Bird R.B., Stewart W.E., Lightfoot E.N. Transport Phenomena
Подождите немного. Документ загружается.

517.3 Theory of Diffusion in Gases at Low Density
527
The dimensionless quantity a9,,,-the "collisional integral" for diffusion-is a func-
tion of the dimensionless temperature KT/&,,. The parameters
aAB
and
EAB
are those ap-
pearing in the Lennard-Jones potential between one molecule of
A
and one of
B
(cf. Eq.
1.4-1 0):
This function In,,,, is given in Table E.2 and Eq. E.2-2. From these results one can com-
pute that
9JAB
increases roughly as the 2.0 power of T at low temperatures and as the 1.65
power of
T
at very high temperatures; see the
p,
-+
0 curve in Fig. 17.2-1. For rigid
spheres, would be unity at all temperatures and a result analogous to Eq. 17.3-10
would be obtained.
The parameters
o,,
and
EAB
could, in principle, be determined directly from accurate
measurements of
9,,
over a wide range of temperatures. Suitable data are not yet avail-
able for many gas pairs, and one may have to resort to using some other measurable
property, such as the viscosity4 of a binary mixture of A and
B.
In the event that there are
no such data, then we can estimate
a,,
and
E,,
from the following combining rules:5
for nonpolar gas pairs. Use of these combining rules enables us to predict values of
9,,
within about 6%
by
use of viscosity data on the pure species A and
B,
or within about
10% if the Lennard-Jones parameters for
A
and
B
are estimated from boiling point data
by use of Eq. 1.4-12.~
For isotopic pairs, u,,*
=
UA
=
a,,
and
EM.
=
.FA
=
that is, the intermolecular
force fields for the various pairs A-A*, A"-A*, and A-A are virtually identical, and the
parameters
a,
and may be obtained from viscosity data on pure A. If, in addition, MA
is large, Eq. 17.3-11 simplifies to
I
The corresponding equation for the rigid-sphere model is given in Eq. 17.3-9.
Comparison of Eq. 17.3-16 with Eq. 1.4-14 shows that the self-diffusivity BAA* and
the viscosity
p
(or kinematic viscosity
v)
are related as follows for heavy isotopic gas
pairs at low density:
in which
0,
=
1.1I1,,,* over a wide range of KT/&,, as may be seen in Table E.2. Thus
9AA*
=
1.32~ for the self-difusivify. The relation between
v
and the
binary
difusivity '?JAB is
not so simple, because
v
may vary considerably with the composition. The Schmidt
number Sc
=
p/p9,,
is in the range from
0.2
to 5.0 for most gas pairs.
Equations 17.3-11, 12, 16, and 17 were derived for monatomic nonpolar gases but
have been found useful for polyatomic nonpolar gases as well. In addition, these equa-
tions may be used to predict QAB for interdiffusion of a polar gas and a nonpolar gas by
using combining laws different7 from those given in Eq. 17.3-14 and 15.
*
S. Weissman and
E.
A.
Mason,
J.
Chem. Pkys.,
37,1289-1300 (1962);
S. Weissman,
J.
Ckem. Pkys.,
40,
3397-3406 (1964).
J.
0.
Hirschfelder,
R.
B.
Bird, and
E.
L.
Spotz,
Ckem. Revs.,
44,205-231 (1949);
S. Gotoh,
M.
Manner,
J.
P. Sdrensen, and W.
E.
Stewart,
I.
Ckem.
Eng.
Data,
19,169-171 (1974).
ti
R.
C.
Reid,
J.
M. Prausnitz, and
B.
E.
Poling,
The Properties
of
Gases and Liquids,
4th
edition,
McGraw-Hill, New York
(1987).
J.
0.
Hirschfelder,
C.
F.
Curtiss, and
R.
B.
Bird,
Molecular Theoy
of
Gases and Liquids,
2nd corrected
printing, Wiley, New York
(1964), #.6b
and p.
1201.
Polar gases and gas mixtures are discussed
by
E.
A.
Mason and
L.
Monchick,
J.
Chem. Pkys.
36,2746-2757 (1962).

528
Chapter
17
Diffusivity and the Mechanisms of Mass Transport
Predict the value of
Eb,,
for the system
CO-CO,
at
296.1K
and
1.0
atm total pressure.
