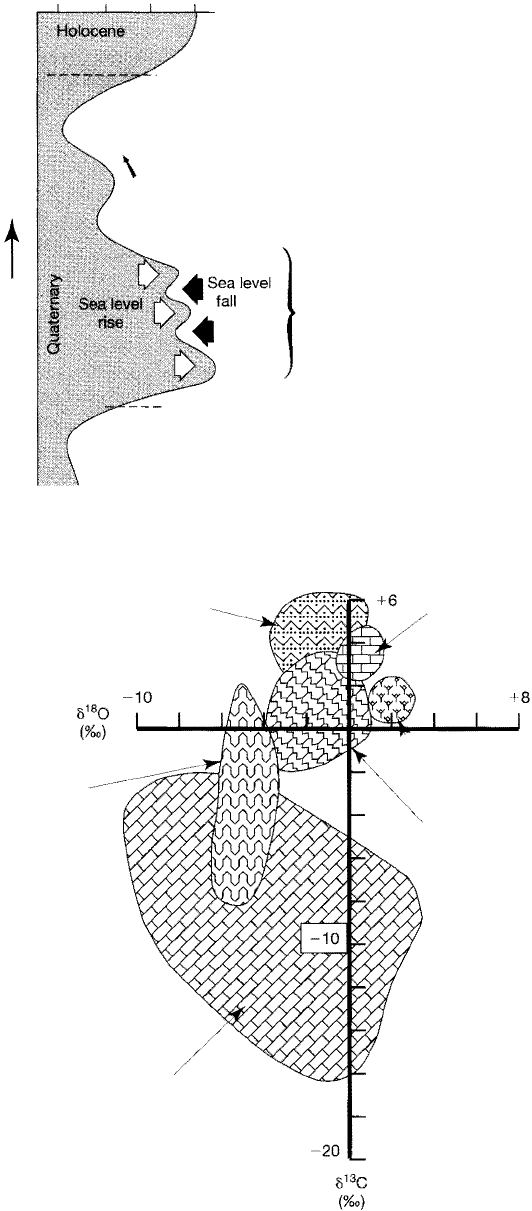
gen water to the oceans. ese principles are illustrated in Figure 15.8.
Decrease temperature of the seawater in which biogenic calcite precipi
tates also causes an increase e a
1s
o values that are built to the calcite. Thus,
during glacial periods both decrease in temperature of ocean water and changes
in isotopic composition of ocean water owing to buildup of ice caps on the conti
nents combine to crease the a
1s
o content of biogenic calcites. Conversely, melt
ing of polar ice caps, with consequent return of light-oxygen water to the oceans,
and increase in ocean temperature will be reflected in a decrease in 8
1s
o values in
marine biogenic carbonates.
Dierent kinds of marine organisms tend to incorporate somewhat different
ratios of oxygen isotopes into their shells (fractionate oxygen isotopes to dierent
degrees), as indicated in Figure 15.9. Therefore, to evaluate changes in oxygen iso
topes in the ocean as a function of time requires that we analyze the same kind of
fossil organism in rocks of different ages. Planktonic foraminifers are the most
common fossil used in oxygen isotope studies of this kind.
d
18
Q (%o}
0
�
0.5 -1 .0 -1 .5
t
E
Hermatypic
corals
Full Interglacial: sea level high stand
Te rmination of glaciation: rapid isotopic
and sea level change
Full Glacial: ice volume maximum, sea level miminum,
18
0 enrichment
in oceans
\
Period of slowly increasing ice volume and
sea level lowering
Last Interglacial: arrows denote
sea level changes
_
Te rmination of glaciation: rapid isotopic and
sea level change
Full Glacial: -150,000 years before present
Green algae
Freshwater
limestone
Shallow-water
marine
limestone
\ Deep-sea
limestone
Shallow-water
molluscs and
foraminifers
15.4 Chronocorrelation 541
Figure 15.8
Schematic illustration of the relationship between
continental glaciation and the o
1
80 content of
ocean water during the last 150,000 years. Note
that
o1
8
0 values become less negative (more
heavy oxygen) during stages of continental glacia
tion and lowered sea level. [After Williams, D. F.,
I. Lerche, and Fult 1988, Isotope chronos
tratigraphy-Theory and methods: Academic
Press, Fig. 30, p. 58. Reproduced by permission.]
