Blum W., Riegler W., Rolandi L. Particle Detection with Drift Chambers
Подождите немного. Документ загружается.


66 2 The Drift of Electrons and Ions in Gases
Case of E Orthogonal to B. The second case concerns the drift volume of the axial
wire drift chamber, where cos
2
φ
= 0; here
c
2
=
eE
mN
σ
2
2
λ
+
ω
2
2N
2
σ
2
2
1/2
−
ω
2
2N
2
σ
2
. (2.49)
We note that the random velocity c is reduced in the presence of a magnetic field
orthogonal to E. The same is true for the drift velocity because of (2.47). Any two
values of E and B that produce the same solution for c in (2.49) lead to the same
drift velocity. In particular, if E
1
is the electric field that produces certain values of
c and u in the absence of magnetic field (B
1
= 0), then there is a corresponding E
2
for the same values of c and u at some non-zero B
2
. In order to find an expression
for E
2
in terms of E
1
and B
2
, we rewrite (2.49) for the two pairs of fields in the
following form:
−
eE
1
mN
σ
2
2
λ
+ c
4
= 0,
ecB
2
mN
σ
2
−
eE
2
mN
σ
2
2
λ
+ c
4
= 0.
(2.50)
Note that λ and
σ
are the same in both cases because they are functions of c alone.
From (2.50) we deduce that
E
2
2
= E
2
1
1+
eB
2
cmN
σ
2
= E
2
1
(1+
ω
2
2
τ
2
), (2.51)
which is the microscopic justification for (2.12) and (2.13). One cannot understand
(2.12) from a derivation using a constant friction term
τ
because
τ
does not remain
constant when E is varied (see (2.7) and Fig. 2.17). Rather, as we have seen,
τ
also
depends on B in such a way that it comes back to its old value for the appropriate
combination of B and E.
Next, we wish to evaluate (2.49) for large values of the B field. In the limit
ωτ
1 the second term in the square root of (2.49) becomes much larger com-
pared to the first term (using (2.46) and (2.47) it can be shown that the two terms
are equal when
ωτ
2.2), and we have to first order in 1/
ωτ
c
2
=
eE
m
ω
2
2
λ
, (2.52)
u
2
=
eE
m
ω
2
, u =
E
B
, (2.53)
which is remarkable because c no longer depends on
σ
.
Equation (2.53) has the following significance. In the presence of a magnetic
field B, orthogonal to E and strong enough for
ωτ
to be large, the drift velocity u
approaches a universal value E/B, which is the same for all gases.
2.2 The Microscopic Picture 67
In order to study the deviations from the universal expression (2.53), we may
carry the expansion of the square root in (2.49) to the second order. Using (2.47)
and (2.53) we find
u =
E
B
1−
1
ω
2
τ
2
(
ωτ
1). (2.54)
This means that the universal limit (2.53) is approached rapidly from below as B is
increased.