Computation of Mass
SOLUTION
Diffusiviiyfor
From Table
E.l
we obtain the following parameters:
Density Gases
co:
co,:
The mixture parameters are then estimated from Eqs.
17.3-14
and
15:
The dimensionless temperature is then
KT/s~~
=
(296.1)/(144.6)
=
2.048. From Table
E.2
we
can find the collision integral for diffusion,
flgpB
=
1.067.
Substitution of the preceding values
in
Eq. 17.3-12
gives
s17.4
THEORY
OF DIFFUSION IN BINARY LIQUIDS
The kinetic theory for diffusion in simple liquids is not as well developed as that for di-
lute gases, and it cannot presently give accurate analytical predictions of diffusivities.'-3
As a result our understanding of liquid diffusion depends primarily on the rather crude
hydrodynamic and activated-state models. These in turn have spawned
a
number of em-
pirical correlations, which provide the best available means for prediction. These corre-
lations permit estimation of diffusivities in terms of more easily measured properties
such as viscosity and molar volume.
The
hydrodynamic
theory
takes as its starting point the Nernst-Einstein equation:
which states that the diffusivity of a single particle or solute molecule
A
through a sta-
tionary medium
B
is given by
=
KT(~A/FA)
(17.4-1)
in which uA/FA is the "mobility" of a particle
A
(that is, the steady-state velocity attained
by the particle under the action of a unit force). The origin of
Eq.
17.4-1
is discussed in
$17.5
in connection with the Brownian motion of colloidal suspensions. If the shape and
size of
A
are known, the mobility can be calculated by the solution of the creeping-flow
equation of motion5 (Eq. 3.5-8). Thus,
if
A
is spherical and if one takes into account the
possibility of "slip" at the fluid-solid interface, one obtains6
R.
J.
Bearman and
J.
G.
Kirkwood,].
Chem. Phys.,
28,136-145 (1958).
R.
J.
Bearman,
J.
Phys. Chem.,
65,1961-1968 (1961).
C.
F.
Curtiss and
R.
B.
Bird,
J.
Chem. Phys.,
111,10362-10370 (1999).
See 517.7 and
E.
A. Moelwyn-Hughes,
Physical Chemistry,
2nd edition, corrected printing, Maanillan,
New York (1964), pp. 62-74. See also
R.
J.
Silbey and
R.
A. Alberty,
Physical Chemisty,
3rd edtion, Wiley,
New York (2001), 520.2. Apparently the Nemst-Einstein equation cannot be generalized to polymeric fluids
with appreciable velocity gradients, as has been noted by
H.
C.
&linger,
AKhE Journal,
35,279-286 (1989).
S. Kim and S.
J.
Karrila,
Microhydrodynamics: Principles and Selected Applications,
Butterworth-
Heinemann, Boston (1991
).
H.
Lamb,
Hydrodynamics,
6th edition, Cambridge University Press (1932), reprinted (1997), 5337.
$1
7.4
Theory of Diffusion in Binary Liquids
529
in which
p,
is the viscosity of the pure solvent, RA is the radius of the solute particle, and
PA,
is the "coefficient of sliding friction" (formally the same as the
p/c
of problem 2B.9).
The limiting cases of
DAB
=
and
DAB
=
0
are of particular interest:
a.
fiAB
=
~4
(no-slip condition)
In this case Eq. 17.4-2 becomes Stokes' law (Eq. 2.6-15) and Eq. 17.4-1 becomes
which is usually called the Stokes-Einstein equation. This equation applies well to the dif-
fusion of very large spherical molecules in solvents of low molecular weight7 and to sus-
pended particles. Analogous expressions developed for nonspherical particles have been
used for estimating the shapes of protein
molecule^.^,'
b.
fiAB
=
0
(complete slip condition)
In this case Eq. 17.4-1 leads to (see Eq. 4B.3-4)
If the molecules
A
and
B
are identical (that is, for self-diffusion) and if they can be as-
sumed to form a cubic lattice with the adjacent molecules just touching, then 2RA
=
and
Equation 17.4-5 has been found'' to agree with self-diffusion data for a number of liq-
uids, including polar and associated substances, liquid metals, and molten sulfur, to
within about 12%. The hydrodynamic model has proven less useful for binary diffusion
(that is, for
A
not identical to
B)
although the predicted temperature and viscosity depen-
dences are approximately correct.
