Blum W., Riegler W., Rolandi L. Particle Detection with Drift Chambers
Подождите немного. Документ загружается.


36 1 Gas Ionization by Charged Particles and by Laser Rays
Table 1.7 Va lu es of N
eff
calculated for various average numbers m of clusters, using the cluster-
size distribution of Fig. 1.7
Average number m 1 5 10 20 50 100 200 500 1000
N
eff
1 2.4 3.7 5.2 8.0 12 15 26 41
This is because the x
i
are created independently of each other so that the mixed
terms x
i
x
j
in the square drop out. The statistical factor that multiplies L
2
/12 has
been denoted 1/N
eff
in (1.73); its value depends on the partitioning of the charge
over the m clusters. If all clusters were equally large, then N
eff
would take on the
value m. It is equal to the equivalent number of independently fluctuating entities
of equal sizes that create a fluctuation that is the same as the one created by the
clustered charges. As these are different in size, N
eff
has to be smaller than m.
For a calculation of N
eff
, which governs the localizability of any cluster ionization
charge, we have to integrate over the cluster-size distribution. Starting from the one
plotted in Fig. 1.7 we calculate, with the Monte Carlo method, the values listed in
Table 1.7.
There are two properties of N
eff
that are remarkable: (i) it is quite small compared
with the average number m of clusters (let alone the total ionization); (ii) it does
not grow as fast as m (see the graph in Fig. 1.22). The reason for the latter is
that the relative contribution of the rare large clusters grows with the increase of
the number of clusters as the tail of the cluster-size distribution is sampled more
often.
The variation of N
eff
with m seen in Fig. 1.22 may be described roughly as
N
eff
= m
0.54
. (1.74)
There is a statistical correlation between N
eff
and the total charge deposited in
L. It can be used to increase N
eff
by cutting on the pulse height, but the gain is not
overwhelmingly large.
If diffusion is taken into account, N
eff
may become considerably larger in time,
owing to the declustering effect. This is treated in Sect. 1.2.
Fig. 1.22 Dependence of N
eff
,
the effective number of inde-
pendently fluctuating entities
of charge, as a function of the
mean number m of clusters.
Result of a calculation using
the cluster-size distribution
in Ar

1.2 Calculation of Energy Loss 37
1.2.10 A Measurement of N
eff
The effective number of independently fluctuating charges along the length L of a
track can be measured. The r.m.s. fluctuation of the centre of gravity of the charge
deposited along L is, by definition of N
eff
, equal to
δ
y
c
=
L
√
12N
eff
.
A particle track whose ionization is sampled over a length L by n wires with
pulse heights P
i
(i = 1,...,n) has the centre of its charge at
y
c
=
∑
P
i
y
i
/
∑
P
i
,
where the y
i
are the wire positions along the track. A measurement of y
c
of many
tracks allows a determination of
δ
y
c
, and hence of N
eff
, to be made.
In an experiment using a model of the ALEPH TPC, cosmic-ray tracks were
collected in a small interval of momentum and angle of incidence. The chamber
contained an Ar (90%)+CH
4
(10%) gas mixture at atmospheric pressure and had a
sense-wire spacing of 0.4 cm. Track lengths L between 0.8 and 20 cm were specified
and N
eff
was calculated for every L. The result is shown in Fig. 1.23. The choice
of L is limited on the low side by the wire spacing and on the high side by the
dynamic range of the pulse-height recording. In the case under consideration, the
overflow bin contained 1.5% of the pulses. For higher values of L this bin is sampled
more often, and the calculated number N
eff
begins to depend on the details of an
extrapolation of the overflow bin. A power-law fit to Fig. 1.23 shows that N
eff
varied
with L according to
N
eff
∝ (L/1cm)
0.45±0.1
.
Comparing Figs. 1.22 and 1.23, we notice that the exponent is identical, within
errors, to that obtained from the theoretical cluster-size distribution. Using the power
law, which is only an empirical relation, we may obtain an estimate of the primary
ionization density, because it can be expected that the function N
eff
(L) becomes
equal to 1 at L = λ, the average distance between clusters.
Fig. 1.23 N
eff
measured with
cosmic rays as a function
of the track length L.The
last data point has the largest
dependence on the treatment
of the overflow bin (see text).