This physical content of (2.53) can best be understood by comparison with the
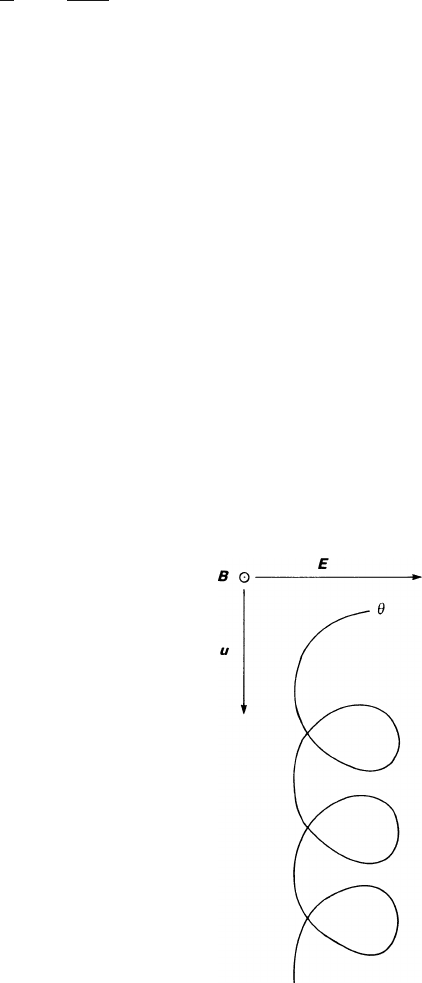
motionofanelectronin vacuo: under the influence of crossed electric and magnetic
fields, the electron is accelerated by the E field and bent by the B field; it performs
a cycloidal motion in the plane orthogonal to B (see (2.43) and Fig. 2.7). When
averaging over the periodic part, one finds that the electron drifts with the constant
speed E/B in the direction of −
ˆ
E ×
ˆ
B. This motion in vacuo is the limit of the
gas drift for large values of
ωτ
, the mean number of turns (expressed in radians)
between collisions. Experimentally it can be reached either by increasing the B field
or by reducing the gas density, which is roughly proportional to 1/
τ
.
2.2.4 Diffusion
As the drifting electrons or ions are scattered on the gas molecules, their drift ve-
locity deviates from the average owing to the random nature of the collisions. In the
simplest case the deviation is the same in all directions, and a point-like cloud of
Fig. 2.7 Motion of an elec-
tron in vacuo under the
influence of orthogonal E
and B fields: B goes out of the
paper, the electron stays in the
plane of the paper

68 2 The Drift of Electrons and Ions in Gases
electrons which then begins to drift at time t = 0 from the origin in the z direction
will, after some time t, assume the following Gaussian density distribution:
n =
1
√
4
π
Dt
3
exp
−r
2
4Dt
, (2.55)
where r
2
= x
2
+ y
2
+(z −ut)
2
; D is the diffusion constant because n satisfies the
continuity equation for the conserved electron current Γ :
Γ = nu −D∇n, (2.56)
∂
n
∂
t
+ ∇·Γ = 0, (2.57)
∂
n
∂
t
+ n∇·u −D∇
2
n = 0. (2.58)
The diffusion constant D, defined by (2.56), makes the mean squared deviation of
the electrons equal to 2Dt in any one direction from their centre. (This is a special
case of (2.75), which deals with the case of anisotropic diffusion.)
In order to express the diffusion constant in terms of the microscopic picture, we
suppose that one electron or ion starts at time t = 0 and has a velocity c; hence,
according to (2.16), there is probability distribution of free path l equal to
g(l)dl =
1
l
0
e
−l/l
0
dl, (2.59)
where l
0
= c
τ
is the mean free path. The simplest case is the one where scattering
is isotropic with respect to the drift direction. We consider this case first. It applies
to ions at low electric fields E(E/(N
σ
) kT) and, to a good first approximation,
to electrons.