Keep in mind that the above formulas apply only to dilute solutions of
A
in
B.
Some
attempts have been made, however, to extend the hydrodynamic model to solutions of
finite concentrations."
The Eyring activated-state theory attempts to explain transport behavior via a quasi-
crystalline model of the liquid state.12 It is assumed in this theory that there is some uni-
molecular rate process in terms of which diffusion can be described, and it is further
assumed that in this process there is some configuration that can be identified as the "ac-
tivated state." The Eyring theory of reaction rates is applied to this elementary process in
a manner analogous to that described in s1.5 for estimation of liquid viscosity.
A
modifi-
-
-
'
A.
Polson,
J.
Pkys. Colloid Ckem.,
54,649-652 (1950).
".
J.
V.
Tyrrell,
Diffusion and Heat Flow
in
Liquids,
Butterworths, London (1961), Chapter 6.
'
Creeping motion around finite bodies in a fluid of infinite extent has been reviewed by
J.
Happel
and H. Brenner,
Low Reynolds Number Hydrodynamics,
Prentice-Hall, Englewood Cliffs,
N.J.
(1965); see
also
S.
Kim and
S.
J.
Karrila,
Microkydrodynarnics: Principles and Selected Applications,
Butterworth-
Heinemann, Boston (1991).
G.
K.
Youngren and
A.
Acrivos,
I.
Ckem. Pkys.
63,3846-3848 (1975) have
calculated the rotational friction coefficient for benzene, supporting the validity of the no-slip condition
at molecular dimensions.
'O
J.
C.
M.
Li and
P.
Chang,
J.
Ckm. Phys.,
23,518-520 (1955).
I'
C. W.
Pyun
and M. Fixman,
J.
Clzem. Pkys.,
41,937-944 (1964).
''
S.
Glasstone, K.
J.
Laidler, and
H.
Eyring,
Tkeoy
of Rate Processes,
McGraw-Hill, New York (1941),
Chapter
IX.

530
Chapter 17 Diffusivity and the Mechanisms of Mass Transport
EXAMPLE
17.4-1
Estimation of Liquid
Diffusivity
cation of the original Eyring model by Ree, Eyring, and
coworker^'^
yields an expression
similar to Eq. 17.4-5 for traces of
A
in solvent
B:
Here
6
is a "packing parameter," which in the theory represents the number of nearest
neighbors of a given solvent molecule. For the special case of self-diffusion,
5
is found to
be very close to
27r,
so that Eqs. 17.4-5 and
6
are in good agreement despite the difference
between the models from which they were developed.
The Eyring theory is based on an oversimplified model of the liquid state, and con-
sequently the conditions required for its validity are not clear. However, Bearman has
shown2 that the Eyring model gives results consistent with statistical mechanics for "reg-
ular solutions," that is, for mixtures of molecules that have similar size, shape, and inter-
molecular forces. For this limiting situation, Bearman also obtains an expression for the
concentration dependence of the diffusivity,
in which
9,,
and
pB
are the diffusivity and viscosity of the mixture at the composition
XA,
and
a,
is the thermodynamic activity of species
A.
For regular solutions, the partial
molar volumes,
VA
and
V,,
are equal to the molar volumes of the pure components.
Bearman suggests on the basis of his analysis that Eq. 17.4-7 should be limited to regular
solutions, and it has in fact been found to apply well only to nearly ideal solutions.
Because of the unsatisfactory nature of the theory for diffusion in liquids, it is neces-
sary to rely on
empirical
expressions. For example, the Wilke-Chang equation14 gives the
diffusivity for small concentrations of
A
in
B
as
Here
6
is the molar volume of the solute
A
in cm3/g-mole as liquid at its normal boiling
point,
p
is the viscosity of the solution in centipoises,
t+!~~
is an llassociation parameter"
for the solvent, and
T
is the absolute temperature in
K.
Recommended values of
$,
are:
2.6
for water; 1.9 for methanol;
1.0
for benzene, ether, heptane, and other unassociated
solvents. Equation 17.4-8 is good only for dilute solutions of nondissociating solutes. For
such solutions, it is usually good within
210%.
Other empiricisms, along with their relative merits, have been summarized by Reid,
Prausnitz, and Poling.I5
Estimate
9,,
for a dilute solution of
TNT
(2,4,6-trinitrotoluene) in benzene
at
15°C.