Figure 15.9
Distribution of o180 and o1
3
C values in various
types of marine carbonates. (After Milliman, J. D.,
1974, Marine carbonates. Fig. 19, p. 33. Reprint
ed by permission of Springer-Verlag.]
542
Chapter 15 I Chronostratigraphy and Geologic Time
Figure 15.10
Oxygen-isotope stratigraphy
of Pleistocene cores from
Deep Sea Drilling Project
(DSDP) sites in the Pacific and
Atlantic oceans. Note the
numbered isotope stages,
which
can be correlated from
one core to another. M/B
refers to the Matuyama/Brun-
hes polarity chrons. [After
Wei, W., 1993, calibration of
upper Pliocene-lower Pleis-
tocene nannofossil events
with oxygen isotope stratigra-
phy: Paleoceanography, v. 8.,
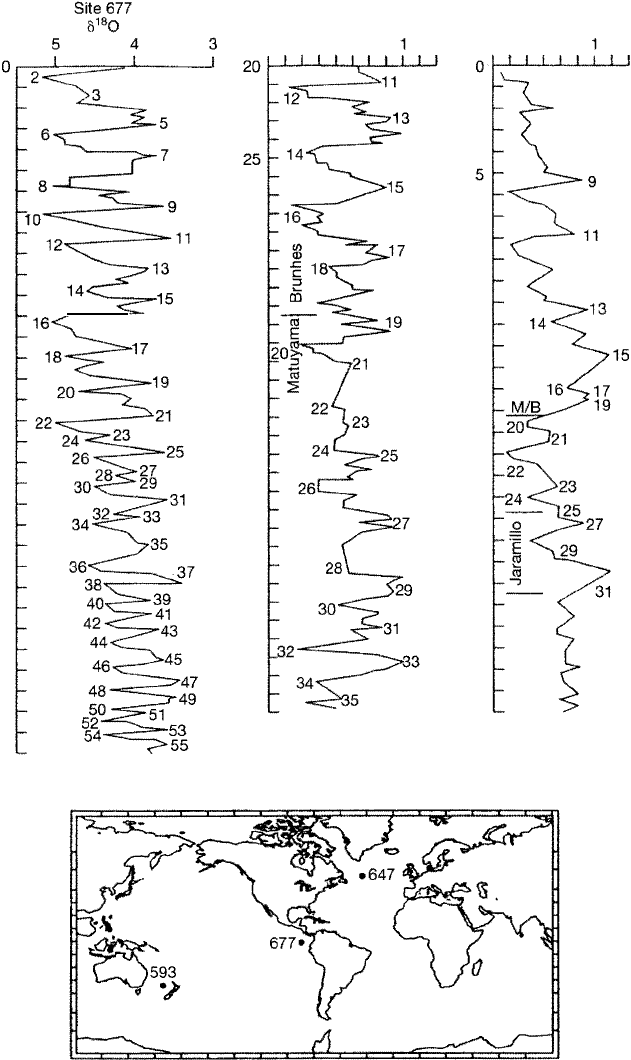
Oxygen isotopes in sediment cores of late Tertiary to Quateary age show
numerous 8180 maxima and minima. Figure 15.10 shows an example of oxygen
isotope values in three Pleistocene cores om widespread localities in e Pacific
and Atlantic oceans (Wei, 1993). The numbers on the isotope curves are oxygen
isotope stage numbers (e.g., Kennett, 1982; Ruddiman et al., 1989; Raymo et al.,
1989). Where available, the magnetostratigraphic chrons are shown also. Once e
isotope stages in a core have been identified and numbered, they can be correlated
10
30
20
0
35
0
30
:
0
40
m
40
45
50
50
60
55
60
40
20
0
20
40
60
Site 647
8
1
80
5 4 3
2 0
10
15
20
25
30
Site 593
31
80
4
3
2 0
Fig. 4, p. 91, published by the
American Geophysical Union.]
120
160E
160W
120
80
40
0
40
80

15.4 Chronocorrelation
543
to e same isotope stages in other cores across the world ocean. These isotopic
events appear to be related to e Milankovich orbital-climate cycles discussed
Chapter 12. us, they provide a record of fourth- and fifth-order cycles that are
presumably driven by changes in climate related to changes in Earth's orbital para
meters. These orbital chges cause uctuations in the intensity of solar radiation
reaching Earth at different latitudes, which, in tum, result in alteate accumula
tion and melting of continental ice sheets, producing rise and fall of sea level.