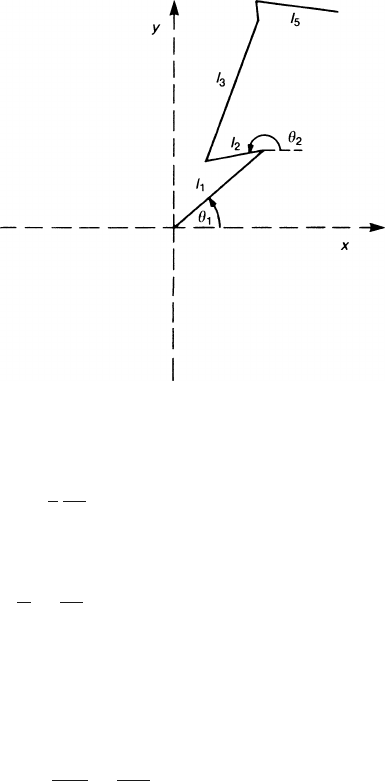
Consider a time t at which a large number, n, of encounters have already oc-
curred; then n = t/
τ
. The mean square displacement in one direction, say x,is
n
∑
1
l
i
cos
θ
i
2
n
∏
1
g(l
k
)dl
k
d cos
θ
k
2
= n
2
3
l
2
0
=
2
3
l
2
0
τ
t. (2.60)
Hence l
i
cos
θ
i
is the displacement along x between the (i −1)th and the ith colli-
sion (Fig. 2.8); the cos
θ
i
are uniformly distributed between −1 and +1 according
to our assumption. The l
i
are distributed with the probability density (2.59). Note
that the mixed terms in the sum vanish. The part proportional to 2t is the diffusion
coefficient D,
D =
l
2
0
3
τ
=
cl
0
3
=
c
2
τ
3
=
2
3
ε
m
τ
. (2.61)
Recalling the expression for the electron mobility
μ
,
μ
=
e
m
τ
,
2.2 The Microscopic Picture 69
Fig. 2.8 Electron paths for
the derivation of (2.60)
we notice that the electron energy can be determined by a measurement of the
ratio D/
μ
:
ε
=
3
2
De
μ
. (2.62)
When the diffusing body has thermal energy,
ε
=(3/2)kT, (2.62) takes the form
D
μ
=
kT
e
,
which is known as the Nernst–Townsend formula,ortheEinstein formula.(Forhis-
torical references, see [HUX 74].)
The energy determines the diffusion width
σ
x
of an electron cloud which, after
starting point-like, has travelled over a distance L:
σ
2
x
= 2Dt =
2DL
μ
E
=
4
ε
L
3eE
. (2.63)
In drift chambers we therefore require small electron energies at high drift fields
in order to have
σ
2
x
as small as possible. In the literature one finds the concept of
characteristic energy,
ε
k
, which is related to our
ε
by the relation
ε
k
=(2/3)
ε
.
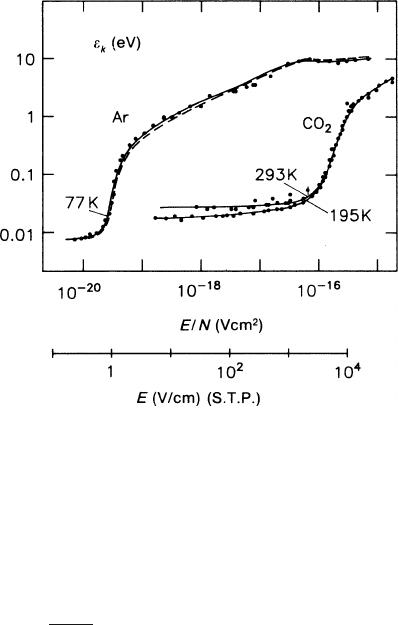
In Fig. 2.9 we show the variation of
ε
k
with the electric field strength, measured
with electrons drifting in the two gases, argon and carbon dioxide, which repre-
sent somewhat extreme cases concerning the change-over from thermal behaviour
to field-dominated behaviour. In argon a field strength as low as 1 V/cm produces
electron energies distinctly larger than thermal (‘hot gas’). In carbon dioxide the
same behaviour occurs only at field strengths above 2 kV/cm (‘cold gas’). The rea-
son is a large value of the relative energy loss λ in CO
2
, due to the internal degrees
of freedom of the CO
2
molecule, which are accessible at low collision energies.
70 2 The Drift of Electrons and Ions in Gases
Fig. 2.9 Characteristic energy
of electrons in Ar and CO
2
as a function of the reduced
electric field. The electric
field under normal condi-
tions is also indicated. The
parameters refer to the dif-
ferent temperatures at which
the measurements were made
[SCH 76]
The fractional energy loss λ was introduced in Eq. 2.15 and played a decisive role
throughout Sect. 2.2.