SOLUTION
Use the equation of Wilke and Chang, taking TNT as component
A
and benzene as compo-
nent
B.
The required data are
p
=
0.705~~ (the viscosity for pure benzene)
VA
=
140 cm3/g-mole (for TNT)
l3
H.
Eyring,
D.
Henderson,
B.
J.
Stover,
and
E.
M.
Eyring,
Statistical Mechanics and Dynamics,
Wiley,
New
York
(1964), 516.8.
'v.
R.
Wilke,
Chem.
Eng.
Pvog.,
45,218-224 (1949);
C.
R.
Wilke
and
P. Chang,
AIChE
Journal,
1,
264-270
(1955).
'5
R.
C.
Reid,
J.
M.
Prausnitz,
and
B.
E.
Poling,
The Properties
of
Gases
find
Liquids,
4th
edition,
McGraw-Hill,
New
York
(1987), Chapter 11.

517.5
Theory of Diffusion in Colloidal Suspensions
531
$B
=
1.0
(for benzene)
MB
=
78.11
(for benzene)
Substitution into
Eq.
17.4-8
gives
This result compares well with the measured value of
1.39
X
cm2/s.
517.5
THEORY
OF
DIFFUSION IN COLLOIDAL SUSPENSIONS~~~
Next we turn to the movement of small colloidal particles in a liquid. Specifically we
consider a finely divided, dilute suspension of spherical particles of material
A
in
a
sta-
tionary liquid
B.
When the spheres of
A
are sufficiently small (but still large with respect
to the molecules of the suspending medium), the collisions between the spheres and the
molecules of
B
will result in an erratic motion of the spheres. This random motion is re-
ferred to as Brownian rnoti~n.~
The movement of each sphere can be described by an equation of motion, called the
Langevin
equation:
in
which u, is the instantaneous velocity of the sphere of mass
m.
The term -luA gives
the Stokes' law drag force:
5
=
6rpBR,
being the "friction coefficient." Finally F(t) is the
rapidly oscillating, irregular Brownian motion force. Equation 17.5-1 cannot be "solved"
in the usual sense, since it contains the randomly fluctuating force F(t). Equations such
as Eq. 17.5-1 are called "stochastic differential equations."
If it is assumed that
(i)
F(t) is independent of
uA
and that (ii) the variations in F(t) are
much more rapid than those of u,, then it is possible to extract from Eq. 17.5-1 the proba-
bility W(uA,t;uA0)duA that at time t the particle will have a velocity in the range of uA to
UA
+
du,. Physical reasoning requires that the probability density W(U~,~;U~~) approach a
Maxwellian (equilibrium) distribution as t
-+
w:
Here,
T
is the temperature of the fluid in which the particles are suspended.
A. Einstein,
Ann. d. Phys,
17,549-560 (1905), 19,371-381 (1906);
Investigations on the Theory of the
Brownian Movement,
Dover, New York (1956).
S. Chandrasekhar,
Rev. Mod. Phys.,
15,l-89 (1943).
W.
B.
Russel, D. A. Saville, and
W.
R.
Schowalter,
Colloidal Dispersions,
Cambridge University Press
(1989);
H.
C.
Ottinger,
Stochastic Processes in Polymeric Fluids,
Springer, Berlin (1996).
Named after the botanist
R.
Brown,
Phil. Mag.
(4), p. 161 (1828);
Ann. d. Phys.
u.
Chem.,
14,294-313
(1828). Actually the phenomenon had been discovered and reported earlier
in
1789 by Jan Ingenhousz
(1730-1799) in the Netherlands.
%s can
be
seen from Example 4.2-1, Stokes' law is valid only for the steady, unidirectional motion
of a sphere through a fluid. For a sphere moving in
an
arbitrary manner, there are, in addition to the
Stokes' contribution, an inertial term and a memory-integral term (the Basset force). See A.
8.
Basset,
Phil. Trans.,
179,43-63 (1887);
H.
Lamb,
Hydrodynamics,
6th edition, Cambridge University Press (1932),
reprinted (1997),
p.
644;
H.
Villat and
J.
Kravtchenko,
Lecons sur les Fluides Visqueux,
Gauthier-Villars,
Paris (1943),
p.
213, Eq.
(62);
L. Landau and
E.
M.