The three values correspond
to extrapolation according
to (a) a1/PH
2
law, (b) the
Landau distribution or (c) no
extrapolation, respectively
38 1 Gas Ionization by Charged Particles and by Laser Rays
1.3 Gas Ionization by Laser Rays
The light of a narrow pulsed laser beam that traverses a volume of gas, under
favourable conditions, is capable of ionizing the gas in the beam so that it imitates
a straight particle track. For this to occur, there must be some ionizable molecules
in the gas, and the energy density must be sufficiently high, depending on the wave-
length. In practice, a small nitrogen laser, emitting pulses of 10
4
W on a few square
millimetres at λ = 337nm, may be sufficient in an ordinary chamber gas that has not
been especially cleansed and therefore contains suitable molecules in some low con-
centration. This technique has the obvious advantage of producing identical tracks
in the same place. Therefore, by taking repeated measurements of the same coor-
dinate, one may form the average, which can be made almost free from statistical
variations, if only the number of repeated measurements is made large enough. In
practice 100 shots are usually sufficient. The technique has been widely used ever
since its first application in 1979 [AND 79].
Chemical compounds used for laser ionization are discussed in Sect. 12.4.
1.3.1 The nth Order Cross-Section Equivalent
The quantum energy of laser light in the visible and the near ultraviolet is much
lower than the ionization energies of molecules. It takes two or more such laser
photons to ionize the organic molecules present in the chamber gas. Multiphoton
ionization processes involving 11 photons have been observed in xenon (see the
review by Lambropoulos [LAM 76]). For the ionization of a molecule to occur, the
n photons have to be incident on the molecule during the lifetime of the intermediate
states. Then the ionization rate varies the nth power of the photon flux because
the photons act incoherently in the gas. The probability of n photons arriving in
a given time interval is equal to the nth power of the probability of each one of
them, and is therefore proportional to the nth power of the photon flux
φ
.Justasthe
concept of ‘cross-section’
σ
describes one-particle collision rates in units of cm
2
,
we have an ‘nth-order cross-section equivalent’
σ
(n)
for n-particle collisions in units
of cm
2n
s
n−1
.InavolumeV containing a density N of molecules, the ionization rate
R is therefore given by the expression
R =
φ
n
NV
σ
(n)
. (1.75)
In fact, n-photon ionization is most easily identified by a measurement of R as a
function of
φ
.
A light pulse with cross-sectional area A and duration T contains m photons if
the flux
φ
and the energy E are given by
φ
= m/(AT ),
E = mh
ν
= mhc/λ.

1.3 Gas Ionization by Laser Rays 39
Considering a traversed volume V = AL, the specific ionization per unit track length
for the n-photon process is
RT
L
=
m
n
(AT )
n−1
N
σ
(n)
=
E
AT
n
AT N
λ
hc
n
σ
(n)
.
(1.76)
In (1.76), (E/AT ) is the power density of the beam.
So far, we have implicitly assumed the duration of the light pulse to be short
compared with the inverse transition frequencies between the states involved. The
more general situation develops towards a dynamic equilibrium between excitation
and de-excitation. The case of two-photon ionization is treated in more detail in
Sect. 1.3.2.
The molecular ions created in the ionization do not as a rule resemble the par-
ent molecules very much, because they are cracked in the process. Fragmentation
patterns are complicated and usually not understood [REI 85].
1.3.2 Rate Equations for Two-Photon Ionization
For the electrons in the molecules, we distinguish the ground level (0), the inter-
mediate level (1), and the continuum ionization level (2). We denote the population
densities in the gas by P
0
, P
1
, and P
2
. The incident radiation stimulates transitions
0 →1, 1 → 2, and 1 →0, but there are also spontaneous transitions 1 →0. Depend-
ing on the circumstances, there may be losses from level 1 into other channels. The
transition rates, denoted by k
1
to k
4
, are determined by the incident flux and by the
internal transition mechanism.
The rate equations are written in the following form (the primes denote the time
derivatives):
P
0
(t)=−k
1
P
0
(t)+(k
1
+ k
2
)P
1
(t),
P
1
(t)=+k
1
P
0
(t) −(k
1
+ k
2
+ k
3
+ k
4
)P
1
(t),
P
2
(t)=k
4
P
1
.
(1.77)
They are symbolized in Fig. 1.24.