For ions and electrons with thermal energy
ε
=(3/2)kT, (2.63) shows that the
diffusion width of a cloud is independent of the nature of the gas and is proportional
to the square root of the absolute temperature: the ‘thermal limit’ is given by
σ
x
=
2kTL
eE
1/2
. (2.64)
2.2.5 Electric Anisotropy
Until 1967 it had always been assumed that the diffusion of drifting electrons in
gases has the isotropic form implied by (2.55). But then Wagner et al. [WAG 67]
discovered experimentally that the value of electron diffusion along the electric field
can be quite different from that in the perpendicular direction. The diffusion of drift-
ing ions is also often found to be non-isotropic.
When ions collide with the gas molecules, they retain their direction of motion
to some extent because the masses of the two collision partners are similar, and
therefore the instantaneous velocity has a preferential direction along the electric
field (see Sect. 2.2.2). This causes the diffusion to be larger in the drift direction; the
mechanism is at work for ions travelling in high electric fields E(eE/(N
σ
) ≥ kT),
and we do not treat it here because it plays no role in the detection of particles.
In the case of electrons, there is almost no preferential direction for the instan-
taneous velocity. We will describe the electron diffusion anisotropy following the

2.2 The Microscopic Picture 71
semiquantitative treatment of Partker and Lowke [PAR 69], which is restricted to
energy loss by elastic collisions. The essential point of the argument is that the mo-
bility of the electrons assumes different values in the leading edge and in the centre
of the travelling cloud if the collision rate is a function of electron energy. This
change of mobility inside the cloud is equivalent to a change of diffusion in the
longitudinal direction.
We express the energy balance (2.15) in the drifting cloud more precisely by
making use of the electron current introduced in (2.51); it now contains the diffu-
sion term D∇n. Let the drift be in the z direction (E = E ˆz). Using the collision
frequency
ν
≡ 1/
τ
instead of
τ
and dropping the index from
ε
, the energy balance
takes the form
n
ν
λ
ε
= eE ·Γ = e
μ
E
2
n −eED
∂
n
∂
z
, (2.65)
where
ν
,
μ
, λ and D are functions of
ε
. In principle, we can solve this equation
for
ε
in terms of (1/n)(
∂
n/
∂
z). Here we will put λ constant, as it is in the case of
elastic scattering, and develop
ν
(
ε
) around the equilibrium point, which is given
when
∂
n/
∂
z = 0:
ε
0
=
1
mλ
eE
ν
0
2
.
The functions D and m are approximated by their expressions (2.61) and (2.62).
Putting
ε
=
ε
0
+
Δε
and
ν
=
ν
0
+(
∂ν
/
∂ε
)
Δε
in (2.65), the variation of energy
inside the cloud can be expressed to first order as
Δε
= −
2
ε
2
0
3eE[1 + 2(
∂ν
/
∂ε
)
0
(
ε
0
/
ν
0
)]
1
n
∂
n
∂
z
, (2.66)
(
∂ν
/
∂ε
)
0
being the derivative of the collision frequency with respect to the energy,
evaluated at
ε
0
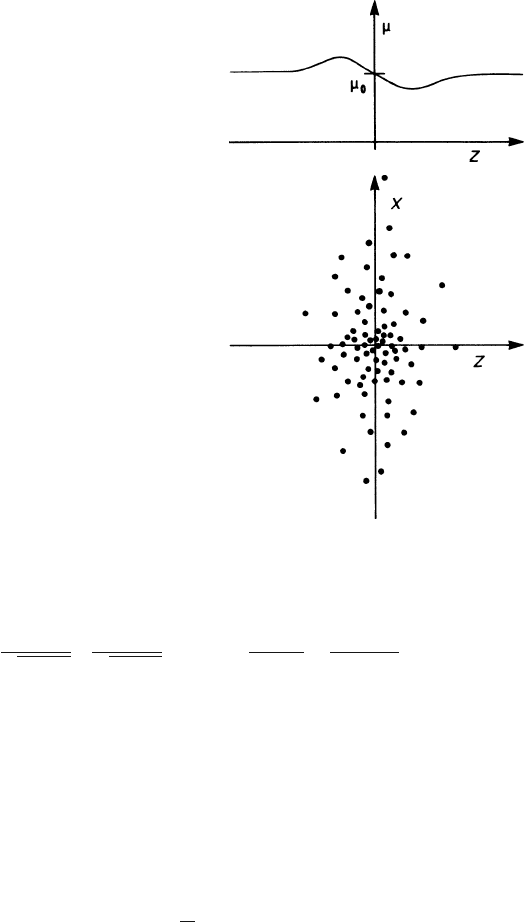
. Equation (2.66) shows that
ε
is larger in the leading edge of the cloud
and smaller in the trailing edge, and from (2.62) it follows that – unless (
∂ν
/
∂ε
)=
0 – the mobility
μ
also is a function of position in the cloud (see Fig. 2.10):
μ
=
μ
0
1
ν
0
∂ν
∂ε
Δε
.