Carbon Isoto
p
es
Carbon-12 and carbon-13 are e nonradioactive isotopes of carbon. Carbon-12 is
much more abundant than carbon-13 and makes up most of the carbon seawater
(Table 15.3). The isotopic
13
C/
1
2
C ratio can expressed terms of per l deviaon
( 8
13
C) from e PDB standard, just as oxygen isotope ratios are expressed. The 8
13
C
values marine carbonates reflect e
13
C/
1
2
C rao of C0
2
dissolved in deep ocean
water;
is ratio, in tum, reects the source of carbon C0
2
• Carbon dioxide dis
solves in the ocean by interchange with the atmosphere, and it is generated also by e
decay of organic matter that originates both in the ocean and on ld. Organisms pref
erenally incorporate light carbon (
1
2
C); therefore, carbon dioxide derived om de
caying orgac matter is shaly depleted of
13
C compared to at derived om e
atmosphere. us, water runo om the continents (where soil organic matter is
abundant) brings orgc-ri waters with low
13
C/
1
2
C ratios into the ocean, sii
cantly lowering the 8
13
C content of surface ocean waters near the continents. (Note
from Fig. 15.9 the low 8
13
C values in freshwater carbonates deposited in lakes.)
Another factor that influces e 8
13
C contt of ocean water, and us the 8
13
C
content in the shells of marine organisms that live in these waters, is the sidence
me of deep-water masses in the oce. Carbon-13 is depleted in deep-water masses
that have long residence times near the ocean bottom, owing to oxidaon of low-8
13
C
marine orgc matter that sinks om the surface. Oxidation of this low-a
13
C organic
matter leads to producon of low-a
13
C dissolved bicarbonate (HC0
3
-). Respiration
by bottom-dwelling organisms also aepartly causes a decrease in a
13
C of deep bot
tom waters (Kennett, 1982). If low-a
L
C bottom water is later circulated to the surface
in some maer, carbonate-secreng organisms will build this low-a
13
C isotope ratio
into their shells.
The a
B
c content of ocean water is also related to the primary productivity of
photosynthesizing marine organisms (e.g., diatoms). During times of high pro
ductivity (large numbers of organisms present), the rate of removal of light carbon
(
1
2
C)
in
C0
2
by these organisms is high. Selective removal of the light carbon
causes surface ocean waters to be relatively enriched in
B
e, resulting in positive
values of a
13
C. The light carbon is delivered to the seafloor when organisms die,
whe it is reoxidized (Holser and Magaritz, 1992). During times of low primary
productivity, this end is reversed and surface waters tend to have negative a
13
C
values. It has been suggested, for example, that the dramatic decrease in a
B
c val
ues across the Cretaceous-Tertiary boundary (see Fig. 15.11) is the result of marked
decrease in marine photosynthesizing organisms owing to a catastrophic mete
orite impact (e.g., Hsu et al., 1982).