The rate per molecule k
1
is taken to be proportional to the incoming flux
φ
of pho-
tons; the constant of proportionality is the cross-section
σ
01
for the process 0 → 1:
k
1
=
σ
01
φ
. (1.78)
We want to calculate the transition rate P
2
(t) with which electrons appear in the
continuum state. It is proportional to the density P
1
(t) of the intermediate state, and
the corresponding rate per molecule is
k
4
=
σ
12
φ
. (1.79)

40 1 Gas Ionization by Charged Particles and by Laser Rays
Fig. 1.24 Scheme of the
five transition rates used in
(1.77a–c)
The first two of the rate equations are a system of homogeneous linear differential
equations. Before we derive the general mathematical solution, we shall consider
some limiting cases in order to better understand the physical content of (1.77).
Before the pulse arrives, P
0
(0) is of the order of 10
13
per cubic centimetre in a
gas having a typical concentration of the ionizing molecules of one part per million,
and P
1
(0)=0. While P
1
(t) is slowly built up at the rate P
1
(t), we may consider
P
0
as a constant as long as P
1
(t) has not developed into a similar order of magni-
tude. The situation of making only a few ionization electrons with the laser shot is
well described by this approximation, which treats the ground state as an infinite
reservoir.
(a) Case of P
0
as an Infinite Reservoir. We may write the first two of (1.77) in the
following form:
P
0
(t)=−k
1
P
0
+(k
1
+ k
2
)P
1
(t), (1.80a)
P
1
(t)=+k
1
P
0
−(k
1
+ k
2
+ k
3
+ k
4
)P
1
(t). (1.80b)
The solution of (1.80b) is
P
1
(t)=
k
1
P
0
∑
k
(1−e
−t
∑
k
), (1.81)
where
∑
k = k
1
+ k
2
+ k
3
+ k
4
. Obviously, our approximation is always fulfilled if
k
1
∑
k or
σ
01
φ
∑
k (using (1.78)). Let us assume that this is the case. Equa-
tion (1.81) leads to a production rate of ionization equal to
P
2
(t)=k
4
P
1
(t)=
k
1
k
4
P
0
∑
k
(1−e
−t
∑
k
). (1.82)
Integrated over the duration T of a constant light pulse, the total density reached at
the end of the pulse is given by

1.3 Gas Ionization by Laser Rays 41
P
2
(T)=
k
1
k
4
P
0
∑
k
T −(1 −e
−T
∑
k
)
∑
k
. (1.83)
In the limit of short pulses, i.e. T 1/
∑
k, the exponential may be expanded to
second order and gives us
P
2
(T) →
1
2
k
1
k
4
P
0
T
2
. (1.84)
In the limit of long pulses, i.e. T 1/
∑
k, the exponential vanishes, so that
P
2
(T) →
k
1
k
4
P
0
∑
k
T. (1.85)
Expression (1.84) is proportional to
φ
2
, the square of the photon flux, because of
(1.78) and (1.79). This is also the case for expression (1.85) unless k
1
or k
2
domi-
nates
∑
k. In our approximation that treats P
0
as an infinite reservoir, we assume that
k
1
∑
k; then a similar condition holds for k
2
(except for special circumstances in
which
σ
12
is orders of magnitude larger than
σ
10
):
P
2
(T) →
1
2
σ
01
σ
12
P
0
φ
2
T
2
(small T, small
φ
), (1.86)
P
2
(T) →
σ
01
σ
12
k
2
+ k
3
P
0
φ
2
T (large T, small
φ
). (1.87)
(b) General Case. If we want to know what happens when the photon flux is strong,
(1.77) have to be solved in a rigorous way. This is achieved by a variable
transformation that separates the equations (see any textbook on differential
equations, for example [KAM 59].) One finds the eigenvalues s
1
,s
2
of the ma-
trix of coefficients from the determinant
−k
1
−sk
1
+ k
2
k
1
−
∑
k −s
= 0, (1.88)
s
1,2
= −
∑
k + k
1
2
±
∑
k + k
1
2
2
−k
1
(k
3
+ k
4
)
1/2
. (1.89)
These two eigenvalues are real and negative; we take s
2
< s
1
< 0.