We now rearrange the terms in the expression for the electron current and find
Γ =
μ
0
Enˆz −D
∂
n
∂
x
ˆx +
∂
n
∂
y
ˆy
−D
1−
γ
1+ 2
γ
∂
n
∂
z
ˆz,
where
γ
≡ (
ε
0
/
ν
0
)(
∂ν
/
∂ε
). Obviously the diffusion in the drift direction has
changed and is no longer equal to the diffusion in the perpendicular direction. We
distinguish the two diffusion coefficients by the indices L and T. The ratio is
D
L
D
T
=
1+
γ
1+ 2
γ
. (2.67)
72 2 The Drift of Electrons and Ions in Gases
Fig. 2.10 Mobility variation
inside an electron cloud trav-
elling in the z direction
Instead of the density distribution (2.55) of the diffusing cloud of electrons, we have
in the anisotropic case
n =
1
√
4
π
D
L
t
1
√
4
π
D
T
t
2
exp
−
x
2
+ y
2
4D
T
t
−
(z −ut)
2
4D
L
t
. (2.68)
2.2.6 Magnetic Anisotropy
Let us now consider the effect of a magnetic field B along z. The electric field is
in the x −z plane and we assume there is no electric anisotropy. The magnetic
field causes the electrons to move in helices rather than in straight lines between
collisions. Projection onto the x −y plane yields circles with radii
ρ
=
c
ω
sin
θ
, (2.69)
where
ω
=(e/m)B is the cyclotron frequency of the electron, c its velocity and
θ
the angle with respect to the z axis. Projection onto the x−z plane yields sinusoidal
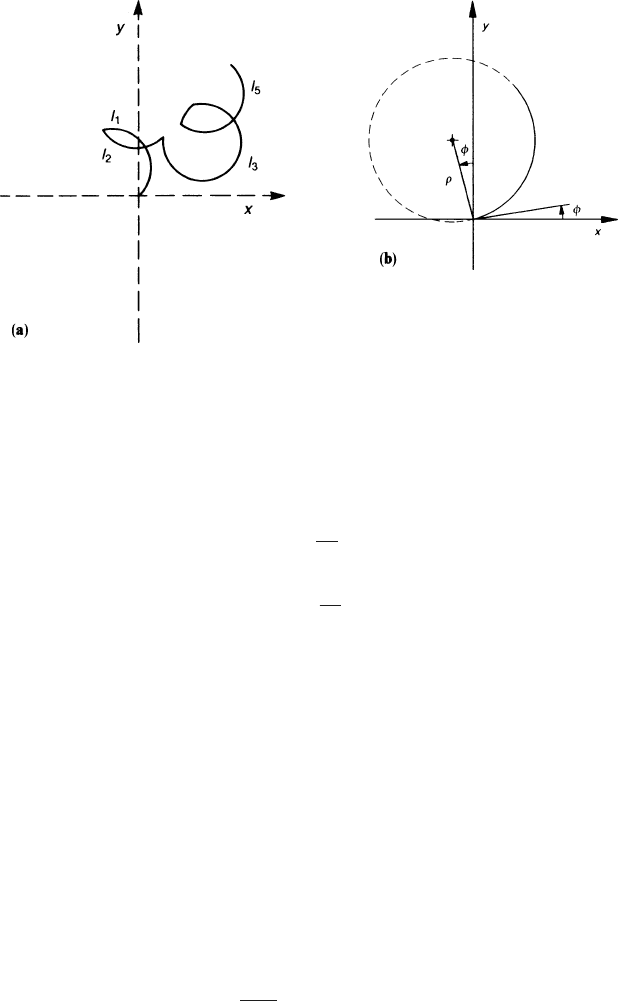
curves. Figure 2.11a, in contrast to Fig. 2.8, shows how the random propagation
of the electron is diminished by the magnetic field. We must repeat our calculation
(2.60) with the new orbits.