Lifshitz,
Fluid Mechanics,
2nd edition, Pergamon, New
York (1987), p. 94. In applying the Langevin equation to polymer kinetic theory, the role of the Basset
force has been investigated by
J.
D. Schieber,
J.
Chem. Phys.,
94,7526-7533 (1991).

532
Chapter
17
Diffusivity and the Mechanisms of Mass Transport
Another quantity of interest that can be obtained from the Langevin equation is the
probability, W(r,t;ro,uAo)dr, that at time
t
the particle will have a position in the range r to
r
+
dr if its initial position and velocity were ro and
UAO.
For long times, specifically t
>>
m/{,
this probability is given by
However, this expression turns out to have just the same form as the solution of Fick's
second law of diffusion (see Eq. 19.1-18 and Problem
20B.5)
for the diffusion from
a
point source. One simply has to identify
W
with the concentration cA, and KT/~ with
B,,.
In this way Einstein (see Ref. 1 on page 531) arrived at the following expression for the
diffusivity of a dilute suspension of spherical colloid particles:
Thus,
is related to the temperature and the friction coefficient
5
(the reciprocal of the
friction coefficient is called the "mobility"). Equation 17.5-4 was already given in
Eq.
17.4-3
for the interdiffusion of liquids.
s17.6
THEORY OF DIFFUSION OF POLYMERS
For a dilute solution of a polymer
A
in a low-molecular-weight solvent
B,
there is a de-
tailed theory,' in which the polymer molecules are modeled as bead-spring chains (see
Fig. 8.6-2). Each chain is a linear arrangement of N beads and
N
-
1 Hookean springs.
The beads are characterized by a friction coefficient
6,
which describes the Stokes' law
resistance to the bead motion through the solvent. The model further takes into account
the fact that, as a bead moves around, it disturbs the solvent in the neighborhood of all
the other beads; this is referred to as hydrodynamic interaction. The theory ultimately
predicts that the diffusivity should be proportional to
N-I'2
for large
N.
Since the num-
ber of beads is proportional to the polymer molecular weight M, the following result is
obtained:
The inverse square-root dependence is rather well borne out by experiment.' If hydrody-
namic interaction among beads were not included, then one would predict
%,,
-
1 /M.
The theory of self-diffusion in an undiluted polymer has been studied from several
points of
vie^.^,^
These theories, which are rather crude, lead to the result that
'
J.
G. Kirkwood,
Macromolecules,
Gordon and Breach, New York (1967),
pp.
13,41,76-77,95,
101-102. The original Kirkwood theory has been reexamined and slightly improved by
H.
C.
&tinger,
J.
Chem. Phys.,
87,3156-3165 (1987).
R.
B. Bird,
C.
F.
Curtiss,
R.
C. Armstrong, and
0.
Hassager,
Dynamics of Polymeuic Liquids, Vol.
2,
Kinetic Theory,
2nd edition, Wiley, New York (19871, pp. 174-175.
P.-G.
de Gennes and
L.
Lbger,
Ann.
Rev. Phys. Chem.,
49-61
(1982);
P.-G.
de Gennes,
Physics Today,
36,3539 (1983). De Gennes introduced the notion of
reptation,
according to which the polymer molecules
move back and forth along their backbones in a snake-like Brownian motion.
R.
B. Bird,
C.
F.
Curtiss,
R.
C.
Armstrong,
and
0.
Hassager,
Dynamics of Polymeric Liquids, Vol.
2,
Kinetic Theory,
2nd edition, Wiley, New York (1987),
pp.
326-327;
C.
F.
Curtiss and
R.
B.
Bird,
Puoc. Nat.
Acad. Sci.,
93,7440-7445 (1996).

s17.7
Mass and Molar Transport
by
Convection
533
Experimental data agree more or less with this result," but the exponent on the molecular
weight may be as great as
3
for some polymers.
Although a very general theory for diffusion of polymers has been de~eloped,~ not
very much has been done with it. So far it has been used to show that, in flowing dilute
solutions of flowing polymers, the diffusivity tensor (see Eq. 17.1-1
0)
becomes anisotropic
and dependent on the velocity gradients. It has also been shown how to generalize the
Maxwell-Stefan equations (see 517.9 and s24.1) for multicomponent polymeric liquids.