Because the a
B
c in the calcareous shells of marine organisms is a function of
the a
B
c content of the waters in which they live, changes in the o
B
c content of
fossil marine organisms indicate changes in ocean water masses. Abrupt decreas
es in the a
13
c in fossil marine calcareous organisms may reflect changes in prima
ry marine productivity, as suggested, or changes in deep ocean paleocirculation
d upwelling patterns that caused low-a
B
c deep waters to spread upward and
outward into other parts of the ocean. Or such decreases may reflect changes in
544 Chapter 15 I Chronostratigraphy and Geologic Time
44
48
52
:
56
l
60
64
68
0
o
1
3
C (%o PDB)
Site 528
Site 525A
+1
+2
+3
+1
+2
+3
+4
+1
+2
+3
+2
+3
+4
Site 527
Site 529
o
1
3
C (%o PDB)
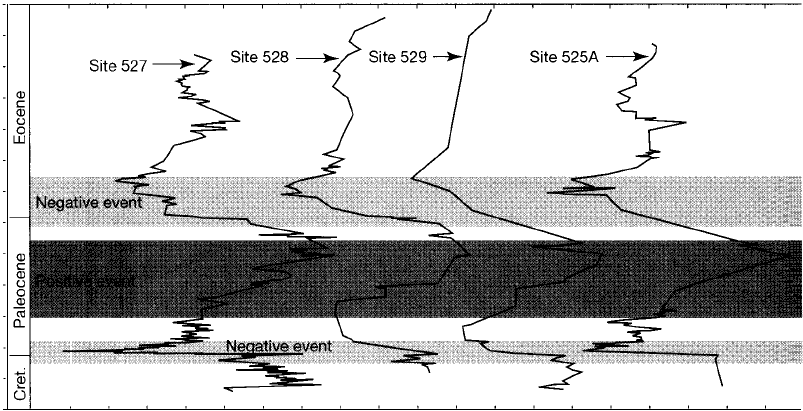
Figure 15.1 1
Carbon isotope stratigraphy of bulk carbonate sediments from Deep Sea Drilling Project
(DSDP) Sites 525A, 527, 528, and 529 in the Atlantic Ocean about 800 km off the coast of
Africa. The sites are about 40 to 70 km apart. [After Shackleton, N. ]., and M. A. Hall, 1984,
Carbon isotope data from Leg 74 sediments, in Moore, T. C., Jr., and P. D. Rabinowitz
et al., Initial Reports DSDP 74, Washington, U.S. Gov. Printing Office., Fig. 1, p. 614.]
surface circulation patterns that brought low-8
13
C surface ocean waters from con
tinental margins into deeper basins. Significant increases in the total biomass pro
duced on the continents during any particular geologic time interval could cause
an increase in runoff of low-8
13
C to the oceans, an increase that may be reflected
also as an episode of low-8
13
C surface water. Increased rates of erosion of organic
rich sediments, such as dark shales and limestones, on land could produce much
the same effect of increasing runoff of low-8
13
C in organic matter to the ocean.
These abrupt changes in circulation patterns or organic carbon runoff from the
continents may have affected the area of a single ocean basin, such as the Pacific,
or in some cases the entire ocean. On the other hand, increased rates of sediment
burial in the ocean may have the opposite effect of removing fine organic matter
containing low-8
13
C from interaction with seawater. This removal would have the
effect of increasing o
13
C in ocean water. Several increases in 8
13
C that took place
the middle Miocene between about 12-18 Ma may be associated with exceptionally
rapid burial of organic carbon and lowered levels of atmospheric C02 (e.g.,
Woodruff and Savin, 1991).
Inasmuch as changes in the 8
13
C content of the ocean are reflected in the 8
13
C
content of marine calcareous organisms, these isotopic events or "excursions" can
be used for correlation, regardless of their exact causes. For example, several
major changes in the Tertiary 8
13
C record include (1) a pronounced negative 8
13
C
event across the Cretaceous-Te rtiary boundary, (2) a positive event from the early
15.4 Chronocorrelation
545
Paleocene into the late Paleocene, and (3) a major a
1
3
C decrease across the Pale
ocene-Eocene boundary (Fig. 15.11). There is also a gradual but significant a
13
c
deterioration from the mid-Miocene to the Pleistocene (e.g., Williams, Lerche, and
Full, 1988, p. 54). Many smaller-scale fluctuations in the Tertiary a
13
C record are
also present. These carbon isotopic excursions are essentially synchronous events
that can be correlated over wide areas of the ocean in DSDP and ODP cores.
Major carbon isotope events are also present in the older sedimentary record.
For example, large changes in carbon isotope content of carbonates have been
measured at the Precambrian/Cambrian boundary and the Permian/Triassic
boundary (Magaritz, 1991).