The solutions to the differential equations (1.77) satisfying the initial conditions
P
1
(0)=0,P
2
(0),aregivenby
P
0
(t)=
P
0
(0)
s
1
−s
2
[−(k
1
+ s
2
)e
s
1
t
+(k
1
+ s
1
)e
s
2
t
], (1.90a)
P
1
(t)=
P
0
(0)k
1
s
1
−s
2
[e
s
1
t
−e
s
2
t
], (1.90b)
P
2
(t)=
P
0
(0)k
1
k
4
s
1
s
2
1+
s
2
s
1
−s
2
e
s
1
t
−
s
1
s
1
−s
2
e
s
2
t
. (1.90c)

42 1 Gas Ionization by Charged Particles and by Laser Rays
Equation (1.90c) represents the general solution to the problem. It describes the
concentration of ionization electrons as a function of time and of the rate coefficients
k
1
to k
4
. For small t we recover our expressions (1.84) and (1.86) by developing the
exponentials to the second order in s
1
t and s
2
t:
P
2
(t) →
1
2
k
1
k
4
P
0
(0)t
2
. (1.91)
For large t, using (1.89), we obtain
P
2
(t) →
k
4
k
3
+ k
4
P
0
(0). (1.92)
This enormous concentration of electrons (saturation) would imply that the ground
state has been emptied, a situation that should not concern us when we study ion-
ization tracks that are similar to particle tracks.
Equation (1.91), identical to (1.84) and (1.86), gives rise to the definition of the
second-order cross-section equivalent described in Sect. 1.3.1. For a laser shot of
duration T, identifying R/V with P
2
/T,wehave
σ
(2)
=
1
2
σ
01
σ
12
T. (1.93)
Under the conditions that lead to (1.87), we have instead
σ
(2)
=
σ
01
σ
12
/(k
2
+ k
3
), (1.94)
whereas under the most general conditions, including saturation,
σ
(2)
depends not
only on T but also on
φ
.
1.3.3 Dependence of Laser Ionization on Wavelength
The ionization yield depends very much on the wavelength. Using tunable dye
lasers, Ledingham and co-workers have found an increase of four orders of mag-
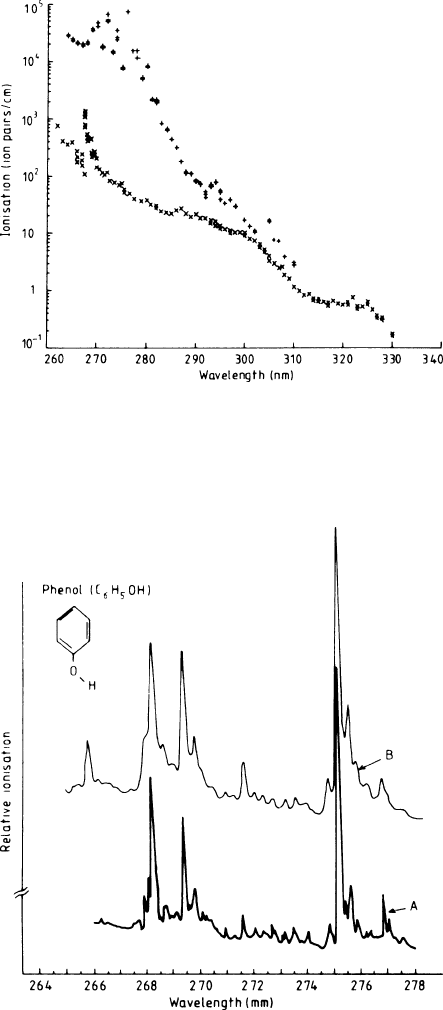
nitude, when going from λ = 330nm to λ = 260nm. In Fig. 1.25 ‘clean’ counter
gas is compared with a gas seeded with a small amount of phenol. The fine
structure visible around 270 nm was resolved using high-resolution techniques
and was compared with the known single-photon UV absorption of phenol
(see Fig. 1.26).
The identical wavelength dependence is apparent. We understand this behaviour
from (1.93) and (1.94): the wavelength dependence resides almost entirely in the
factor
σ
01
, implying that the cross-section for ionization from the intermediate level
does not vary so much. The amount of characteristic structure in the spectrum of
laser-induced resonant two-photon ionization (‘R2PI’) makes it a sensitive tool for
the identification of molecules [HUR 79].
1.3 Gas Ionization by Laser Rays 43
Fig. 1.25 Ionization induced in untreated counter gas [Ar (90%)+CH
4
(10%)] by a 1 ×1mm
2
pulsed laser beam of 1μJ(×), compared with the same gas seeded with a small amount of phenol
(+) [TOW 86]
Fig. 1.26 A comparison of the laser-induced R2PI spectrum of counter gas doped with a trace of
phenol (B) and the single-photon UV absorption spectrum of phenol [TOW 86]
44 1 Gas Ionization by Charged Particles and by Laser Rays
1.3.4 Laser-Beam Optics
A beam of laser light that is to create ionization as if from a particle must have an
approximately constant cross-sectional area along the beam. Relations (1.75) and
(1.76) imply that, for example, in two-photon ionization a light pulse with a given
energy produces half as many ions per unit track length when it has twice the cross-
sectional area. In the interest of a constant high yield and of a narrow deposit of
ionization, one would like to have the beam width as small as possible over the
largest possible track length.