Further advances in this subject can be expected through use of molecular
simulation^.^
517.7
MASS
AND MOLAR TRANSPORT
BY
CONVECTION
In 517.1, the discussion of Fick's (first) law of diffusion was given in terms of
mass
units:
mass concentration, mass flux, and the mass average velocity. In this section we extend the
previous discussion to include molar units. Thus most of this section deals with questions
of notation and definitions. One might reasonably wonder whether or not this dual set of
notation is really necessary. Unfortunately, it really is. When chemical reactions are in-
volved, molar units are usually preferred. When the diffusion equations are solved to-
gether with the equation of motion, mass units are usually preferable. Therefore it is
necessary to acquire familiarity with both. In this section we also introduce the concept of
the convective flux of mass or moles.
Mass and Molar Concentrations
Earlier we defined the mass concentration p, as the mass of species
a
per unit volume of
solution. Now we define the molar concentration c,
=
p,/M, as the number of moles of
a
per unit volume of solution.
Similarly, in addition to the mass fraction
o,
=
pJp, we will use the mole fraction
x,
=
c,/c.
Here p
=
Zap,
is the total mass of all species per unit volume of solution, and c
=
Z,c,
is the total number of moles of all species per unit volume of solution. By the word
"solution" we mean a one-phase gaseous, liquid, or solid mixture. In Table 17.7-1 we
summarize these concentration units and their interrelation for multicomponent systems.
It is necessary to emphasize that p, is the mass concentration of species
a
in a mix-
ture. We use the notation
p'"i
for the density of pure species
a
when the need arises.
Mass Average and Molar Average Velocity
In a diffusing mixture, the various chemical species are moving at different velocities. By v,,
the "velocity of species
a,"
we do not mean the velocity of an individual molecule of species
a.
Rather, we mean the average of all the velocities of molecules of species
a
within a small
volume. Then, for a mixture of
N
species, the local mass average velocity v is defined as
P. F.
Green, in
Diffusion in Polymers
(P.
Neogi, ed.), Dekker, New York
(1996),
Chapter
6.
According to
T.
P.
~odge,
Phys.
Rev. Letters,
86,3218-3221 (1999),
measurements on undiluted polymers
show that the exponent on the molecular weight should be about