Sulfu r Isoto
p
es
Sulfur has four stable isotopes (Table 15.3); sulfur-32 is the most abundant, fol
lowed by sulfur-. The
3
4
S/
32
S ratio is used in most stratigraphic studies involv
ing sulfur isotopes and is expressed terms of a
3
4
S, which is per mil deviation of
the
3
4
S/
32
S ratio relative to a meteorite standard, troilite (a FeS mineral) from the
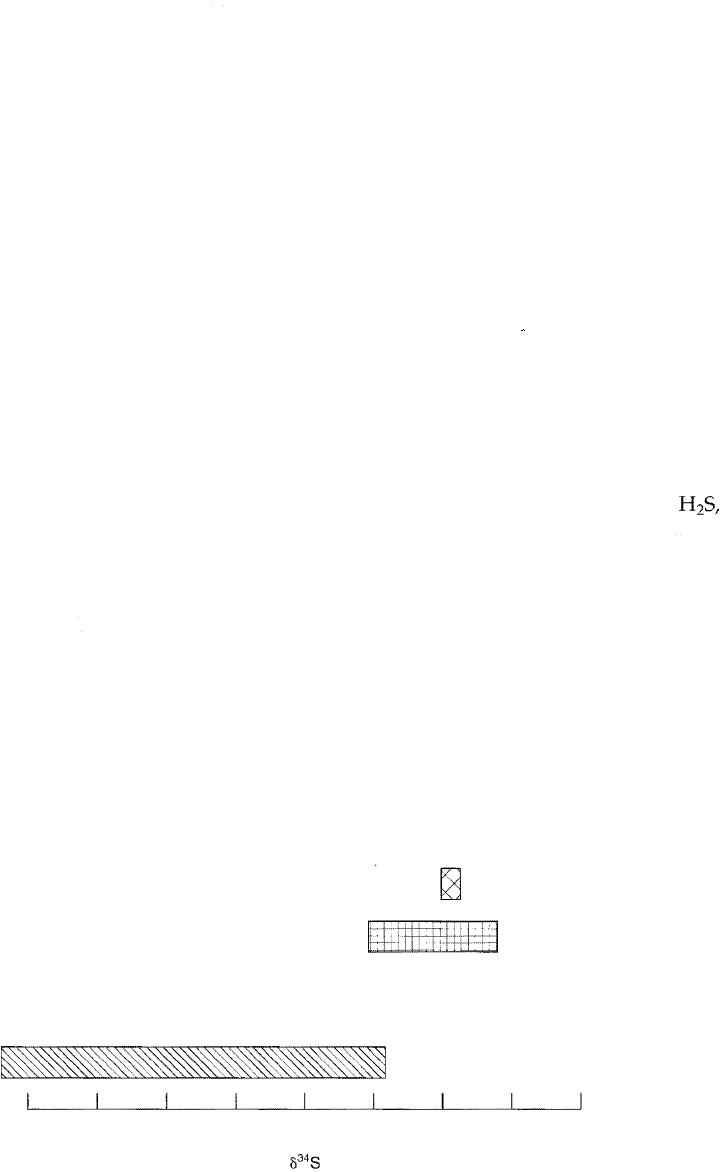
Canyon Diablo meteorite. Figure 15.12 shows the a
3
4
S values in various materials
relative to the Canyon Diablo standard (CDT).
The major means of sulfur isotope fractionation in the oceans is bacterial re
duction of sulfate (S0
4
2
-) in seawater to sulfides (H
2
S, Hs- , HS0
4
-). Bacterial re
duction of dissolved seawater sulfate at the sediment-seawater interface causes
isotopic fractionation of the sulfate, thus enriching the remaining seawater sulfate
in a
3
4
S by about +20%o and depletion in the reduced sulfide by about -9%o (e.g.,
Schopf, 1980; Canfield, 2001). Precipitation of evaporites from dissolved marine
sulfates introduces an additional fractionation (�+1.65%o), causing the a
3
4
S of
evaporites to be higher than that of dissolved marine sulfates. Other minor factors
that can influence the a
3
4
S content of seawater include oxidation of bacterial
which produces sulfates depleted of
3
4
S relative to original sulfates, and local em
anations of sulfate or sulfide through volcanic activity.