2.2 The Microscopic Picture 73
Fig. 2.11 (a) Electron paths causing the magnetic anisotropy of diffusion. (b) Firstpartofthe
trajectory showing the direction projected into the x−y plane
Looking at Fig. 2.11b we describe the motion of the electron which has suffered
a collision at the origin by the orbit
x(l)=
ρ
sin
ω
l
c
−
φ
+ sin
φ
,
y(l)=
ρ
cos
ω
l
c
−
φ
−cos
φ
,
z(l)=l cos
θ
,
(2.70)
where l is the length of the trajectory and
φ
is the starting direction in the x −y
plane. More precisely, the derivatives with respect to l at the origin, giving the initial
direction of the electron, are the following:
x
(0)=sin
θ
cos
φ
,
y
(0)=sin
θ
sin
φ
,
z
(0)=cos
θ
.
The mean square displacement of the electron after the first collision is given
by an integration over the solid angle and over the distribution (2.59) of path
lengths:
x
2
=
1
4
π
l
0
e
−l/l
0
x
2
(l)d
φ
dcos
θ
dl (2.71)
and similarly for y
2
and z
2
. It is not difficult to integrate (2.71), using (2.70) and
(2.69). The result is
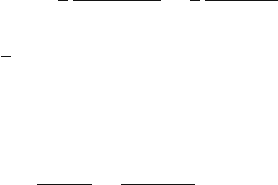
74 2 The Drift of Electrons and Ions in Gases
x
2
= y
2
=
2
3
l
2
0
1+
ω
2
l
2
0
/c
=
2
3
l
2
0
1+
ω
2
τ
2
,
z
2
=
2
3
l
2
0
.
In comparison to (2.60), the magnetic field has caused the diffusion along x and y
(then perpendicular to the magnetic field) to be reduced by the factor
D
T
(
ω
)
D
T
(0)
=
1
1+
ω
2
τ
2
, (2.72)
whereas the longitudinal diffusion is the same as before:
D
L
(
ω
)=D
L
(0). (2.73)
If there is an E field as well as a B field in the gas, the electric and magnetic
anisotropies combine. In the most general case of arbitrary field directions, the diffu-
sion is described by a 3×3 tensor: let the B field be along the z axis of a right-handed
coordinate system S, and let the drift direction ˆu, which is at an angle
β
with respect
to B, have components along ˆz and ˆx. The electric anisotropy is along ˆu, and the dif-
fusion tensor is diagonal in the system S
which is rotated around ˆy by the angle
β
.