2.3.
". F.
Curtiss and
R.
B.
Bird,
Adv.
Polym.
Sci., 125,l-101 (1996)
and
J.
Chem.
Phys.,
111,10362-10370
(1999).
D.
N. Theodorou,
in
Diffusion in Polymers
(P.
Neogi,
ed.),
Dekker, New York
(19961,
Chapter
2.
534
Chapter
17
Diffusivity and the Mechanisms of Mass Transport
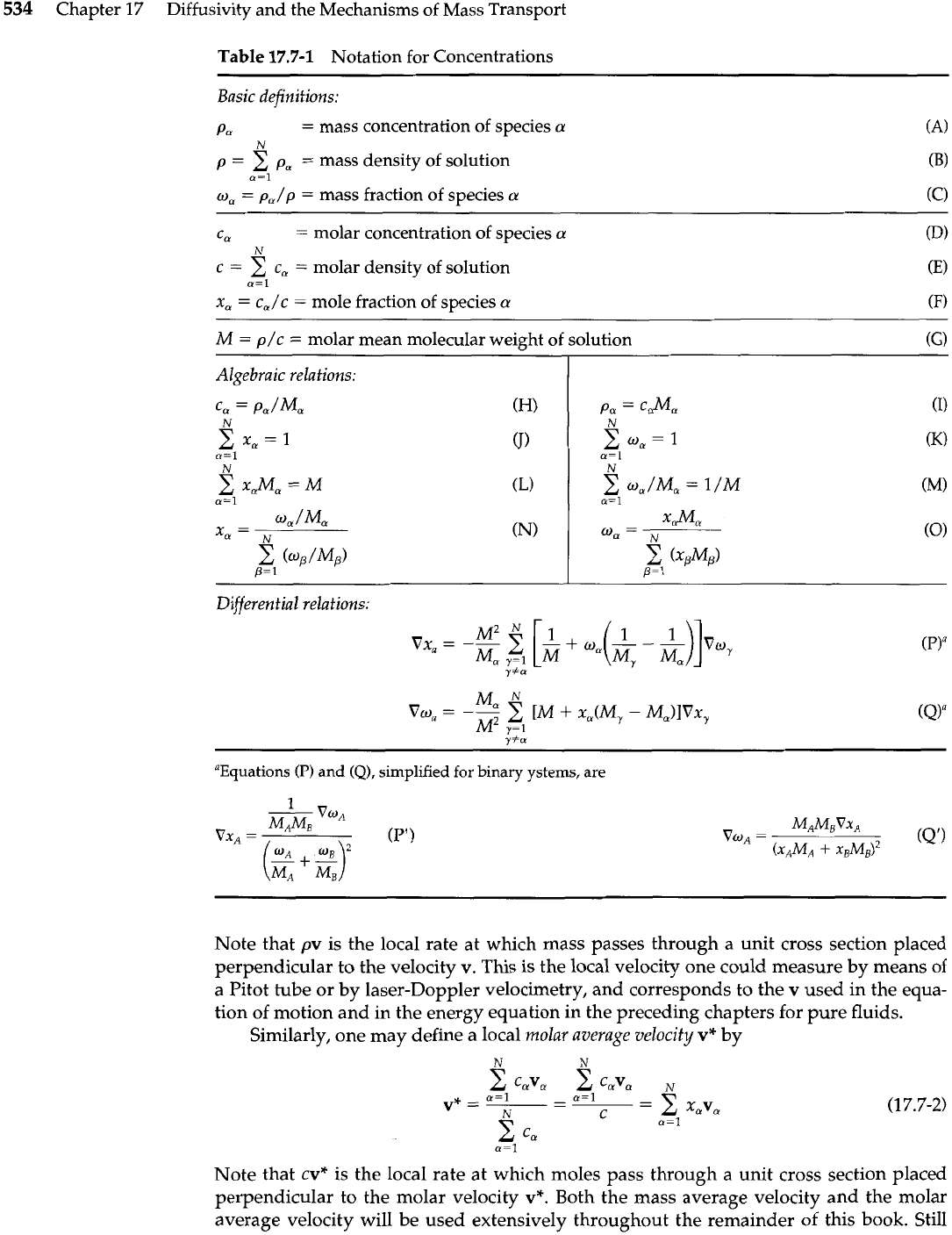
Table
17.7-1
Notation for Concentrations
Basic definitions:
Po
=
mass concentration of species
a
(A)
N
p
=
2
pa
=
mass density of solution
a=l
ma
=
p,/p
=
mass fraction of species
a
(C)
Ca
=
molar concentration of species
a
A'
c
=
2
c,
=
molar density of solution
a=l
x,
=
c,/c
=
mole fraction of species
a
M
=
p/c
=
molar mean molecular weight of solution
(GI
Algebraic relations:
Differential relations:
"Equations
(P)
and
(Q),
simplified for binary ystems, are
Note that
pv
is the local rate at which mass passes through a unit cross section placed
perpendicular to the velocity
v.
This is the local velocity one could measure by means
of
a Pitot tube or by laser-Doppler velocimetry, and corresponds to the
v
used in the equa-
tion of motion and in the energy equation in the preceding chapters for pure fluids.
Similarly, one may define a local
molar average velocity
v*
by
Note that
cv*
is the local rate at which moles pass through
a
unit cross section placed
perpendicular to the molar velocity
v*.
Both the mass average velocity and the molar
average velocity
will
be used extensively throughout the remainder of this book. Still

g17.7 Mass and Molar Transport by Convection
535
Table
17.7-2
Notation for Velocities in Multicomponent Systems
Basic
definitions:
V,
velocity of species
a
with respect to fixed coordinates
(A)
N
v
=
2
O,V,
mass average velocity
a=l
N
V*
=
x
X,V,
molar average velocity
a=l
(C)
v,
-
v
diffusion velocity of species
a
with respect to the mass average
velocity
v
(D)
va
-
v*
diffusion velocity of species
a
with respect to the molar average
velocity
v*
(E)
Additional relations:
other average velocities are sometimes used, such as the
volume average velocity
(see
Problem 17C.1). In Table 17.7-2 we give a summary of the various relations among
these velocities.
Molecular Mass and Molar Fluxes
In 517.1 we defined the molecular mass flux of
a
as the flow of mass of
a
through a unit
area per unit time:
j,
=
p,(v,
-
v).
That is, we include only the velocity of species
a
rela-
tive to the mass average velocity
v.