The photons in a beam occupy a volume in the four-dimensional phase space
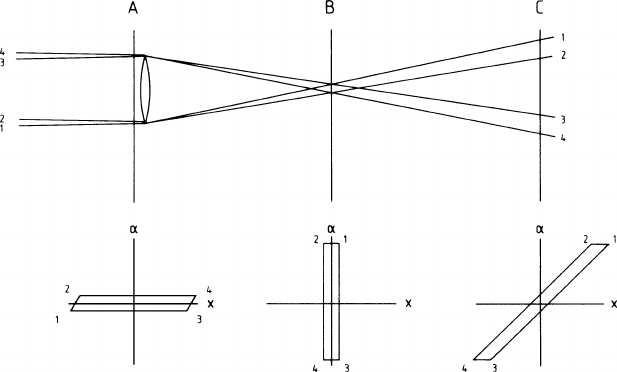
at each point along the beam. For illustration, we consider the horizontal plane in
Fig. 1.27, where an almost parallel beam is shown to be focused into a narrow
waist by a lens. After the focus, the beam opens up again. Each photon trajectory
is represented by a point in the phase-space diagrams below where the distance
from the axis is plotted against the small angle of the trajectory with the axis. Here
we deal with geometrical optics only; the wave optical aspects are treated later. In
this sense we may regard the optical elements of the beam (lenses, mirrors, light
guides, free space, apertures) as determining a trajectory for each photon in a time-
independent fashion. Now, Liouville’s theorem in statistical mechanics states that
in such a passive system the density of photons in phase space is a constant of
motion, meaning that it does not change along the beam. As long as there are no
photons lost, the occupied volume of cross-sectional area times solid angle is the
same everywhere along the beam and the same as just behind the laser. In the phase-
space diagrams of Fig. 1.27 the envelopes containing the beam do not change their
area. A concentration of the beam in space by a focusing lens is accompanied by a
corresponding extension of the solid angle.
Fig. 1.27 Phase-space diagrams at three points along a light-beam, which is almost parallel at the
left and is focussed into B by a lens at A

1.3 Gas Ionization by Laser Rays 45
There is a principal limit of the size, in phase space, of a photon beam.
Heisenberg’s uncertainty principle of quantum mechanics states that, in each of the
two transverse dimensions separately, the limits are given by
Δ
x
Δ
p
x
> h/4
π
, (1.95)
where
Δ
x and
Δ
p
x
are the root mean square widths in space and in momentum
[MES 59] and h is Planck’s constant. Since the r.m.s. opening angle is
Δα
=
Δ
p
x
/p =
Δ
p
x
λ/h 1,
we have
Δ
x
Δα
> λ/4
π
. (1.96)
An example not very far from the principal limit is provided by the situation where a
plane light-wave is delimited by an aperture, say a slit of width ±A. The diffraction
caused by the slit will open the range of the angles
β
of propagation behind the slit
so that approximately
|±
β
|≈
1
A
λ
2
π
. (1.97)
Many lasers can be tuned to emit beams near the fundamental limit. If their wave-
length is subsequently halved by a frequency doubler, the limit refers to the old
wavelength unless the phase space and, inevitably, the power of the beam are re-
duced afterwards.
For practical purposes we work with approximate full widths W
x
and W
α
,
ignoring the exact distribution of intensity across the beam, and use the order-of-
magnitude relation
W
x
W
α
> λ. (1.98)
The highest spatial concentration of light is reached in a beam focus; let it have a
width W
x
. Ideally, the arrival angles of the photons do not depend on the point across
the focus; let the angular width be W
α
. At a distance D behind the focus, every such
point has reached a width
α
D. The statistical ensemble of all the photons has a
combined width of W
tot
given by
W
2
tot
= W
2
x
+(W
α
D)
2
. (1.99)
The quadratic addition of these two widths is correct in the approximation that the
light intensity in the focus is a Gaussian function of both the space and the angular
coordinates. For most applications this is not far from reality.
Applying (1.98) and (1.99), the total width of a focused beam, at a distance D
away from the focus, is not smaller than
W
min
tot
=
W
2
x
+
λD
W
x
2
1/2
. (1.100)
This is plotted in Fig. 1.28 as a function of D for various values of W
x
.