Marine sulfates in the present ocean have a mean a
3
4
S of about +21 %a,
however, the a
3
4
S of ancient marine evaporites ranges from about + 10 to +30
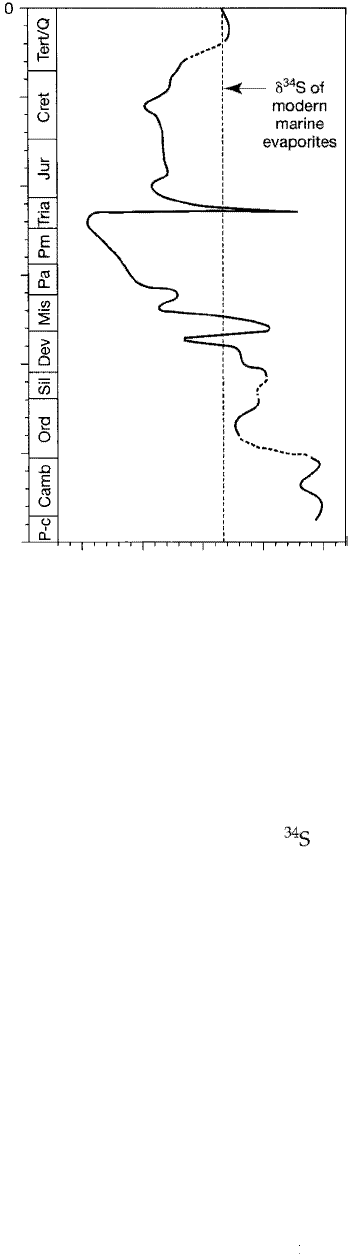
(Fig. 15.13). On the basis of sulfur isotope ratios in ancient evaporite deposits, it
appears that the sulfur isotope ratios in the surface waters of the world ocean have
undergone major changes, or excursions, at various times. These major excursions
are characterized by sharp rises in a
3
4
S in the surface waters of the world ocean fol
lowed by significant drops. The early Paleozoic exhibits high a
3
4
S levels, dicative
I
Canyon Diablo meteorite
(standard)
Marine sulfates, recent
Marine sulfates, ancient
�Seawater sulfate
Rainwater sulfate
-
Sedimentary sulfides
�
40
�
30
�
20
�
10
0
10
20
30
40
Figure 15.12
a
34
S values of marine sulfates shown rela
tive to that of the Canyon Diablo mete
orite. Values for seawater sulfate, rainwater
sulfate, and sedimentary sulfides are shown
for comparison. [Data from Degens, 1965.]
546
Chapter 15 I Chronostratigraphy and Geologic Time
0
100
200
6 300
�
400
Figure 15.13
Sulfur isotope age curve for Phanerozoic marine evaporites. Dashed
portions of the curve indicate lack of data. The dashed vertical line
indicates the o
34
S composition of marine evaporites in the modern
ocean. [After Holser, W. T., M. Magaritz, and j. Wright, 1986, Chem
ical and isotopic variations in the world ocean during Phanerozoic
time, in Walliser, 0. (ed.), Global bio-events, Lecture Notes in Earth
Sciences, v. 8, Fig. 4, p. 69, Springer-Verlag.]
500
u
600
.
'
-
-
+10
+20
8
3
48, 0/00 COT
+30
of a high net flux of sulfide from ocean to sediments; 8
34
5 drops to a minimum in
the Permian, and it returns to intermediate levels in the Mesozoic (Holser, Maga
ritz, and Wright, 1986). Many of these sulfur isotope excursions appear to have af
fected the ocean worldwide.
Chemical events characterized by sharply increased 8
34
5 may be caused by
catastrophic mixing of deep
34
5-rich brines with surface waters. Brines generated
by evaporite deposition are stored in deep basins. Underneath the brines, bacteri
al reduction of sulfates to form pyrite builds up a store of brine heavy in sul
fate. Catastrophic mixing of these
34
-rich brines with surface waters, owing to
destruction of the storage basin by tectonism, causes a sharp rise in the 8
34
5 of sur
face ocean waters and consequently in the evaporite deposits formed from these
surface waters (e.g., Holser, 1977; 1984). Gradual decrease in the 8
34
5 of surface
ocean waters with time after a catastrophic event is attributed to on-land erosion
of
predominantly sulfide materials into the ocean in an amount that exceeds evap
orite deposition (e.g., Claypool et al., 1980).
In any case, these sulfur isotope excursions con.titute a sulfur isotope age
cur
ve because each catastrophic chemical event occurred within a very short in
terval of geologic time. Each major event thus represents a synchronous stra
graphic marker that can be correlated in marine evaporite deposits from one area
to another. Some of the events can be correlated on a global basis. Thus, they pro
vide an important method for inteational chronostratigraphic correlation of
evaporite deposits, which commonly cannot be correlated by other means because
they do not contain fossils or other datable materials. On the other hand, only a
few well-defined, correlatable points are present on the sulfur-isotope curve; thus,
sulfur isotopes are less useful overall for correlation purposes than are oxygen and
carbon isotopes.