Similarly, we define the molecular molar flux of
species
a
as the number of moles of
a
flowing through a unit area per unit time:
J:
=
cA(vA
-
vr).
Here we include only the velocity of species
a
relative to the molar average
velocity
v*.
Then in 517.1 we presented Fick's (first) law of diffusion, which describes how the
mass of species
A
in a binary mixture is transported by means of molecular motions.
This law can also
be
expressed in molar units. Hence we have the pair of relations for
bi-
nary
systems:
The differences
v~
-
v
and
v,
-
v*
are sometimes referred to as
diffusion velocities.
Equa-
tion 17.7-4 can be derived from
Eq.
17.7-3 by using some of the relations in Tables 17.7-1
and
2.
Convective Mass and Molar Fluxes
In addition to transport by molecular motion, mass may also be transported
by
the bulk
motion of the fluid. In Fig. 9.7-1 we show three mutually perpendicular planes of area
dS
at
a
point
P
where the fluid
mass average velocity
is
v.
The volume rate of flow across the
plane perpendicular to the surface element
dS
perpendicular to the x-axis is
v,dS.
The
rate at which mass of species
a
is being swept across the same surface element is then
p,v,dS.
We
can write similar expressions for the mass flows of species
a
across the sur-
face elements perpendicular to the
y-
and z-axes as
p,v,dS
and
p,v,dS,
respectively. If we
536
Chapter
17
Diffusivity and the Mechanisms of Mass Transport
now multiply each of these expressions by the corresponding unit vector, add them, and
divide by dS, we get
as the convective mass flux vector, which has units of kg/m2
.
s.
If one goes back and repeats the story of the preceding paragraph, but using every-
where molar units and the molar average velocity v*, then we get
as the convective molar flux vector, which has units of kg-mole/m2 s.
To get the convective mass and molar fluxes across a unit surface whose normal unit
vector is n, we form the dot products (n
.
p,v) and (n
.
c,v*), respectively.
517.8
SUMMARY OF MASS AND MOLAR FLUXES
In Chapters 1 and
9
we introduced the combined momentum flux tensor
+
and the com-
bined energy flux vector e, which we found useful in setting up the shell balances and
equations of change. We give the corresponding definitions here for the mass and molar
flux vectors. We add together the molecular mass flux vector and the convective mass
flux vector to get the combined mass flux vector, and similarly for the combined molar
flux
vector:
Com bined mass flux:
Combined molar flux:
In the first three lines of Table 17.8-1 we summarize the definitions of the mass and
molar fluxes discussed so far.
In
the shaded squares we also give the definitions of the
fluxes
j:
(mass flux with respect to the molar average velocity) and
J,
(molar flux with re-
spect to mass average velocity). These "hybrid" fluxes should normally not be used.
In the remainder of Table 17.8-1 we give a summary of other useful relations, such
as the sums of the fluxes and the interrelations among the fluxes. By using Eqs.
0)
and
(M)
we can rewrite Eqs. 17.8-1 and
2
as
When simplified for binary systems, these relations can be combined with Eqs. 17.7-3
and 17.7-4, to get Eqs.
(C)
and
(D)
of Table 17.8-2, which are equivalent forms of Fick's
(first) law. The forms given in Eqs.
(E)
and (F) of Table 17.8-2, in terms of the relative ve-
locities of the species, are interesting because they involve neither v nor vr.
In Chapter
18
we will write Fick's law exclusively in the form of Eq.
(D)
of Table
17.8-2. It is this form that has generally been used in chemical engineering. In many
problems something is known about the relation between
NA
and
N,
from the stoi-
chiometry or from boundary conditions. Therefore
N,
can be eliminated from Eq.
(D),
giving
a
direct relation between
NA
and VxA for the particular problem.
In s1.7 we pointed out that the total molecular momentum flux through a surface of
orientation n is the vector In
m].
In 59.7 we mentioned the analogous quantity for the mol-
ecular heat flux-namely, the scalar (n
.
q).
The analogous mass transport quantities are
the scalars (n
*
j,)
and (n
.
J:),
which give the total mass and molar fluxes through
a
surface
of orientation
n.
Similarly, for the combined fluxes through a surface of orientation n, we
have for momentum [n
+I,
for energy (n e), and for species
(n
.
n,) and (n
.
N,).