In order to describe the two anisotropies in S, we must transform the diagonal
tensor S
to S before we multiply by the diagonal tensor that represents the magnetic
anisotropy. If the electric anisotropy is equal to D
L
/D
T
and the magnetic one is
equal to D(0)/D(
ω
)=1/
η
, we get for the combined tensor S
D
ik
=
⎛
⎝
cos
β
0sin
β
010
−sin
β
0 cos
β
⎞
⎠
⎛
⎝
D
T
00
0 D
T
0
00D
L
⎞
⎠
⎛
⎝
cos
β
0 −sin
β
01 0
sin
β
0 cos
β
⎞
⎠
×
⎛
⎝
η
00
0
η
0
001
⎞
⎠
,
D
ik
=
⎛
⎝
η
(D
T
cos
2
β
+ D
L
sin
2
β
) 0 (D
L
−D
T
)sin
β
cos
β
0 D
T
0
η
(D
T
−D
T
)sin
β
cos
β
0 D
T
sin
2
β
+ D
L
cos
2
β
⎞
⎠
. (2.74)
The importance of diffusion for drift chambers is in the limitation for the co-
ordinate measurement. Hence, we are interested in the deviation along a given
direction of an electron that has been diffusing for a time t. We treat the gen-
eral case of a diffusion tensor D
ij
and a direction ˆα given by the three cosine
α
k
(
α
2
1
+
α
2
2
+
α
2
3
= 1), both expressed in the same coordinate system. We make
use of the continuity equation (2.58) for the density n(x
1
,x
2
,x
3
), normalized so that
n dx = 1. For a time-independent and homogeneous field the drift velocity is
constant, and we have
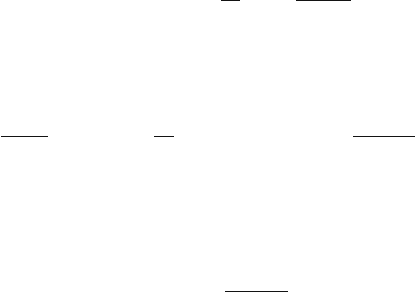
2.2 The Microscopic Picture 75
dn
dt
= D
ik
∂
2
n
∂
x
i
∂
x
k
(summation over identical indices is always understood). The rate of the mean
square deviation along x
=
α
i
x
j
is given by
dx
2
dt
=
+∞
−∞
(
α
i
x
i
)
2
dn
dt
dx =
+∞
−∞
α
i
α
j
x
i
x
j
D
nm
∂
2
n
∂
x
n
∂
x
m
dx = 2
α
i
α
j
D
ij
.
In the last integral all the 81 terms vanish except when the powers of the x
i
match
the powers of the derivatives: one shows by two partial integrations that
+∞
−∞
x
i
x
j
∂
2
n
∂
x
n
∂
x
m
dx = 2
δ
im
δ
jn
,
where
δ
ik
= 1ifi = k, and zero otherwise. We have used the fact that electron density
and its derivatives vanish at infinity
If a point-like ensemble of electrons begins to diffuse at time zero, then after a
time t it has grown so that the mean square width of the cloud in the direction α has
the value
x
2
= 2
α
i
α
j
D
ij
t. (2.75)
The isotropic case in which x
2
is independent of
ˆ
α
is obviously given by a D
ij
which is the unit matrix multiplied with the isotropic diffusion constant. The factor
2 in (2.75) is also present in (2.55) and in the comparison between (2.60) and (2.61).
Equation (2.75) implies that the width of the cloud is calculated only from the sym-
metric part of D
ij
; furthermore, the diffusion tensor must be positive definite, other-
wise our cloud would shrink in some direction–a thermo-dynamic impossibility.
2.2.7 Electron Attachment
During their drift, electrons may be absorbed in the gas by the formation of negative
ions. Whereas the noble gases and most organic molecules can form only stable
negative ions at collision energies of several electronvolts (which is higher than the
energies reached during the drift in gas chambers), there are some molecules that
are capable of attaching electrons at much lower collision energies. Such molecules
are sometimes present in the chamber gas as impurities. Among all the elements,
the largest electron affinities, i.e. binding energies of an electron to the atom in
question, are found with the halogenides (3.1–3.7 eV) and with oxygen (∼0.5eV).
Therefore we have in mind contaminations due to air, water, and halogen-containing
chemicals.
Our account must necessarily be brief; for a thorough discussion of the atomic
physics of electron attachment, the reader is referred to Massey et al. [MAS 69].
